Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2894
Revised: March 15, 2024
Accepted: April 17, 2024
Published online: June 6, 2024
Processing time: 112 Days and 0.5 Hours
For patients with acute paraplegia caused by spinal giant cell tumor (GCT) who require emergency decompressive surgery, there is still a lack of relevant reports on surgical options. This study is the first to present the case of an acute para
A 27-year-old female patient who underwent single-level TES in an emergency presented with sudden severe back pain and acute paraplegia due to a thoracic spinal tumor. After emergency TES, the patient's spinal cord function recovered, and permanent paralysis was avoided. The postoperative histopathological examination revealed that the excised neoplasm was a rare GCT. Unfortunately, the tumor recurred 9 months after the first surgery. After 12 months of deno
This patient with acute paraplegia underwent TES twice, including once in an emergency, and achieved good therapeutic results. TES in emergency surgery is feasible and safe when conditions permit; however, it may increase the risk of tumor recurrence.
Core Tip: For patients with acute paraplegia caused by spinal giant cell tumor (GCT) who require emergency decompressive surgery, there is still a lack of relevant reports on surgical options. This study is the first to present the case of an acute paraplegic patient with a thoracic spinal GCT who underwent an emergency total en bloc spondylectomy (TES). Despite tumor recurrence, three-level TES was repeated after denosumab therapy. The patient returned to an active lifestyle 1 month after the second surgery, and no recurrence of GCT was found at the last follow-up.
- Citation: Liang HF, Xu H, Zhan MN, Xiao J, Li J, Fei QM. Thoracic giant cell tumor after two total en bloc spondylectomies including one emergency surgery: A case report. World J Clin Cases 2024; 12(16): 2894-2903
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2894.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2894
Giant cell tumor (GCT) of the bone were newly classified as intermediate malignant tumors with high local infiltration ability by the World Health Organization in 2020[1]. Only approximately 1.4%-15% of GCT occur in the spine and most commonly appear in patients aged 20-40 years, with female sex predominance[2]. The complications of spinal GCT include pathologic fracture, lung metastases, nerve root compression in vertebral location, and high postoperative recurrence rate[3]. Patients with a spinal GCT most commonly present with persistent back pain and varying degrees of neurological dysfunction[3]. A large cervical/thoracic GCT will result in sensory and motor deficits, or even paraplegia of extremities secondary to spinal cord compression. A lumbar GCT with the involvement of the sacral plexus may induce bladder and bowel symptoms.
Total spondylectomy is the treatment of choice for eradicating spinal GCT. The Spinal Tumor Research Group (2009) recommended total en bloc spondylectomy (TES) for better control of spinal GCT[4]. Although total spondylectomy might be the best strategy to avoid recurrence, surgical technique is challenging with the possibility of severe complications. Because spinal GCT is adjacent to the spinal cord, nerve root, aorta, vena cava, and vertebral artery[5]. It is difficult and risky to achieve the goal of en bloc resection, leading to higher incidence of complications (vascular injury, pleural effusion, cerebrospinal fluid leakage, neurological deterioration, etc.) compared with other spine surgeries.
Some patients with spinal GCT may experience rapid neurological compromise owing to tumor compression, resulting in paraplegia, which often requires immediate emergency surgery to relieve spinal cord compression. To the best of our knowledge, emergency TES for sudden paraplegia caused by spinal GCT has not yet been reported. Emergency surgery must be performed under extreme stress and time constraints, sometimes without a thorough understanding of the patient's condition and clinical information, which further increases the difficulty of performing TES. Therefore, the efficacy and prognosis of emergency TES should be evaluated.
The surgical treatment of recurrent spinal GCT requires special attention. Repeated surgical treatment may be difficult because of the extent of adhesions surrounding the recurrent lesions and disorganized anatomical structures[3]. Regarding treating spinal GCT recurrence after TES, few reports have described a second TES for recurrent multilevel spinal GCT. This is because procedures with high technical requirements often discourage orthopedist from performing multilevel revision surgeries and leave patients with palliative care.
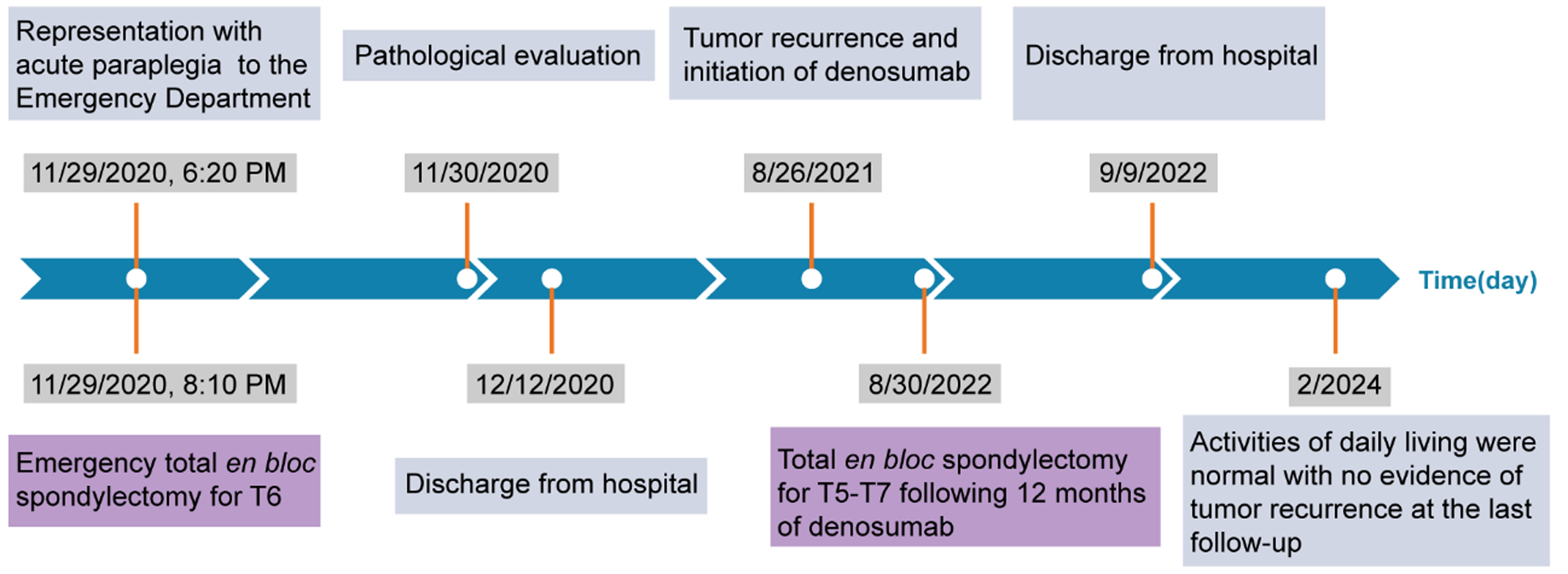
This report describes a case of a 27-year-old female patient who experienced acute paraplegia due to a thoracic spine GCT and underwent single-level TES in the emergency department, successfully recovering spinal cord function and avoiding permanent paralysis. Although the spinal GCT recurred 9 months after the first surgery, we performed three-level TES again after 12 months of neoadjuvant denosumab therapy. The patient returned to an active lifestyle 1 month after the second surgery, and no recurrence of the GCT was observed at the 18-month of the second postoperative follow-up. The timeline of the major clinical events during treatment and follow-up is shown in Figure 1.
A 27-year-old female patient presented to a local hospital with sudden severe back pain and complete paraplegia.
After insertion of an indwelling urethral catheter, the patient was transferred to the emergency department of our hospital by ambulance the following day.
The patient had no history of trauma, fever, weight loss, or infection.
Physical examination revealed bilateral lower limb paraplegia with a power of 0 out of 5, loss of knee reflexes, and a negative Babinski’s sign. Superficial sensory function was lost below the T8 level. Fortunately, deep sensory function was still present.
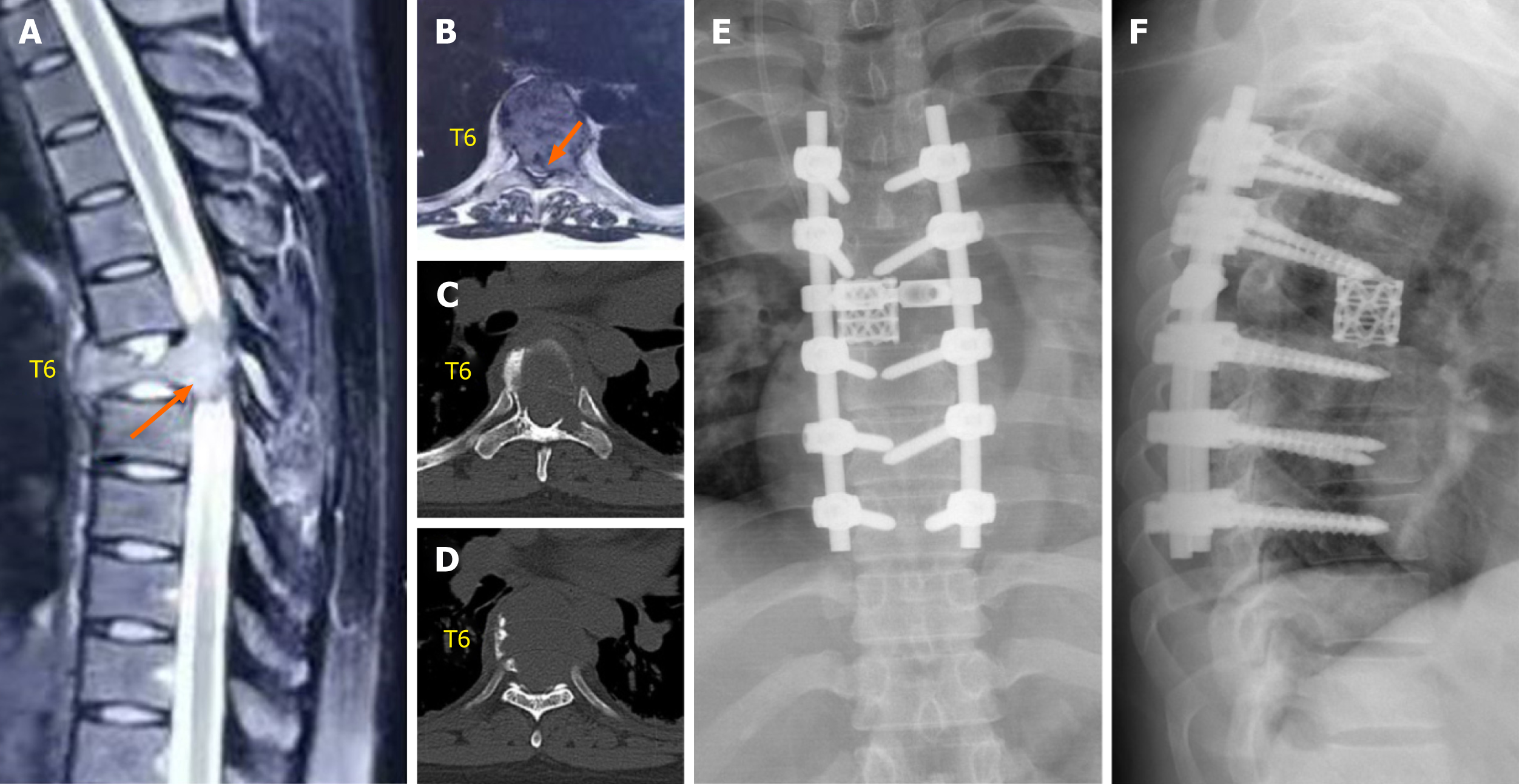
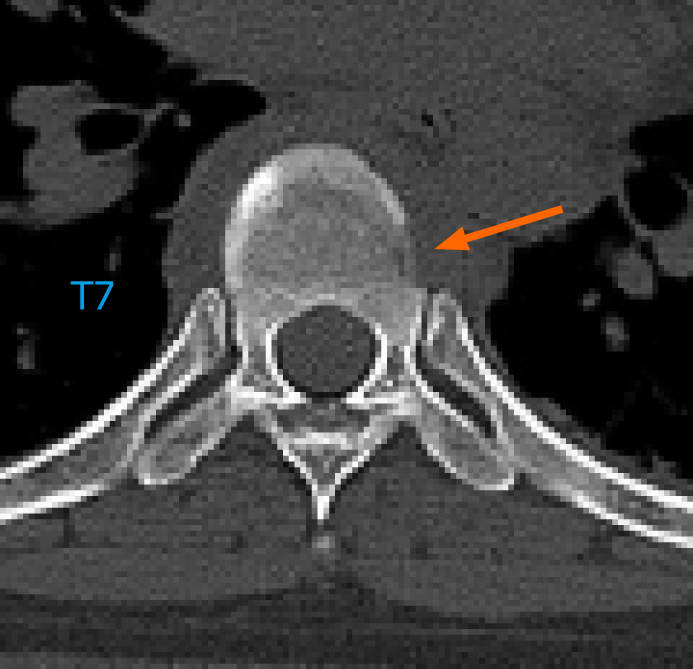
Emergency magnetic resonance imaging (MRI) revealed a vertebral tumor and pathological fracture of the T6 vertebra, and the spinal cord was severely compressed (Figure 2A and B). Emergency chest, abdominal, and pelvic computed tomography (CT) performed at our hospital revealed an osteolytic lesion involving the T6 vertebra that extended into its accessory process (Figure 2C and D).
Based on the patient’s age, medical history, imaging data, and physical signs, the possibility of a primary spinal tumor was considered.
To relieve spinal cord compression as soon as possible and rescue spinal cord function, we performed emergency TES for T6 through a single posterior approach 8 h after the patient developed complete paraplegia. The patient was placed in a prone position for pedicle screw placement.
After pedicle screws were inserted into the T4-5 and T7-9 bilateral vertebral pedicles, the T5-7 costovertebral joint was exposed, and the ribs were transected approximately 4 cm laterally from the T6 costotransverse joint. Intercostal blood vessels were ligated. The superior articular processes of T7 and inferior articular processes of T5 were removed. The laminae at T5, T6, and T7 were resected using an ultrasonic bone knife to expose the spinal canal and dural membrane. The dural pulsation was weak. Subsequently, gentle blunt dissection of the anterior T6 vertebral body and careful electrocoagulation of segmental blood vessels were performed.
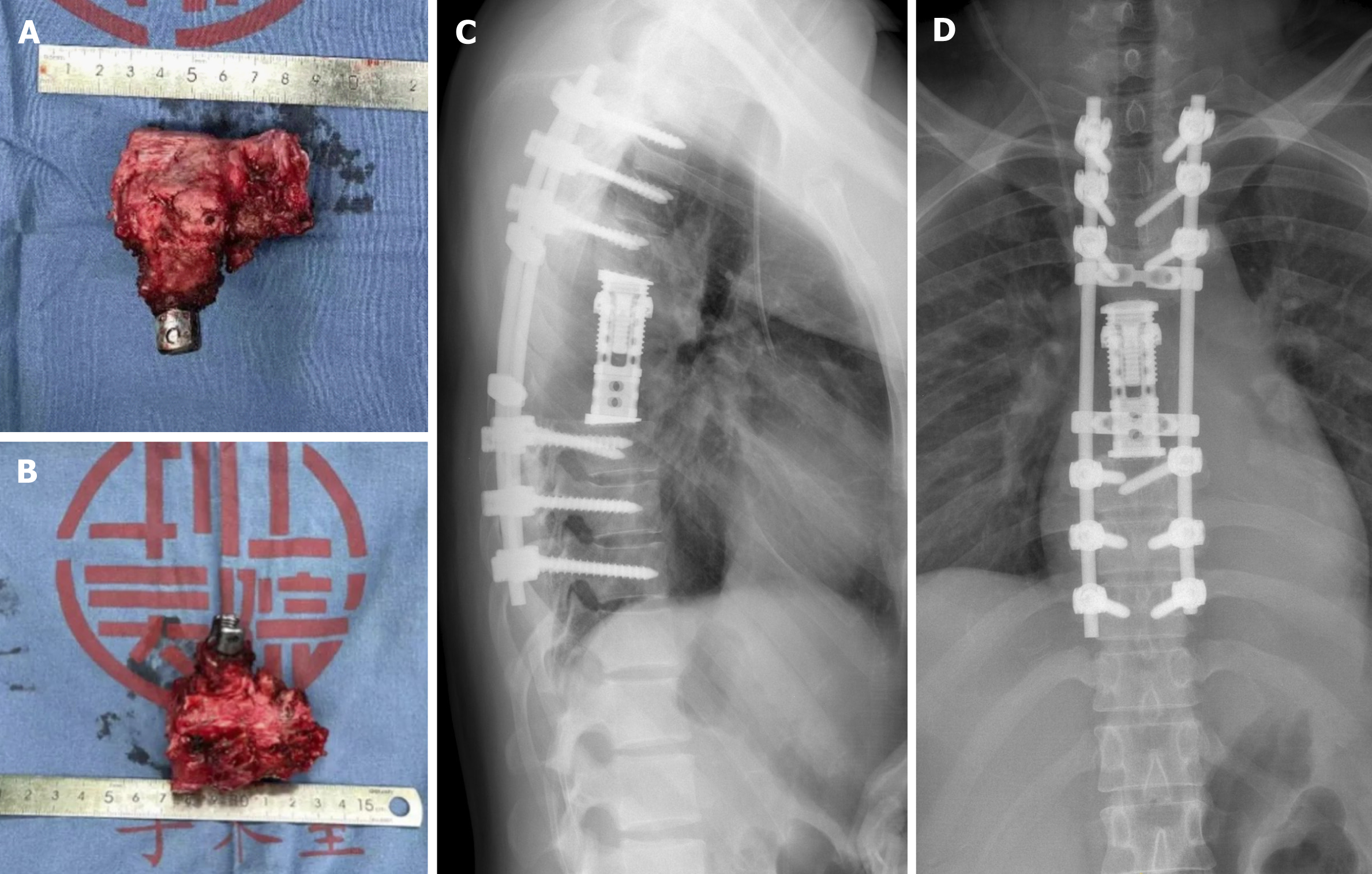
A pair of long, homemade, S-shaped protective retractors were inserted, with their anterior tips overlapping at the anterior T6 vertebral body. Before bilateral resection of T6 pedicles, temporary stability was maintained by using a posterior fixation rod on the left side. At the level of the T5-6 and T6-7 intervertebral discs, cross-puncture needles were used on both sides, and the intervertebral discs were cut with a stainless-steel thread wire saw (T-saw) in a reciprocating motion. After all disc dissections were completed, the freed anterior column was rotated around the spinal cord and carefully removed from the right side. A total en bloc resection of the vertebral tumor was performed. The residual disc and cartilaginous endplates were removed. Subsequently, a titanium mesh cylinder of appropriate length was selected and filled with autogenous bone graft. It was then placed in the middle of the cutting space. A connecting rod and transverse connection were used for fixation (Figure 2E and F). Finally, the wound was closed after the drain insertion.
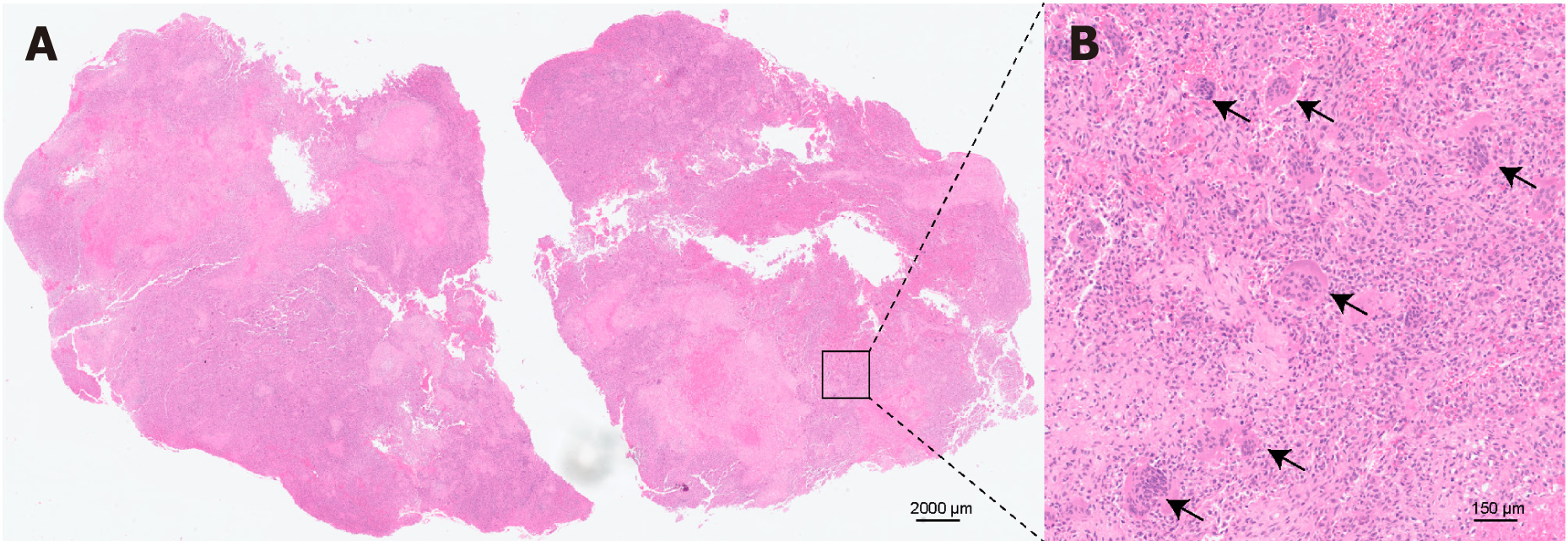
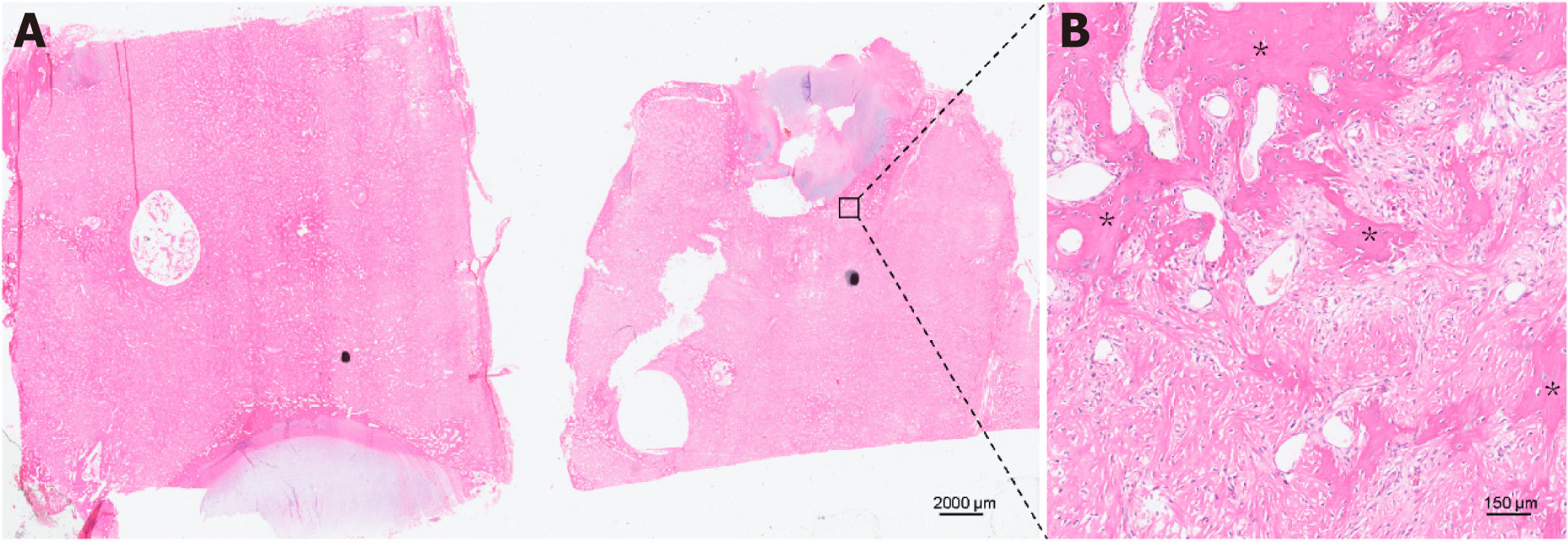
The total surgical time was 6 h, and the total amount of blood loss was 800 mL. Hematoxylin and eosin staining and immunohistochemical analyses confirmed a GCT characterized by multinucleate giant cells surrounded by neoplastic mononuclear mesenchymal stromal cells with H3F3A mutations (Figure 3). The patient was discharged on the 13th postoperative day with superficial sensory restoration approximately 10 cm below the knee joint.
One month postoperatively, the patient's neurological deficits had recovered, and she was able to ambulate with crutches. The patient was able to walk without crutches 2 months postoperatively. After 3 months of postoperative follow-up, activities of daily living were normal, with no evidence of local recurrence.
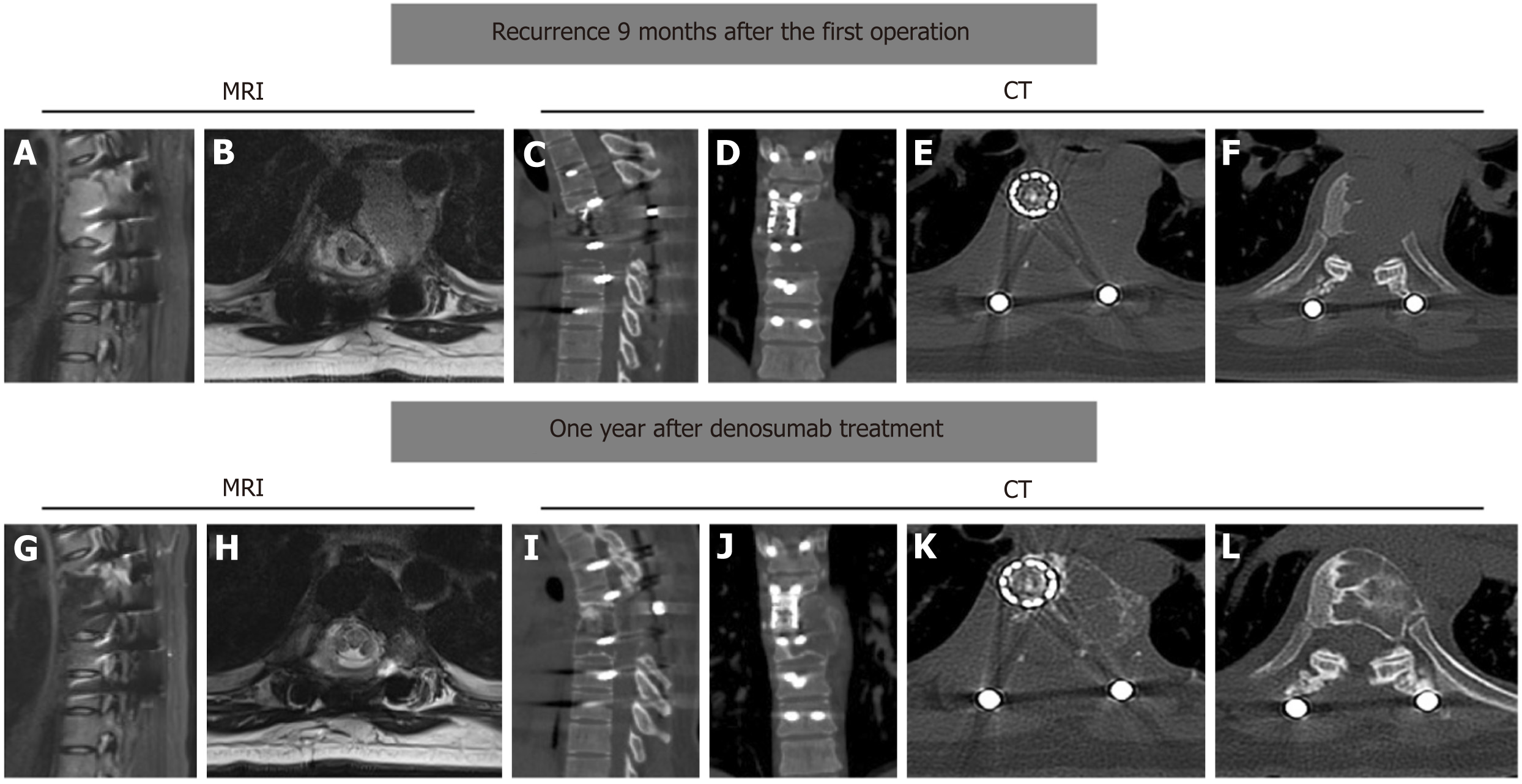
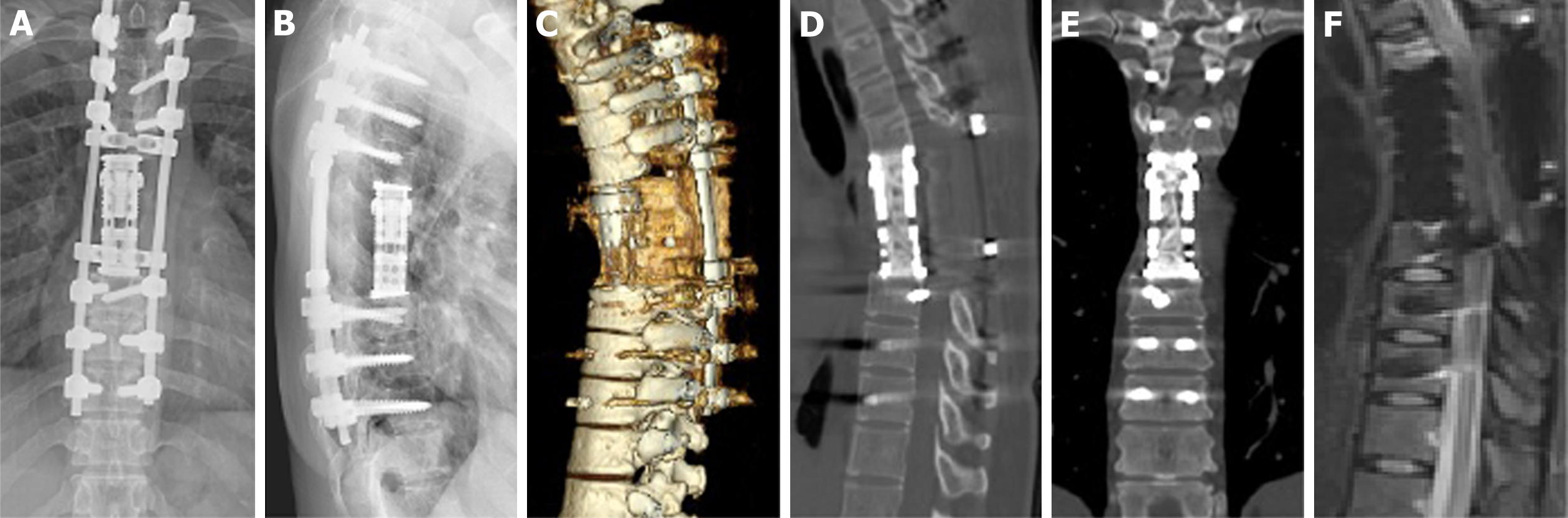
Approximately 9 months after the initial surgery, the patient started experiencing mild back pain. She was followed-up at our outpatient clinic, and tumor recurrence was noted. Radiographic analysis revealed osteolytic destruction of the T7 vertebral body and formation of soft tissue masses beside the titanium mesh cylinder without additional metastatic lesions (Figure 4A-F).
The mass pushed the thoracic aorta laterally. Considering the extensive soft tissue involvement of the recurrent GCT, the patient received denosumab 120 mg subcutaneous injections weekly for 3 wk, followed by monthly subcutaneous injections of 120 mg for 11 months. The back pain disappeared after initial denosumab treatment. One year after denosumab treatment, MRI and CT revealed significant sclerotic rim formation at the tumor site and tumor shrinkage (Figure 4G-L).
To prevent further progression of the recurrent thoracic spinal GCT, TES was performed at three levels (T5-T7) of the thoracic spine using a single posterior approach following denosumab treatment. Preoperative angiography and embolization of the segmental artery from T5 to T7 were performed the day before the surgery to reduce intraoperative bleeding. A skin incision was made along the surgical scar on the patient’s back. Lateral dissection was performed to expose the lamina at T2-10. Pedicle screws were placed at T2, T3, and T10, and a posterior fixation rod was used to maintain temporary stability.
The previously placed tail caps, connecting rods, and transverse connections on the left side were removed from the spine. Surgeons carefully exfoliated the scar tissue and exposed the T5-8 costovertebral joint. The ribs were transected approximately 4 cm lateral to the costotransverse joint. Parts of the T4 spinous process, T4 laminae, and T8 laminae were excised, and the spinal canals at the levels of T4 and T8 were fully exposed. Dissection between the scar tissue and the dura was carefully performed, and good dural pulsation was observed. Gentle blunt dissection was performed on the left vertebral of T5-T7 to cross the median line, and segmental arteries were electrocoagulated. A temporary fixation rod was placed on the left side. Blunt dissection was continued on the right side of the vertebral body until the hands joined the front of the vertebral body. Expansive changes in the T7 tumor mass and a limited paravertebral tumor mass on the left side of T6 were observed that did not break through the capsule. S-shaped protective retractors were placed in front of the vertebral body to separate and protect the pleura and the great vessels. After the two discs were thoroughly cut from T4-5 to T7-8 using sharp-pointed knives and a T-saw, the fixation rod was replaced on the right side, and en bloc laminectomy was performed after T5 and T7 pediculotomy. The freed vertebral bodies at T5-7 were rotated around the spinal cord and carefully removed from the left side. A suitable artificial vertebral body filled with autogenous bone is placed at the middle of the cutting space. After verification of placement with the C-arm on radiographs, the posterior instrumentation was slightly adjusted to compress the inserted mesh. The wound was closed following inserting a drain. Thus, total en bloc resection of the three vertebrae involved in the tumor was achieved (Figure 5).
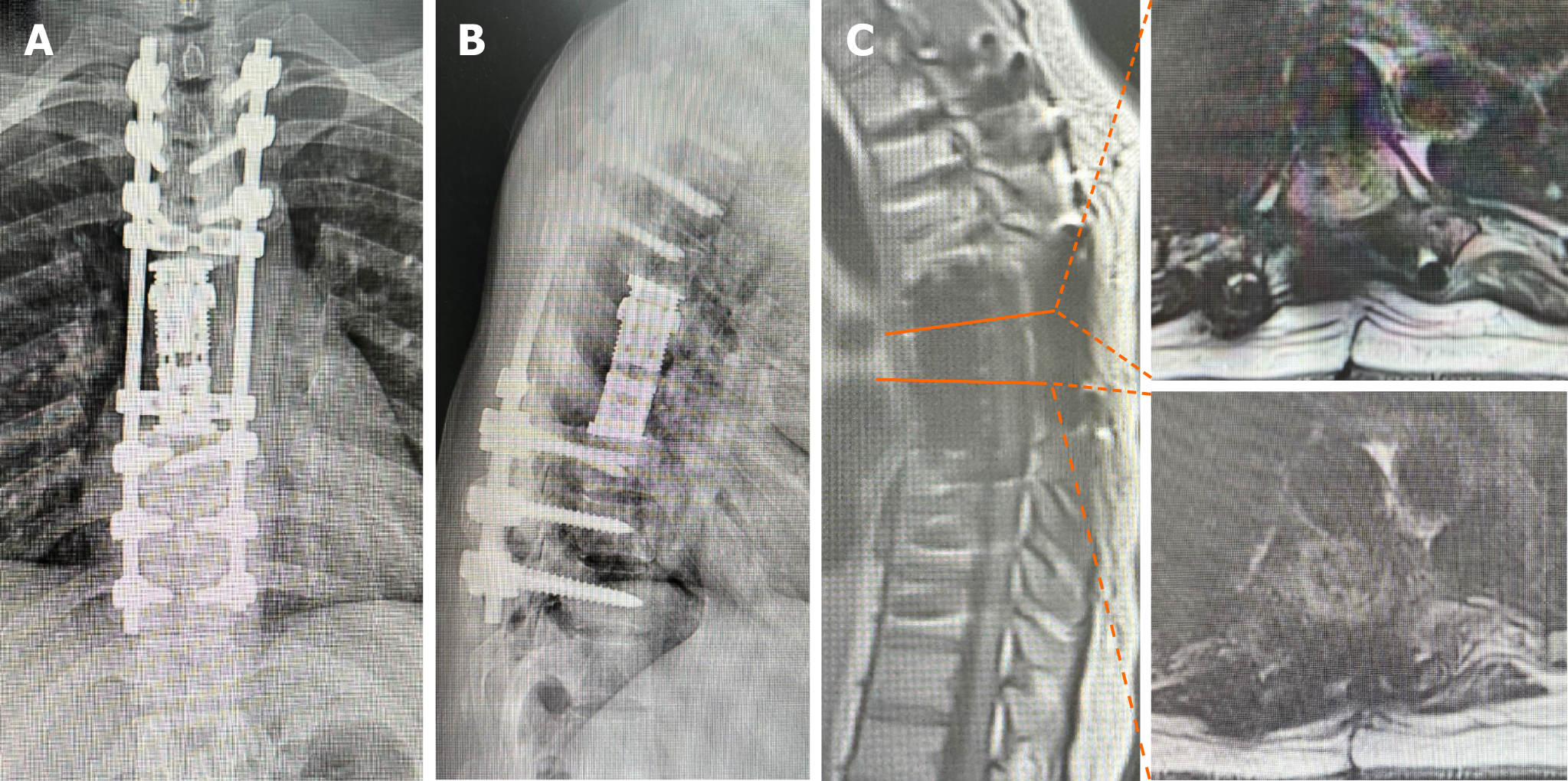
The operative time was 11 h, with a blood loss of 1500 mL. Pathological findings showed a significant increase in woven bone at the peripheral lesion in transverse vertebral sections (Figure 6). Multinucleated giant cells were not observed (Figure 6). The patient was discharged on the 10th postoperative day and was able to ambulate without neurological deficits. Furthermore, the patient returned to an active lifestyle 1 month after surgery. Three months postoperatively, radiography showed a stable construct. No local tumor recurrence was detected at the follow-up after the second surgery (Figures 7 and 8).
GCT is a rare, locally aggressive primary bone tumor recently classified as an intermediate malignancy, with an estimated incidence of 1.3 per million per year, and is less common in the spine[6,7]. The most common clinical presentation in patients with spinal GCT is back pain, but the symptoms often progress slowly[7]. Surgical treatment is the foundational treatment strategy for spinal GCT, aiming to preserve functionality, relieve pain, control local recurrence, and prolong survival[2]. A variety of surgical procedures are applicable, from the simplest subtotal resection (curettage) to the most complex TES[4]. Due to the complex anatomy of the spine, subtotal resection is a common surgical option for surgeons but has proven to be inadequate for the treatment of spinal GCT[2]. Compared with subtotal resection (50%-80%), both total en bloc spondylectomy and total piecemeal spondylectomy could significantly reduce the recurrence rate of spinal GCT[8,9]. Further studies on the two types of total spondylectomy found that total piecemeal spondylectomy (14.8%-26.9%) was associated with a higher recurrence rate than TES (0%-15%)[4,9-13]. Hence, TES is recommended for spinal GCT because it can result in better oncological control, lower risk of local recurrence, and obviation of comorbidities associated with repeat surgery[1,14,15].
There is still a lack of relevant reports on surgical options for patients with acute paraplegia caused by spinal GCT who require emergency decompressive surgery. TES remains a challenge in emergency surgeries. Evidence regarding the use of TES shows its theoretical feasibility in emergency surgery; however, the safety of surgery and patient prognosis remains poor. The safety of TES in emergency surgery under extreme stress and time constraints requires further investigation. Table 1 gives a summary of case reports of spinal GCT treated with total spondylectomy. None of these patients had acute progression of paraplegia secondary to a spinal GCT and did not undergo total spondylectomy in emergency surgery[10,16-20]. Lucasti et al[7] reported a patient with T8 GCT presented with acute paraplegia of bilateral lower extremities, but this patient only received laminectomy and excisional biopsy of the epidural mass. Therefore, to our knowledge, this is the first case report of a patient who suffered sudden back pain and acute paraplegia secondary to a GCT of the thoracic spine and underwent emergency TES, successfully recovering spinal cord function and avoiding permanent paralysis. Unfortunately, the tumor recurred 9 months after initial TES. Careful examination of the emergency CT scan before the first operation revealed signs of bone destruction in the lateral wall of the adjacent vertebra (T7) below T6 (Figure 9). Therefore, we believe that the first surgery was eager to gain time for spinal cord decompression, leaving hidden dangers in determining the extent of tumor invasion. Another possible reason may be that because the tumor involves T6 bilateral vertebral pedicles, it is difficult to avoid exposure of the tumor, resulting in tumor cell contamination in the surgical field. In addition, surgeons should pay attention to the possibility of multiple GCTs of bone to avoid missed diagnosis.
| Ref. (country) | Age (yr), gender | Site of GCT | Treatment | Follow-up (month) |
| Hua et al[16] (China) | 41, male | T5 vertebra | TES | 24 |
| Kinoshita et al[10] (Japan) | 20, female | L3 vertebra | TES | 24 |
| Al-Shamary et al[17] (Saudi Arabia) | 29, male | T1 vertebra | Total spondylectomy | 10 |
| Yonezawa et al[18] (Japan) | 51, male | T12 vertebra | Total spondylectomy | 12 |
| Inoue et al[19] (Japan) | 35, female | T11 vertebra | TES | 3 |
| Matsumoto et al[20] (Japan) | 47, female | T5 vertebra | Total spondylectomy | 30 |
Management of recurrent GCT in the spine is challenging. Owing to the complicated anatomical structure, serious tissue adhesions, and significant surgical obstacles after repeated surgeries, revision surgery for recurrent multisegmental GCT of the thoracic spine is technically demanding[14,21]. However, if the condition permits, patients with recurrent spinal GCT would benefit from TES because surgically removing the GCT in a intact piece could significantly reduce the recurrence of spinal GCT and improve the survival rate[14,22]. In order to avoid lethal complications, our experience is to use an ordinary wire saw from the intervertebral foramen to the inner wall of the vertebral pedicle to cut off the pedicle, or use a nerve stripper to stick to the inner wall of the vertebral pedicle to protect the nerves and dura mater, and use a special curved osteotome to cut off vertebral pedicles. Moreover, our hospital has made a self-made foldable front large blood vessel baffle, and used a self-made wire saw or long surgical knife to cut the intervertebral disc by two-step technique. In addition, unlike the emergency situation of the first TES, our patient was well prepared for the second TES 1 year after denosumab treatment. Selective arterial embolization was performed the day before surgery to reduce intraoperative bleeding. Our patient recovered well after the second TES operation, and the results of the last follow-up were satisfactory.
Denosumab, as a neoadjuvant therapy or standalone treatment for GCT, has been used to inhibit progressive bone destruction by replacing multinuclear giant cells with non-proliferative, differentiated, densely woven new bone[23,24]. Denosumab can harden the edges of GCT and facilitate tumor calcification[25]. According to previous studies, denosumab is suitable for neoadjuvant use before the wide resection of GCT, including spinal lesions, and for main
However, denosumab treatment of GCT remains controversial. Yonezawa et al[18] presented a case of T12 GCT that was completely removed by total spondylectomy following 10 courses of denosumab therapy. They found that, although the administration of denosumab resulted in shrinkage of the epidural extraosseous tumor, the progression of vertebral collapse and massive callus formation made the tumor margins unclear and increased the difficulty of performing posterior-approach TES. In addition, high-grade sarcomas arising from bone GCT in patients treated with denosumab have been reported[28,29]. The preoperative and postoperative durations of denosumab treatment, long-term safety, and maintenance dose remain to be elucidated.
This is the first report of a patient with sudden back pain and acute paraplegia secondary to thoracic spinal GCT who underwent emergency TES, successfully recovering spinal cord function and avoiding permanent paralysis. Although GCT recurred after the first surgery, we performed a second three-level TES after denosumab treatment to avoid tumor recurrence as much as possible. Therefore, under permitted conditions, such as the patient’s general condition, the surgical experience of the treatment team, and adequate blood preparation in the hospital, TES in emergency surgery is feasible and safe but may increase the risk of spinal GCT recurrence.
| 1. | Tsukamoto S, Mavrogenis AF, Kido A, Errani C. Current Concepts in the Treatment of Giant Cell Tumors of Bone. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Yin H, Cheng M, Li B, Wang P, Meng T, Wang J, Zhou W, Yan W, Xiao J. Treatment and outcome of malignant giant cell tumor in the spine. J Neurooncol. 2015;124:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Lin P, Lin N, Teng W, Wang SD, Pan WB, Huang X, Yan XB, Liu M, Li HY, Li BH, Sun LL, Wang Z, Zhou XZ, Ye ZM. Recurrence of Giant Cell Tumor of the Spine after Resection: A Report of 10 Cases. Orthop Surg. 2018;10:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Zhou H, Wu F, Dang L, Li Y, Liu X, Liu Z, Wei F. Comparison of the prognostic factors of total en bloc spondylectomy and total piecemeal spondylectomy in patients with Enneking stage III giant cell tumor in the thoracic and lumbar spine. Eur Spine J. 2023;32:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Yonezawa N, Murakami H, Demura S, Kato S, Yoshioka K, Takeuchi A, Shinmura K, Yokogawa N, Shimizu T, Oku N, Kitagawa R, Handa M, Tsuchiya H. Morphologic Changes After Denosumab Therapy in Patients with Giant Cell Tumor of the Spine: Report of Four Cases and a Review of the Literature. World Neurosurg. 2019;127:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Rockberg J, Bach BA, Amelio J, Hernandez RK, Sobocki P, Engellau J, Bauer HC, Liede A. Incidence Trends in the Diagnosis of Giant Cell Tumor of Bone in Sweden Since 1958. J Bone Joint Surg Am. 2015;97:1756-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Lucasti C, Patel D, Hawayek B, Maraschiello M, Kowalski J. Giant cell tumor of the thoracic spine causing acute paraplegia-a case report. J Spine Surg. 2021;7:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Xu W, Li X, Huang W, Wang Y, Han S, Chen S, Xu L, Yang X, Liu T, Xiao J. Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Ann Surg Oncol. 2013;20:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Ouyang HQ, Jiang L, Liu XG, Wei F, Yang SM, Meng N, Jiang P, Yu M, Wu FL, Dang L, Zhou H, Zhang H, Liu ZJ. Recurrence Factors in Giant Cell Tumors of the Spine. Chin Med J (Engl). 2017;130:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Kinoshita H, Orita S, Yonemoto T, Ishii T, Iwata S, Kamoda H, Tsukanishi T, Inage K, Abe K, Inoue M, Norimoto M, Umimura T, Fujimoto K, Shiga Y, Kanamoto H, Furuya T, Takahashi K, Ohtori S. Successful total en bloc spondylectomy of the L3 vertebra with a paravertebral giant cell tumor following preoperative treatment with denosumab: a case report. J Med Case Rep. 2019;13:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Luksanapruksa P, Buchowski JM, Singhatanadgige W, Bumpass DB. Systematic Review and Meta-analysis of En Bloc Vertebrectomy Compared with Intralesional Resection for Giant Cell Tumors of the Mobile Spine. Global Spine J. 2016;6:798-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Yin H, Yang X, Xu W, Li B, Wang T, Meng T, Wang P, Liu T, Zhou W, Xiao J. Treatment and outcome of primary aggressive giant cell tumor in the spine. Eur Spine J. 2015;24:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Tomita K, Kawahara N, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006;11:3-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Ma Y, Li J, Pan J, Yan W, Huang Q, Liu T, Yang X, Yang C, Xu W, Wei H, Xiao J. Treatment options and prognosis for repeatedly recurrent giant cell tumor of the spine. Eur Spine J. 2016;25:4033-4042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yokogawa N, Murakami H, Demura S, Kato S, Yoshioka K, Shimizu T, Oku N, Kitagawa R, Tsuchiya H. Total spondylectomy for Enneking stage III giant cell tumor of the mobile spine. Eur Spine J. 2018;27:3084-3091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Hua W, Guo T, Li X, Wu Q, Yang C. Total en bloc spondylectomy of thoracic giant cell tumor with secondary aneurysmal bone cyst: case reports and review of literature. Int J Neurosci. 2023;133:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Al-Shamary E, Al-Dhafeeri W, Al-Sharydah A, Al-Suhibani S, Kussaibi H, Al-Issawi W. Total Spondylectomy for Upper Thoracic Spine Giant Cell Tumor: A Case Report. Case Rep Oncol. 2019;12:131-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Yonezawa N, Murakami H, Kato S, Takeuchi A, Tsuchiya H. Giant cell tumor of the thoracic spine completely removed by total spondylectomy after neoadjuvant denosumab therapy. Eur Spine J. 2017;26:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Inoue G, Imura T, Miyagi M, Saito W, Tazawa R, Nakazawa T, Takaso M. Total en bloc spondylectomy of the eleventh thoracic vertebra following denosumab therapy for the treatment of a giant cell tumor. Oncol Lett. 2017;14:4005-4010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Matsumoto M, Ishii K, Takaishi H, Nakamura M, Morioka H, Chiba K, Takahata T, Toyama Y. Extensive total spondylectomy for recurrent giant cell tumor in the thoracic spine. Case report. J Neurosurg Spine. 2007;6:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Duan PG, Li RY, Jiang YQ, Wang HR, Zhou XG, Li XL, Wang YC, Dong J. Recurrent adamantinoma in the thoracolumbar spine successfully treated by three-level total en bloc spondylectomy by a single posterior approach. Eur Spine J. 2015;24 Suppl 4:S514-S521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Boriani S, Bandiera S, Casadei R, Boriani L, Donthineni R, Gasbarrini A, Pignotti E, Biagini R, Schwab JH. Giant cell tumor of the mobile spine: a review of 49 cases. Spine (Phila Pa 1976). 2012;37:E37-E45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Nagano A, Urakawa H, Tanaka K, Ozaki T. Current management of giant-cell tumor of bone in the denosumab era. Jpn J Clin Oncol. 2022;52:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Li H, Gao J, Gao Y, Lin N, Zheng M, Ye Z. Denosumab in Giant Cell Tumor of Bone: Current Status and Pitfalls. Front Oncol. 2020;10:580605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 25. | de Carvalho Cavalcante RA, Silva Marques RA, dos Santos VG, Sabino E, Fraga AC Jr, Zaccariotti VA, Arruda JB, Fernandes YB. Spondylectomy for Giant Cell Tumor After Denosumab Therapy. Spine (Phila Pa 1976). 2016;41:E178-E182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Agarwal A, Larsen BT, Buadu LD, Dunn J, Crawford R, Daniel J, Bishop MC. Denosumab chemotherapy for recurrent giant-cell tumor of bone: a case report of neoadjuvant use enabling complete surgical resection. Case Rep Oncol Med. 2013;2013:496351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Zhang RZ, Ma TX, Qi DW, Zhao M, Hu T, Zhang GC. Short-term Preoperative Denosumab With Surgery in Unresectable or Recurrent Giant Cell Tumor of Bone. Orthop Surg. 2019;11:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL. A High-grade Sarcoma Arising in a Patient With Recurrent Benign Giant Cell Tumor of the Proximal Tibia While Receiving Treatment With Denosumab. Clin Orthop Relat Res. 2015;473:3050-3055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Broehm CJ, Garbrecht EL, Wood J, Bocklage T. Two Cases of Sarcoma Arising in Giant Cell Tumor of Bone Treated with Denosumab. Case Rep Med. 2015;2015:767198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |