Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2881
Revised: March 4, 2024
Accepted: April 10, 2024
Published online: June 6, 2024
Processing time: 115 Days and 0.1 Hours
Granulomatosis with polyangiitis (GPA) is one of the most prevalent forms of the antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. GPA is characterized histologically by necrotizing granulomatous inflammation in addition to vasculitis. The diagnosis of GPA depends on clinical presentation, serological evidence of a positive ANCA, and/or histological evidence of necro
The patient, a 52-year-old male, presented with unexplained nasal congestion, tinnitus, and hearing loss. After a duration of 4 months experiencing these symptoms, the patient subsequently developed fever and headache. The imaging examination revealed the presence of bilateral auricular mastoiditis and partial paranasal sinusitis, and the ANCA results were negative. The anti-infective the
For GPA patients with negative ANCA, there is a potential for early missed diagnosis. The integration of histopathological results and multidisciplinary communication plays a crucial role in facilitating ANCA-negative GPA.
Core Tip: In this case, otomastoiditis was the initial clinical manifestation, and then the clinical manifestations of central system involvement gradually appeared. Laboratory examination showed that proteinase 3-antineutrophil cytoplasmic antibody (ANCA) changed from negative to positive 3 months later. Therefore, for patients with clinical suspicion of granulomatosis with polyangiitis (GPA), repeated examination of ANCA should be considered, and obtained pathological evidence whenever possible. Meanwhile, the importance of early participation of the rheumatological immunology team is emphasized. Through multidisciplinary communication, the probability of early diagnosis of GPA can be improved, and timely drug intervention can be carried out to prevent disease progression and reduce the risk of local and systemic sequelae.
- Citation: Zhang Y, Dai QD, Wang JA, Xu LP, Chen Q, Jin YZ. Dynamically changing antineutrophil cytoplasmic antibodies in granulomatosis with polyangiitis: A case report. World J Clin Cases 2024; 12(16): 2881-2886
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2881.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2881
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis is an autoimmune disease characterized by necrotizing vasculitis of small and medium-sized vessels, comprising granulomatosis with polyangiitis (GPA), mi
GPA is characterized histologically by necrotizing granulomatous inflammation in addition to vasculitis. Clinical features typically include destructive sinonasal lesions, lower respiratory tract involvement with pulmonary hemorrhage and granulomatous inflammation, and necrotizing glomerulonephritis[2]. Diagnosis of GPA depends on clinical pre
We present a case of GPA with bilateral otomastoiditis and recurrent fever as the initial presentation. ANCA was negative in the first determination. The ANCA profile was re-examined 3 months later, and c-ANCA was positive and PR3 had a high titer. Right-sided migraine, increased cerebrospinal fluid (CSF) protein, and other manifestations of central nervous system (CNS) vasculitis were found.
A 52-year-old male patient with symptoms of chronic nasal congestion, tinnitus, hearing loss lasting > 8 months, and recurrent fever persisting > 4 months.
A 52-year-old male presented with nasal congestion and tinnitus, accompanied by bilateral conduction and sensorineural hearing loss in both ears. After enduring these symptoms for 4 months, the patient subsequently developed a fever, which was accompanied by excessive weight loss > 8 kg. Tympanic puncture was performed and dexamethasone was injected into the tympanic membrane. Two days later, tympanic catheter drainage was performed. The results of the pathogen culture of the tympanic hydrops showed Staphylococcus aureus (S. aureus), and the patient was given an intravenous injection of linezolid glucose 0.6 g once every 12 h. After 1 wk of anti-infective treatment, the patient continued to experience fever. S. aureus may have been a contaminating bacterium rather than a causative factor in fever and otomastoiditis, and given the insufficient diagnostic evidence for fungal or active tuberculosis infection, a trial of intravenous methylprednisolone at 40 mg once daily was initiated for 1 wk. Following treatment, there was a significant reduction in hypersensitive C-reactive protein (hs-CRP) and erythrocyte sedimentation rate (ESR) levels, accompanied by resolution of fever. The symptoms of ear tightness and tinnitus were also alleviated. Following discharge, the patient commenced oral administration of methylprednisolone tablets at 8 mg twice daily, gradually tapering down by 4 mg/wk until reaching a maintenance dose of 2 mg twice daily.
After 3 months of glucocorticoid maintenance treatment, the patient developed fever again, with body temperature fluctuating between 36.9 °C and 38.0 °C, accompanied by right-sided migraine, stuffiness in the right ear, poor ventilation in the right nostril. Otorhinolaryngological examination showed a clear tympanic catheter and a negative meningeal stimulation sign. The Ear, Nose and Throat Department referred the patient to the Rheumatology Department.
The patient was diagnosed with chronic nephritis 20 years ago based on of positive occult blood in urine, however, biopsy to determine the pathological type of the kidney was not performed. Additionally, it should be noted that the patient had a history of raising parrots 1 year ago.
The personal and family history did not reveal any notable features.
Temperature: 37.5 °C, pulse rate: 112 beats/min, respiratory rate: 18 beats/min, blood pressure: 121/85 mmHg. The ear canal was clear and unobstructed. There was no tenderness observed in the sinuses, and both lungs showed no abnormalities upon auscultation, and there was no edema observed in both lower limbs.
During the first stage of the disease, laboratory examination showed that hs-CRP was 55 mg/L and ESR was 58 mm/h. Active urinary sediment examination revealed a urinary protein level of 1+, urinary erythrocyte count of 66.7/μL, and 24-h total protein excretion rate of 284 mg/L. Additionally, urinary pathogen culture and renal ultrasonography showed no abnormalities. The results of p-ANCA, c-ANCA, antinuclear antibody detected by indirect immunofluorescent assay and PR3 and MPO detected by enzyme-linked immunosorbent assay were all negative as confirmed by two medical agencies.
After relapse, laboratory examination results showed that hs-CRP was 55 mg/L, ESR was 71 mm/h, a urinary protein level of 1+, a urinary erythrocyte count of 89.0/μL, and a 24-h total protein excretion rate of 373 mg/L, c-ANCA was positive, PR3 was 100.8 RU/mL, p-ANCA and MPO were negative. CSF pressure was 130 mmH2O, the Pandy test was positive, and the total number of nucleated cells was 6.0/μL. Total protein in CSF was 89.4 mg/dL, and chlorine and sugar levels were normal. ANCA in CSF was negative. Cryptococcal antigen and Xpert mycobacterium tuberculosis/rifampicin resistance detection of tuberculosis in CSF were normal. No clinically significant pathogenic microorganisms were detected by next-generation sequencing (NGS) in the blood.
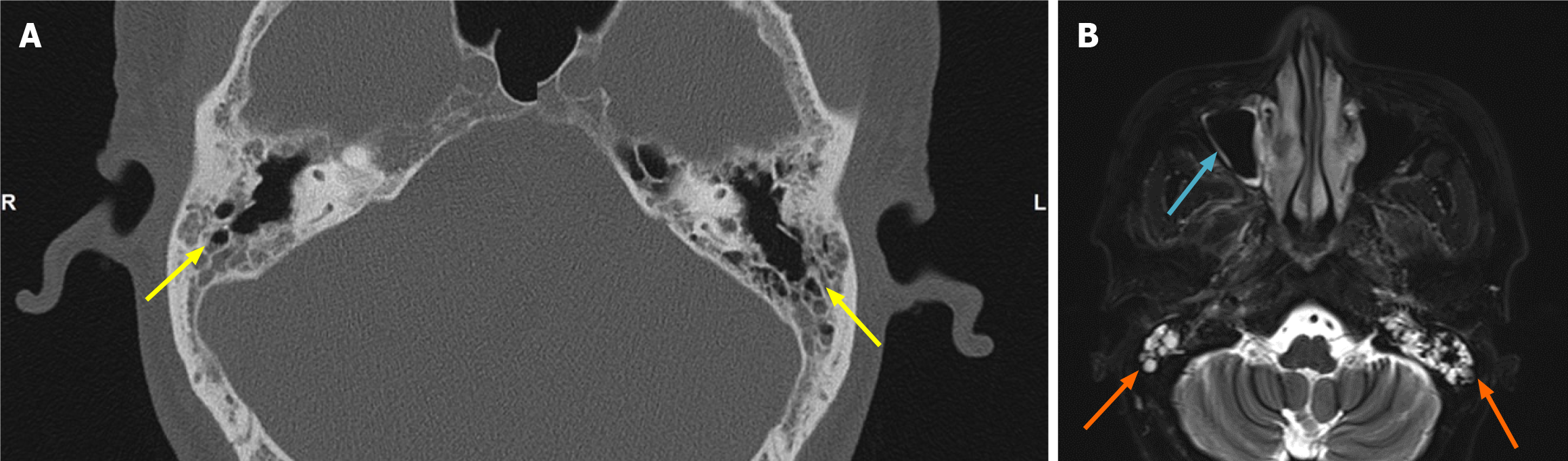
During the first stage of the disease, computed tomography (CT) showed bilateral otomastoiditis with auditory ossicles and partial paranasal sinusitis (Figure 1A). Nasopharyngeal enhanced magnetic resonance imaging (MRI) showed bilateral otomastoiditis and right maxillary sinus inflammation (Figure 1B).
After relapse, head enhanced MRI and CT angiography (CTA) revealed no significant abnormalities, except for the presence of bilateral otomastoiditis and right maxillary sinusitis.
During the first stage of the disease, otolaryngological examination revealed a deviated nasal septum, purulent discharge in the right middle nasal passage, and absence of any masses in the nasopharynx. Otoscopy showed bilateral tympanic membrane invagination and bilateral tympanic effusion.
After relapse, otolaryngological examination revealed a clear ear canal with no presence of pus or fluid discharge.
Combined with the patient’s medical history, the final diagnosis was GPA with bilateral mastoiditis, active glomerulonephritis and suspected CNS vasculitis.
According to the patient's condition, the Birmingham Vasculitis Activity Score was 23[5]. The patient received intra
At present, the patient is taking methylprednisolone tablets 4 mg once a day, and the cumulative dose of CYC was 7.2 g (until July 2023). c-ANCA and PR3 became negative, and there was no recurrence.
The patient did not exhibit purulent or bloody nasal discharge, and the presence of right maxillary sinusitis on imaging does not necessarily indicate granulomatous sinusitis. Furthermore, there was no clinical evidence of lung invasion or pathological evidence of granulomatous inflammation. Therefore, due to the challenges associated with procuring pathological specimens and obtaining negative ANCA results, early-stage diagnosis can be easily overlooked, particularly in cases where it is not the initial assessment within the rheumatology department. However, considering the patient's otomastoiditis, increased albuminuria, microscopic hematuria, positive response to glucocorticoid therapy, as well as conversion to positive c-ANCA/PR3, there was substantial evidence supporting the diagnosis of GPA in this patient. The patient's "chronic nephritis" condition was stable for a long time, with only a small number of red blood cells in the urine. During the course of this patient’s disease, progressive increases in urinary erythrocytic cells and urinary proteins were observed, as well as fever, otomastoiditis, suspected central vasculitis, and PR3-ANCA positivity. Therefore, we considered that the patient's kidney damage was related to GPA. This patient had the characteristic clinical manifestations of GPA, and PR3-ANCA became positive during the course of the disease; therefore it was still identified as GPA despite the lack of pathological support.
Up to 90% of GPA patients can be involved in the ears and nose, with sinus involvement being the most common[6]. Local involvement in the above respiratory tract and ears is more likely to occur in young patients[7]. The literature reports that GPA patients negative for c-ANCA may have a positive result after 4 years[8], and it is more commonly seen in patients with lesions limited to the upper and/or lower respiratory tract that do not affect renal GPA[9]. However there are also cases of ANCA negative GPA patients with renal biopsy showing necrotizing inflammation with crescent formation[10]. A study summarizing the data of Wegener's Granulomatosis Etanercept Trial and Rituximab in ANCA-Associated Vasculitis Trial found that ANCA negative patients with GPA more commonly had relapsing disease at trial entry (87% vs 57%)[11,12], but the rate of relapse during follow-up was similar in the two groups[13]. Disease damage did not differ between the two groups.
Our patient's serum ANCA spectrum showed "cANCA-PR3 positive" at re-examination after a 3-month interval, accompanied by right migraine, and there was no abnormal neurological examination. The patient had a history of raising birds, so in order to detect infectious diseases such as cryptococcosis and chlamydiasis, blood NGS and CSF puncture were performed to eliminate infectious diseases. Although the patient's cranial enhanced MRI and intracranial vascular CTA did not suggest the presence of hypertrophic cranial pachymeningitis, cranial nerve involvement, hypophysitis, and cerebral ischemia/hemorrhage lesions, the presence of headache and significantly elevated CSF protein levels suggested a potential diagnosis of CNS vasculitis. Among GPA patients with central involvement, 89% were found to be ANCA positive, of whom, 84% were PR3 positive, and most patients presented with clinical manifestations of headache and hearing loss[14]. CSF analysis may demonstrate nonspecific abnormalities such as elevated protein and pleocytosis, but it can help to rule out infectious, neoplastic, or other diseases in the differential diagnosis. One study reported three young patients with biopsy-proven active generalized GPA who were consistently ANCA negative over observation times ranging from 58 to 114 months, indicating that severe CNS manifestations could represent a clinical hallmark of ANCA negative GPA[15]. Although treatment with glucocorticoids and immunosuppressants may affect ANCA results, ANCA titers/levels may not be parallel to important organ involvement in GPA[16]. For GPA patients with unexplained headaches, sensory and/or movement disorders, diabetes insipidus, cranial enhanced MRI and/or intracranial vascular CTA should be performed in a timely manner to assess CNS involvement.
In the present case, otomastoiditis was the initial clinical manifestation, and then the clinical manifestations of CNS involvement gradually appeared. Laboratory examination showed that c-ANCA and PR3 changed from negative to positive 3 months later. Therefore, for patients with clinical suspicion of GPA, repeated examination of ANCA should be considered, and pathological evidence obtained whenever possible. We emphasize the importance of early participation of the rheumatological immunology team. Through multidisciplinary communication, the probability of early diagnosis of GPA can be improved, and timely drug intervention can be carried out to prevent disease progression and reduce the risk of local and systemic sequelae.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade A
P-Reviewer: Kosalka-Wegiel J, Poland S-Editor: Zheng XM L-Editor: A P-Editor: Zhao S
| 1. | Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, Archer AM, Conn DL, Full KA, Grayson PC, Ibarra MF, Imundo LF, Kim S, Merkel PA, Rhee RL, Seo P, Stone JH, Sule S, Sundel RP, Vitobaldi OI, Warner A, Byram K, Dua AB, Husainat N, James KE, Kalot MA, Lin YC, Springer JM, Turgunbaev M, Villa-Forte A, Turner AS, Mustafa RA. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Care Res (Hoboken). 2021;73:1088-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Domínguez-Quintana M, Alba MA, Hinojosa-Azaola A. Classification of ANCA-associated vasculitis: differences based on ANCA specificity and clinicopathologic phenotype. Rheumatol Int. 2021;41:1717-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, Kullman J, Lyons PA, Merkel PA, Savage COS, Specks U, Kain R. ANCA-associated vasculitis. Nat Rev Dis Primers. 2020;6:71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 560] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 4. | McCarthy E, Mustafa M, Watts M. ANCA-negative Granulomatosis with Polyangiitis: A Difficult Diagnosis. Eur J Case Rep Intern Med. 2017;4:000625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, Flossmann O, Hall C, Hollywood J, Jayne D, Jones R, Lanyon P, Muir A, Scott D, Young L, Luqmani RA. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 2009;68:1827-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 817] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 6. | Cannady SB, Batra PS, Koening C, Lorenz RR, Citardi MJ, Langford C, Hoffman GS. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope. 2009;119:757-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Stone JH; Wegener's Granulomatosis Etanercept Trial Research Group. Limited versus severe Wegener's granulomatosis: baseline data on patients in the Wegener's granulomatosis etanercept trial. Arthritis Rheum. 2003;48:2299-2309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener's granulomatosis--a review of diagnosis and treatment in 53 subjects. Rhinology. 1998;36:188-191. [PubMed] |
| 9. | Felicetti M, Cazzador D, Padoan R, Pendolino AL, Faccioli C, Nardello E, Berti A, Silvestrini M, Paolazzi G, Brunori G, Zanoletti E, Emanuelli E, Martini A, Schiavon F. Ear, nose and throat involvement in granulomatosis with polyangiitis: how it presents and how it determines disease severity and long-term outcomes. Clin Rheumatol. 2018;37:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Gangireddy M, Kanderi T, Chan Gomez J, Kundoor V, Cunningham J. When Anti-Neutrophil Cytoplasmic Antibody Fails: A Case of Anti-Neutrophil Cytoplasmic Antibody Negative Granulomatosis With Polyangiitis. Cureus. 2020;12:e8883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Wegener's Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener's granulomatosis. N Engl J Med. 2005;352:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 589] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 12. | Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U; RAVE-ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2156] [Cited by in RCA: 1856] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 13. | Miloslavsky EM, Lu N, Unizony S, Choi HK, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Tchao NK, Fervenza F, Monach PA, Specks U, Stone JH. Myeloperoxidase-Antineutrophil Cytoplasmic Antibody (ANCA)-Positive and ANCA-Negative Patients With Granulomatosis With Polyangiitis (Wegener's): Distinct Patient Subsets. Arthritis Rheumatol. 2016;68:2945-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Fragoulis GE, Lionaki S, Venetsanopoulou A, Vlachoyiannopoulos PG, Moutsopoulos HM, Tzioufas AG. Central nervous system involvement in patients with granulomatosis with polyangiitis: a single-center retrospective study. Clin Rheumatol. 2018;37:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Reinhold-Keller E, de Groot K, Holl-Ulrich K, Arlt AC, Heller M, Feller AC, Gross WL. Severe CNS manifestations as the clinical hallmark in generalized Wegener's granulomatosis consistently negative for antineutrophil cytoplasmic antibodies (ANCA). A report of 3 cases and a review of the literature. Clin Exp Rheumatol. 2001;19:541-549. [PubMed] |
| 16. | Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, Archer AM, Conn DL, Full KA, Grayson PC, Ibarra MF, Imundo LF, Kim S, Merkel PA, Rhee RL, Seo P, Stone JH, Sule S, Sundel RP, Vitobaldi OI, Warner A, Byram K, Dua AB, Husainat N, James KE, Kalot MA, Lin YC, Springer JM, Turgunbaev M, Villa-Forte A, Turner AS, Mustafa RA. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2021;73:1366-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 322] [Article Influence: 80.5] [Reference Citation Analysis (0)] |