Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2869
Revised: April 10, 2024
Accepted: April 18, 2024
Published online: June 6, 2024
Processing time: 139 Days and 20 Hours
Vascular malformations (VMs) arise as a result of errors in the process of angio
A 12-year-old girl was admitted to the hospital with findings of an enlarged right temporal scalp. After admission, computed tomography (CT) angiography of cerebral ateries showed a right occlusal gap and a right temporal artery venous malformation. Furthermore, cerebral angiography showed a right temporal lobe VM with multiple vessels supplying blood. The patient underwent surgery to remove the malformed vessels and the eroded skull. Two hours after the surgery, the patient's right pupil was dilated, and an urgent CT scan of the skull showed a right subdural haematoma under the incision, which was urgently removed by a second operation. After surgery, we gave continuous antibiotic anti-infection treatment, and the patient recovered well and was discharged two weeks later.
Surgical removal of giant haemangiomas is risky and adequate preoperative (including interventional embolisation) and intraoperative preparations should be made.
Core Tip: We present a rare case of giant vascular malformation (VM) of the head and face. Combined with our study suggests that surgical resection of giant VMs is a great challenge for surgeons and should be adequately prepared preoperatively and intraoperatively. One of the preoperative preparations for surgical resection is interventional embolisation.
- Citation: Xie MC, Wang FX, Xu J. Giant vascular malformations invading the skull: A case report. World J Clin Cases 2024; 12(16): 2869-2875
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2869.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2869
Vascular malformations (VMs) are generated due to errors during angiogenesis. They are often present at birth but may not be apparent until later. These malformations are common in the head and neck but can occur anywhere in the body[1]. A thorough clinical history and physical examination may help accurately diagnose VMs. Its primary characteristics are the timing of onset, the amount of growth at the lesion site, ulceration, and bleeding. Routine angiography is not required but plays a key role in deeper lesions and difficult diagnosis cases[2]. We describe a case of a massive trabecular VM of the head where, despite the lack of symptoms, aggressive surgical intervention was essential to the patient's prognosis. The literature on clinical information, available therapies, and disease prognosis is also reviewed.
A 12-year-old Chinese female child was admitted to the hospital in February 2023 with right temporal subscalp enlargement.
The patient found right temporal subscalp enlargement 1 year ago with no apparent cause. She was unconcerned by it at the time. The patient reported no nausea, vomiting, numbness in the extremities or trunk, muscle weakness, or urinary disruption. However, there was a sense of fluctuation in the contact pressure of the mass, with no pain, redness, or swelling.
There is no obvious history of past illness related to this disease.
There is no obvious personal or family history related to this disease.
On admission to the hospital, she was conscious; his vital signs were stable, and no enlarged lymph nodes were discovered in the neck or behind the ears. Both muscle strength and muscle tension of the extremities were normal, and voluntary activities were normal. The bilateral Babinski sign and neck stiffness were negative. Localised enlargement of the right inferotemporal scalp; the enlargement was approximately 8 cm × 4 cm in extent, fluctuating to touch, with no surrounding erythema or oozing.
Routine blood tests, blood biochemistry, immune markers, infection markers, routine urine tests and routine stool tests showed no significant abnormalities.
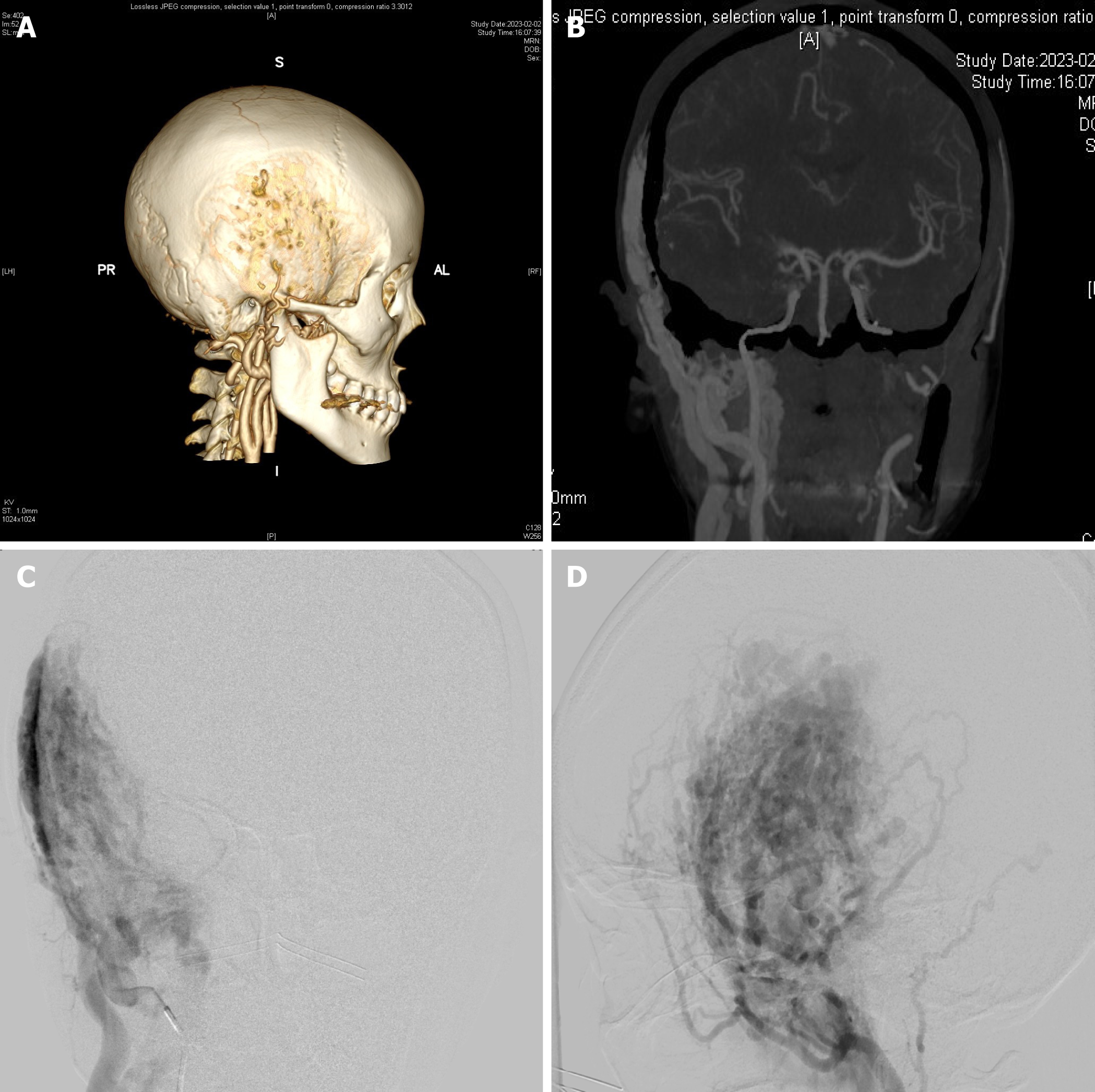
The computed tomography (CT) angiography of cerebral arteries showed multiple tortuous vessels in the right occlusal and temporal subcutaneous areas. Also, the blood supply from the right external carotid artery and lesions invading the adjacent cranial artery and prominence of the epidural area (Figure 1A and B). The digital subtraction angiography showed that the right external carotid artery had a VM of the maxillary artery and a superficial temporal artery supplying blood, which were approximately 10 cm × 6 cm in size (Figure 1C and D).
VMs.
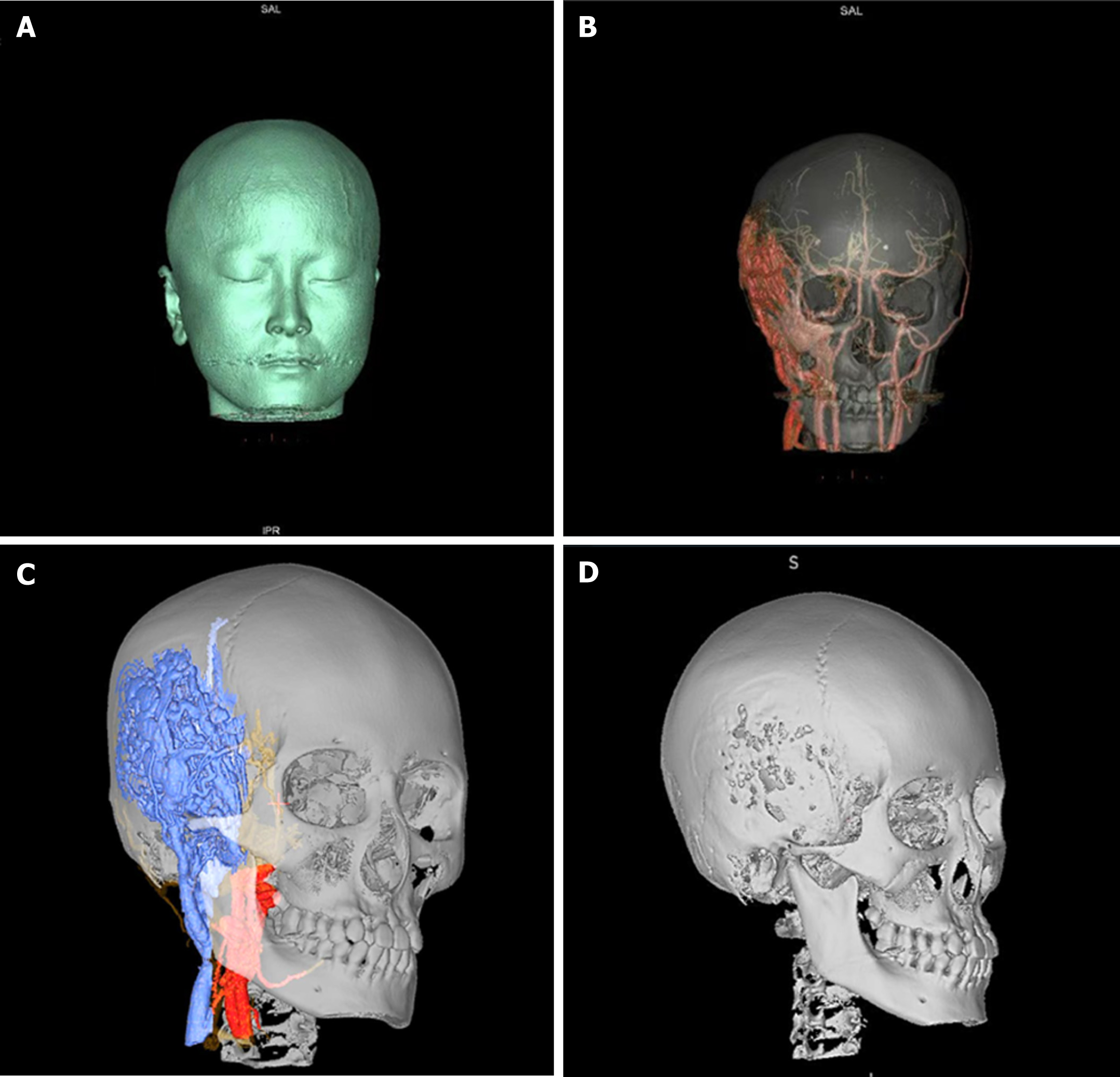
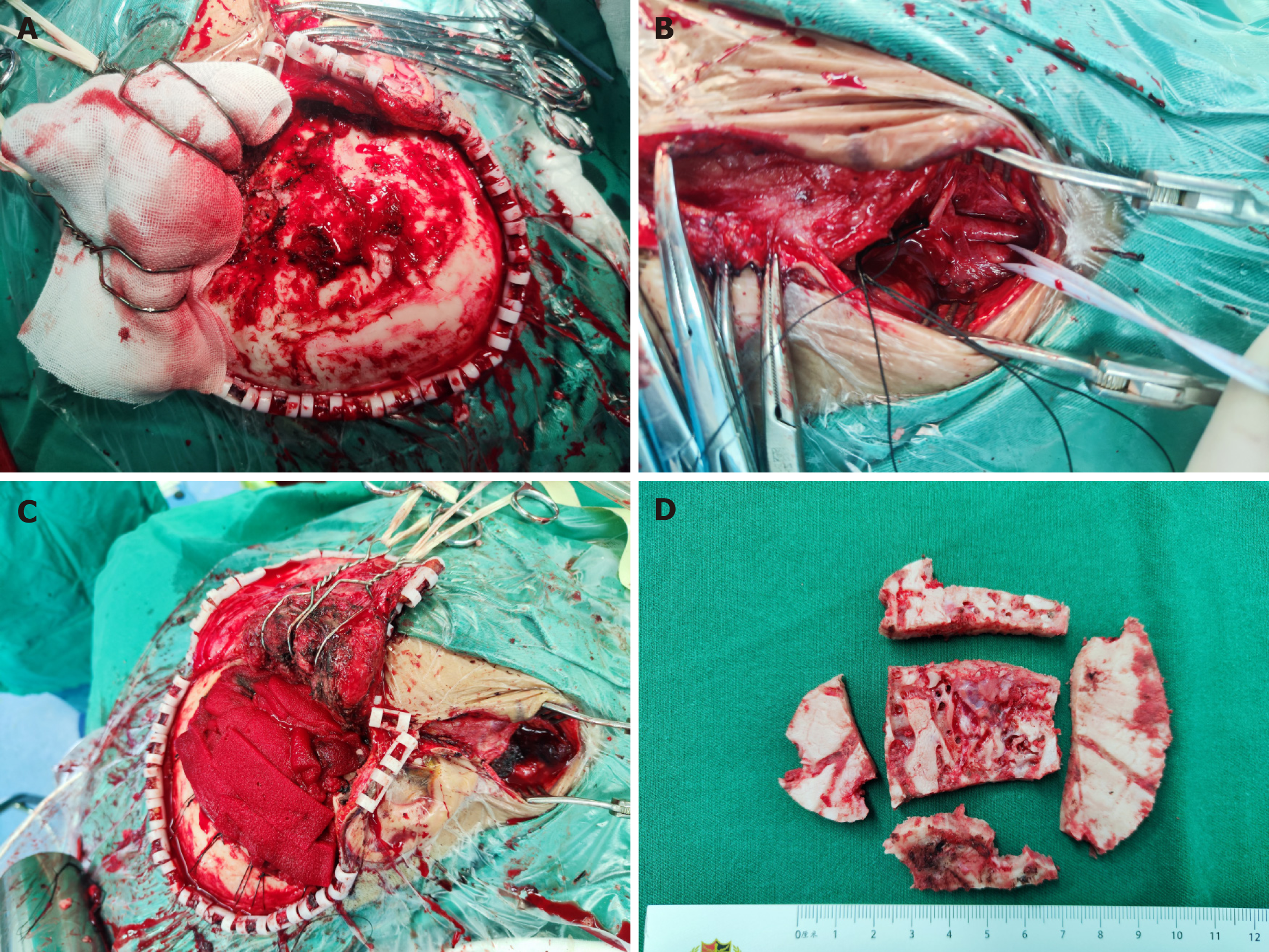
Based on the preoperative examination we constructed the VM and its surrounding structures by means of a neuronavigation system, thus visualising the anatomical relationship of the VM with the skull, peripheral blood vessels and other structures (Figure 2). The patient was operated supine under general anesthesia (Figure 3), with the head deviated to the left and the right anterior cervical + right frontotemporal enlarged pterygoid point approach. First, the right anterior cervical incision was made, and the external carotid artery was freed and ligated. The scalp was incised along the marked incision of the right frontal chin to the skull layer by layer, the temporalis muscle was separated along the skull, and multiple worm-like changes were seen in the skull with raging bleeding. After hemostasis, 6 holes were made in the skull using an electric cranial drill, the dura was removed from between the bone holes, and the bone flap was excised in pieces. The meningeal artery was thickened, and hemostasis was difficult.
The malformed vessels were electrocoagulated and ligated to halt the bleeding. After the dura was suspended, the surgical area was hemostatic. The dura was tightly sutured and repaired with artificial dura. An epidural drain was inserted, and after sufficient hemostasis, the muscular layer, subcutaneous tissue, and all scalp layers were each sutured separately. Bleeding was about 3000 mL after the operation. It was challenging to halt the bleeding and significant blood loss during the operation because of the coarse, deformed arteries and widespread invasion. Throughout the procedure, 1300 mL of autologous blood, 450 mL of plasma, and 1.5 U of suspended red blood cells were transfused.
Due to the raging intraoperative bleeding, the patient was at risk of hemorrhagic shock. The preoperative examination was perfect, and the lesion was identified as a VM; hence no pathological specimen was retained intraoperatively. The patient's right pupil was dilated two hours after the operation but remained unharmed. An emergency cranial CT scan was carried out and reported an epidural hematoma behind the surgical incision. A second surgical procedure was performed to remove the hematoma, close the skull after meticulous hemostasis, and implant one drainage strip. The vital signs were stable following surgery, and a postoperative blood test revealed hemoglobin levels of 90 g/L. After receiving blood transfusion therapy, the patient was released from the hospital two weeks later.
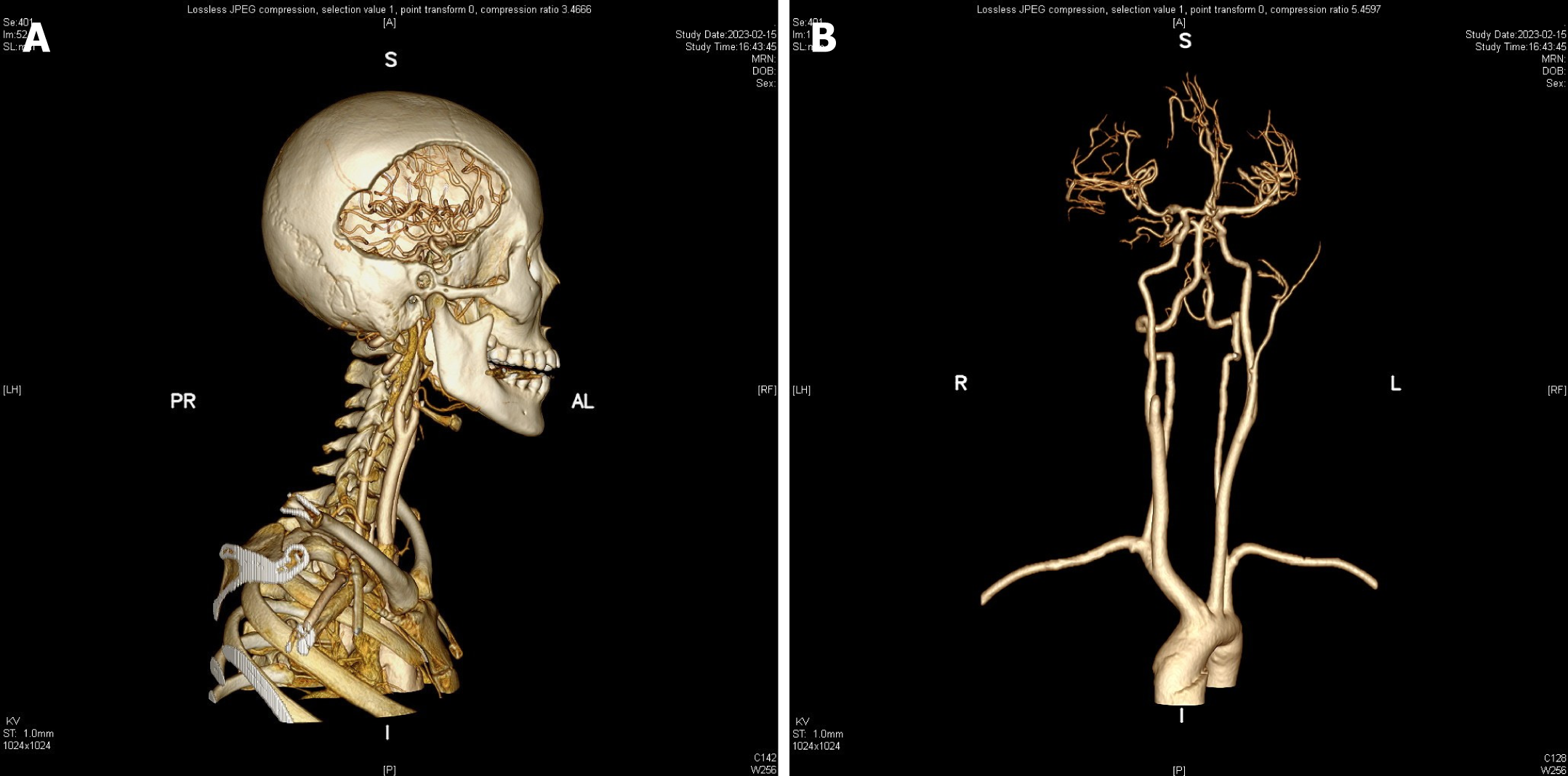
The patient returned to the hospital for a follow-up exam a month after discharge, and no signs of vascular abnormalities (VAs) had returned (Figure 4). Generally, the patient is healthy, showing no neurological, pulmonary, or other pain signs.
VAs are a group of lesions caused by errors in the formation of blood vessels during embryo development. They often manifest in childhood and can affect any region of the body[3]. The International Society provides the most standardized and widely used classification for the Study of Vascular Anomalies. VAs are divided into two types: Vascular tumors and VMs[4]. Vascular tumors are neoplasms that grow rapidly after birth and regress slowly in late childhood. In contrast, congenital VMs do not regress or enlarge and grow at the same rate as children. They include arteriovenous malformations (AVMs), arteriovenous fistulas, capillary malformations, venous malformations, and lymphatic malformations. Despite VM's presence at birth, they strongly manifest later in life with varying symptoms depending on their location and extent, including swelling, facial changes, pain, and bleeding. Superficial VMs is present as swelling caused by compression of soft tissues, increasing in size with Valsalva maneuvers and changes in position. Deep VMs typically do not cause the patient any great discomfort. The rapid growth of the lesion may be caused by thrombosis inside the lesion and hormonal changes associated with puberty and pregnancy[5]. The patient in this instance was a young woman, and hormonal changes occurring in the body may be what caused the development of this massive trabecular VM.
Errors in vascular development cause AVMs during embryogenesis. The absence of the capillary bed results in the direct shunting of blood from the arteries to the venous circulation through abnormal channels[6]. The patient belongs to this malformation based on the imaging presentation. Extracranial AVM intervention time is debatable and typically determined by clinical indicators and the stage of development of the tumor. The widely used staging system is the Schobinger classification, which determines the stage of AVM based on clinical evidence of (1) Dormancy; (2) Dilatation; (3) Destruction; or (4) Decompensation[7]. However, all AVMs eventually progress during adolescence or adulthood hence the need for early intervention[8]. Interventional embolization, surgical resection, or a combination of treatments can achieve this goal[9]. As long as the surgical procedure does not produce functional or cosmetic outcomes worse than the disease, it is a viable alternative. Following up after the intervention has begun through repeated staging is crucial to achieving the optimum treatment outcome. Longer treatment intervals may be used after the lesion has been controlled.
Embolization is often preoperatively to reduce blood loss during resection or relieve unresectable lesions[8]. Many studies have reported using arteriography and embolization with different substances, such as glue, coils, and alcohol. Due to cytotoxicity toward vascular endothelial cells, with the potential to create vascular growth factors, alcohol is considered the most efficient embolization substance. Unfortunately, alcohol causes severe tissue swelling, potential nerve paralysis, and damage to the cardiovascular system. Due to cytotoxicity, alcohol cannot be used to treat intracranial AVMs[10,11]. Instead, Onyx (ethylene vinyl alcohol copolymer) has been effectively utilized for over 10 years to treat intracranial AVM and has the benefit of permanent vascular blockage. Complications include mucosal surface ulcers, skin pigmentation, and tissue nodules. In addition, Onyx is expensive and requires additional fluoroscopy time[12]. Embolization has been performed in huge VMs, with no success. The interventionalists decided against embolization in this example of a large VM because of the numerous and tortuous blood supply channels. We ligated the right external carotid artery at the beginning of the procedure to block the primary blood supply vessel to the lesion to limit surgical bleeding, even though we abandoned embolization. The patient was still vulnerable to hemorrhagic shock during surgery. After intraoperatively removing the lesion from the skull, we sutured the scalp since we didn't think it was too intrusive to require plastic surgery for the skin flap.
Gamma Knife radiosurgery has a long history of treating intracranial AVMs. However, larger AVMs require multiple procedures, with treatment intervals ranging from 3 to 6 months. The occlusion rate ranges from 33%-72% in treating larger AVMs[13]. We discontinued this treatment because of its lengthy duration and unpredictable outcomes.
Some large AVMs are difficult to resect and must be performed by an experienced surgeon. The surgery may be staged and require a vascular flap to reconstruct the defect. Hussain et al[14] reported a case of a large head and neck AVM. The patient underwent two surgeries and was followed up for 6 months after surgery with no recurrence or complications.
The recurrence of AVMs is common. Liu et al[8] retrospectively analyzed 272 patients with AVMs and found that the risk of recurrence was 14.2 times higher in patients treated with embolization alone than those treated with resection and that recurrence was unlikely when treating lower-stage lesions. Some AVMs demonstrate significant recurrence within weeks or months, while other recurrences may not manifest for up to 10 years. Therefore, follow-up arteriography post-surgery is recommended for residual AVMs in recurrent patients.
VMs typically manifest in adolescence, and in this report, we discuss a rare case of large trabecular VM of the head and face and review the existing literature. Surgical resection of giant VMs is a great challenge for surgeons, and adequate preoperative and intraoperative preparations should be made. Interventional embolization is used as one of the preoperative preparations for surgical resection. The possibility of VM recurrence persists even with early intervention. After embolization or surgical resection, a thorough follow-up should be done to monitor for recurrence.
| 1. | Carqueja IM, Sousa J, Mansilha A. Vascular malformations: classification, diagnosis and treatment. Int Angiol. 2018;37:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | DeHart A, Richter G. Hemangioma: Recent Advances. F1000Res. 2019;8:F1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Snyder E, Puttgen K, Mitchell S, Ahlawat S, Tekes A. Magnetic Resonance Imaging of the Soft Tissue Vascular Anomalies in Torso and Extremities in Children: An Update With 2014 International Society for the Study of Vascular Anomalies Classification. J Comput Assist Tomogr. 2018;42:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Sadick M, Müller-Wille R, Wildgruber M, Wohlgemuth WA. Vascular Anomalies (Part I): Classification and Diagnostics of Vascular Anomalies. Rofo. 2018;190:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Hussein A, Malguria N. Imaging of Vascular Malformations. Radiol Clin North Am. 2020;58:815-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Greene AK, Orbach DB. Management of arteriovenous malformations. Clin Plast Surg. 2011;38:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Lee BB, Do YS, Yakes W, Kim DI, Mattassi R, Hyon WS. Management of arteriovenous malformations: a multidisciplinary approach. J Vasc Surg. 2004;39:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Johnson AB, Richter GT. Surgical Considerations in Vascular Malformations. Tech Vasc Interv Radiol. 2019;22:100635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Jeong HS, Baek CH, Son YI, Kim TW, Lee BB, Byun HS. Treatment for extracranial arteriovenous malformations of the head and neck. Acta Otolaryngol. 2006;126:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Bertino F, Trofimova AV, Gilyard SN, Hawkins CM. Vascular anomalies of the head and neck: diagnosis and treatment. Pediatr Radiol. 2021;51:1162-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Panagiotopoulos V, Gizewski E, Asgari S, Regel J, Forsting M, Wanke I. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol. 2009;30:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Hasegawa H, Yamamoto M, Shin M, Barfod BE. Gamma Knife Radiosurgery For Brain Vascular Malformations: Current Evidence And Future Tasks. Ther Clin Risk Manag. 2019;15:1351-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Hussain T, Farooqui F, Jehan M. Arteriovenous malformation of head and neck: A case report. J Pak Med Assoc. 2022;72:360-362. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |