Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2847
Revised: February 25, 2024
Accepted: April 11, 2024
Published online: June 6, 2024
Processing time: 157 Days and 1 Hours
The prognosis of hepatocellular carcinoma (HCC) combined with portal and hepatic vein cancerous thrombosis is poor, for unresectable patients the combin
In our center, a patient with giant HCC combined with portal vein tumor thro
In this case, atezolizumab, bevacizumab, icaritin soft capsules combined with radiotherapy and TACE had a good effect. For patients with hepatocellular carcinoma combined with hepatic vein/inferior vena cava tumor thrombus, adopting a high-intensity, multimodal proactive strategy under the guidance of multidisciplinary team (MDT) is an important attempt to break through the current treatment dilemma.
Core Tip: In this case, atezolizumab, bevacizumab, icaritin soft capsules combined with radiotherapy and transcatheter arterial chemoembolization had a good effect. For patients with hepatocellular carcinoma combined with hepatic vein/inferior vena cava tumor thrombus, adopting a high-intensity, multimodal proactive strategy under the guidance of multidisciplinary team is an important attempt to break through the current treatment dilemma.
- Citation: Song WJ, Xu J, Nie Y, Li WM, Li JP, Yang L, Wei MQ, Tao KS. Conversion therapy of a giant hepatocellular carcinoma with portal vein thrombus and inferior vena cava thrombus: A case report and review of literature. World J Clin Cases 2024; 12(16): 2847-2855
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2847.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2847
The prognosis of hepatocellular carcinoma (HCC) combined with portal and hepatic vein cancerous thrombosis is poor, with a median survival time of only about 2.7-3 months[1], and treatment requires the cooperation of the multidisciplinary team. Prof. Cheng's team gives treatment recommendations based on the classification of portal vein thrombosis and hepatic vein thrombosis. For portal vein tumor thrombus involving the main trunk of the portal vein [portal vein tumor thrombus (PVTT) III] and hepatic vein tumor thrombus involving the inferior vena cava, a combination of radiotherapy, transcatheter arterial chemoembolization (TACE), and surgical resection is recommended according to the patient's liver function[2,3]. The combination of targeted therapy and immune therapy has progressed to become the first-line recommended treatment for advanced HCC[4]. Conversion therapy or conversion surgery (CS) is a surgical strategy developed to improve long-term survival in patients with initially unresectable tumors, aiming at R0 resection after stage reduction by non-surgical treatment[5]. CS has also been reported in HCC, radical resection of partially unresectable HCC (UR-HCC) has been achieved through TACE, portal vein embolization, and oral administration of molecular targeted drugs[6,7]. In recent years, the efficacy of atezolizumab combined with bevacizumab (Atez+Bev) in the treatment of UR-HCC has been confirmed[8,9]. The above therapeutic advances have brought hope for advanced HCC, but the efficacy is still limited.
The patient was a 52-year-old man who was admitted to the hospital on April 24, 2022, with discomfort in the right upper abdomen.
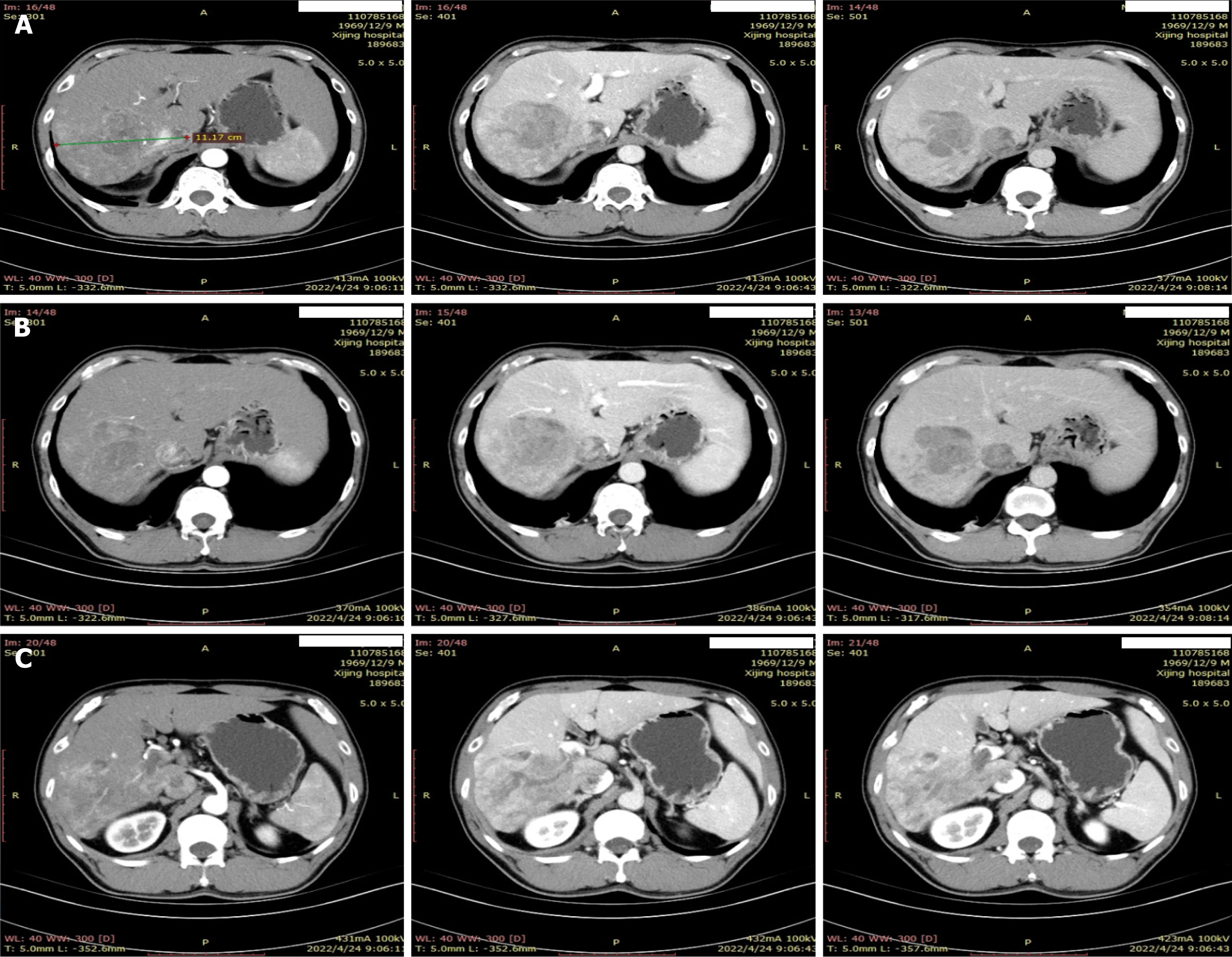
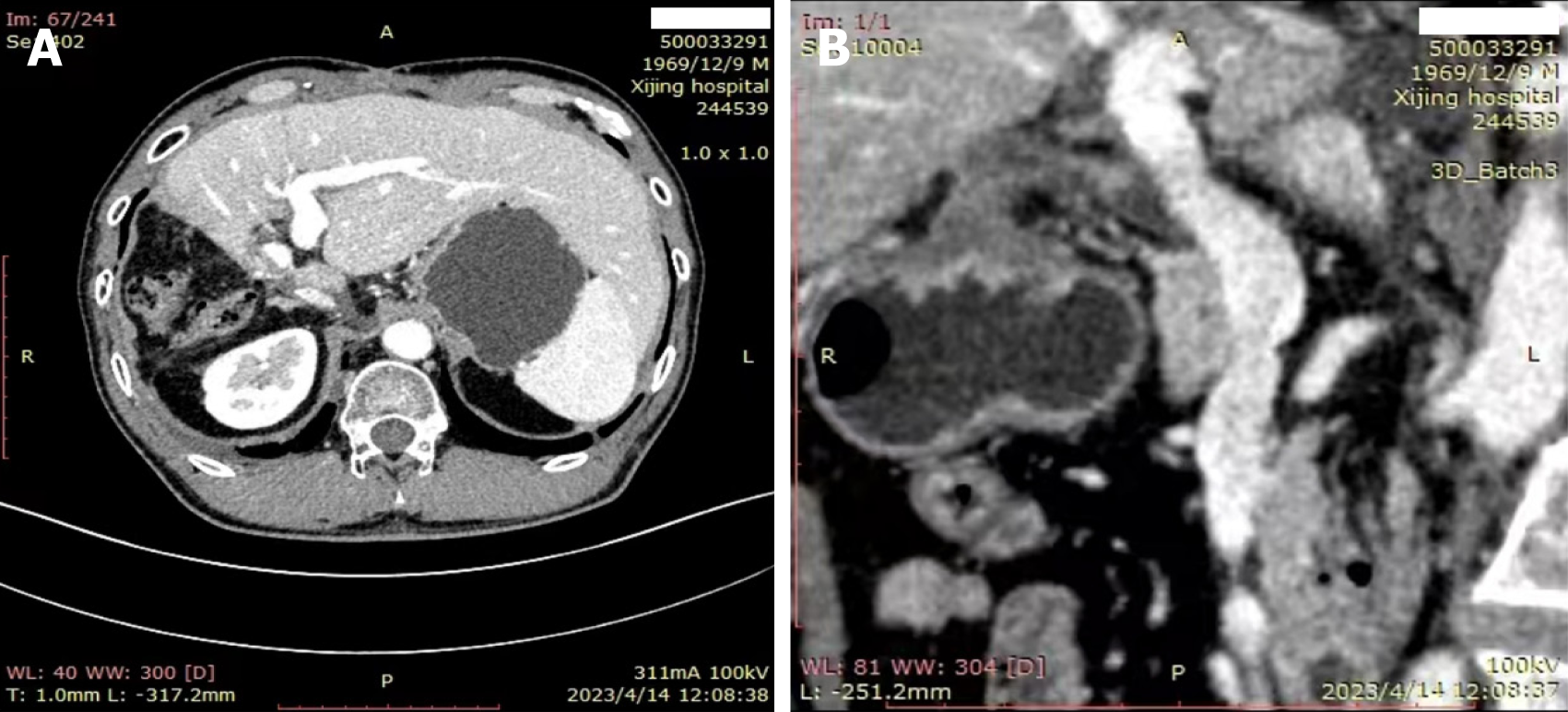
He had a history of hepatitis B for more than 40 years, and a solid mass of about 11.2 cm × 9.5 cm in the right lobe of the liver. The patient was considered to be a hepatocellular carcinoma (CNLC Stage IIIa) combined with a portal vein tumor thrombus (IIIa) and an inferior vena cava tumor thrombus (II), with cirrhosis of the liver (Figure 1).
He had a history of hepatitis B for more than 40 years.
There was no other relevant personal or family history.
he patient’s body temperature was 36.3C, heart rate 127 bpm, respiratory rate 67 breaths/min, fingertip oxygen saturation (SpO2) 89%, and body weight 77 kg. Upon examination, no swollen superficial lymph nodes were noted throughout the body, respiratory sounds in both lungs were rough, and fine moist rales were heard in both lungs. Physical examination of the heart, abdomen and nervous system did not reveal any abnormalities.
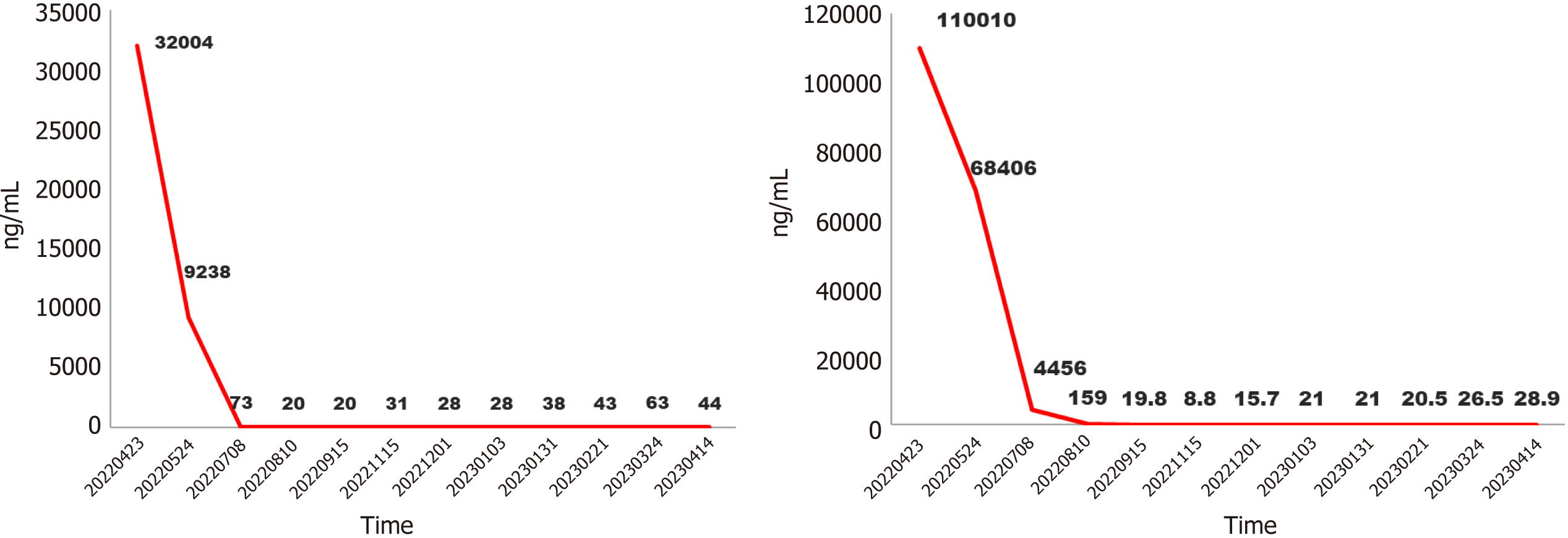
Blood tests revealed that his erythrocyte sedimentation rate, liver and kidney function, and C-reactive protein level were normal. The myocardial enzyme spectrum was normal. The tumor biomarker were elevated, protein induced by vitamin K absence or antagonist-II (PIVKA-II) 32004 ng/mL, alpha fetoprotein (AFP) 110010 ng/mL.
Abdominal computed tomography plain scan showed a significant node in the right of liver (Figure 1).
HCC (CNLC Stage IIIa) combined with a portal vein tumor thrombus (IIIa) and an inferior vena cava tumor thrombus (II), with cirrhosis of the liver.
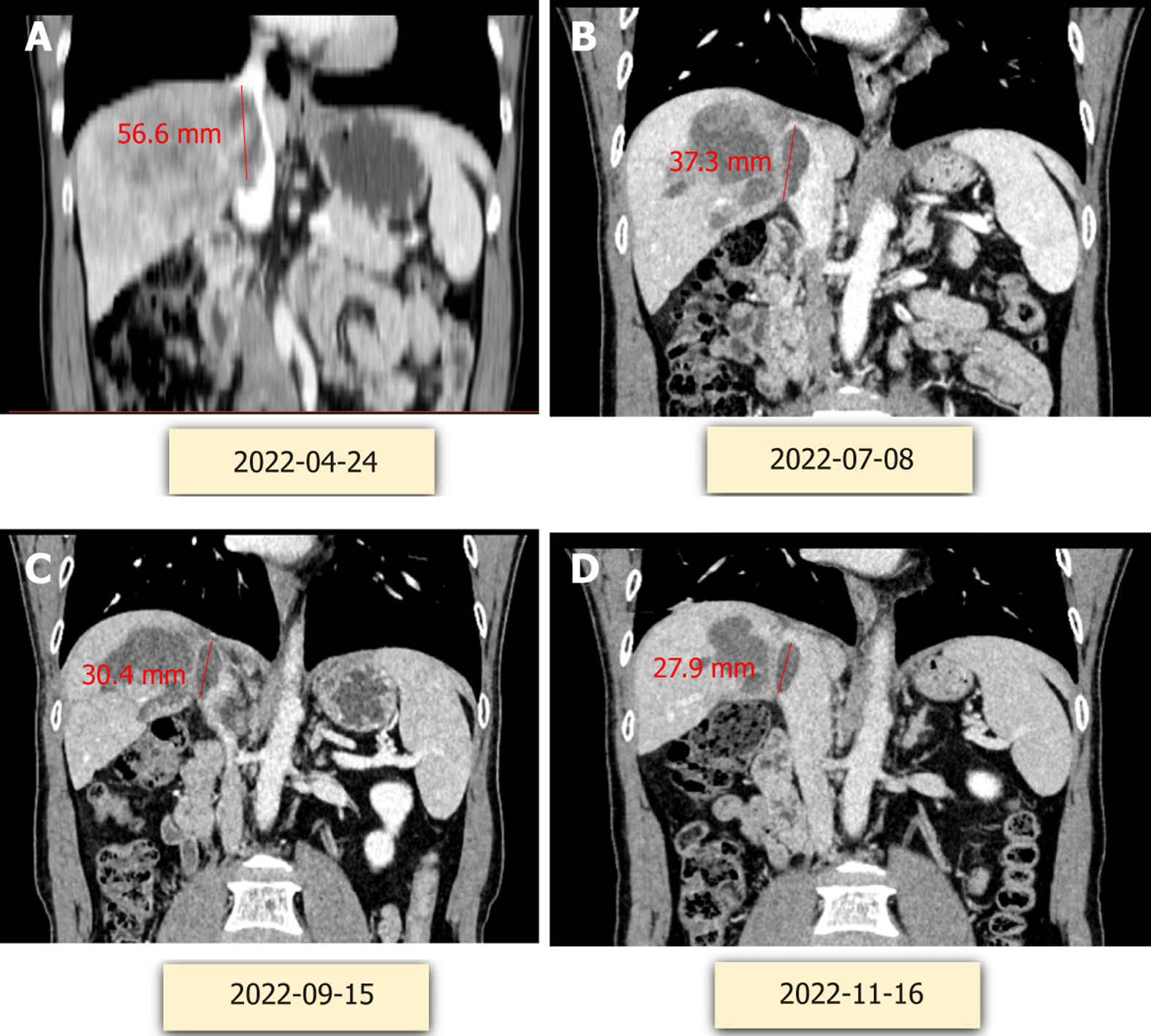
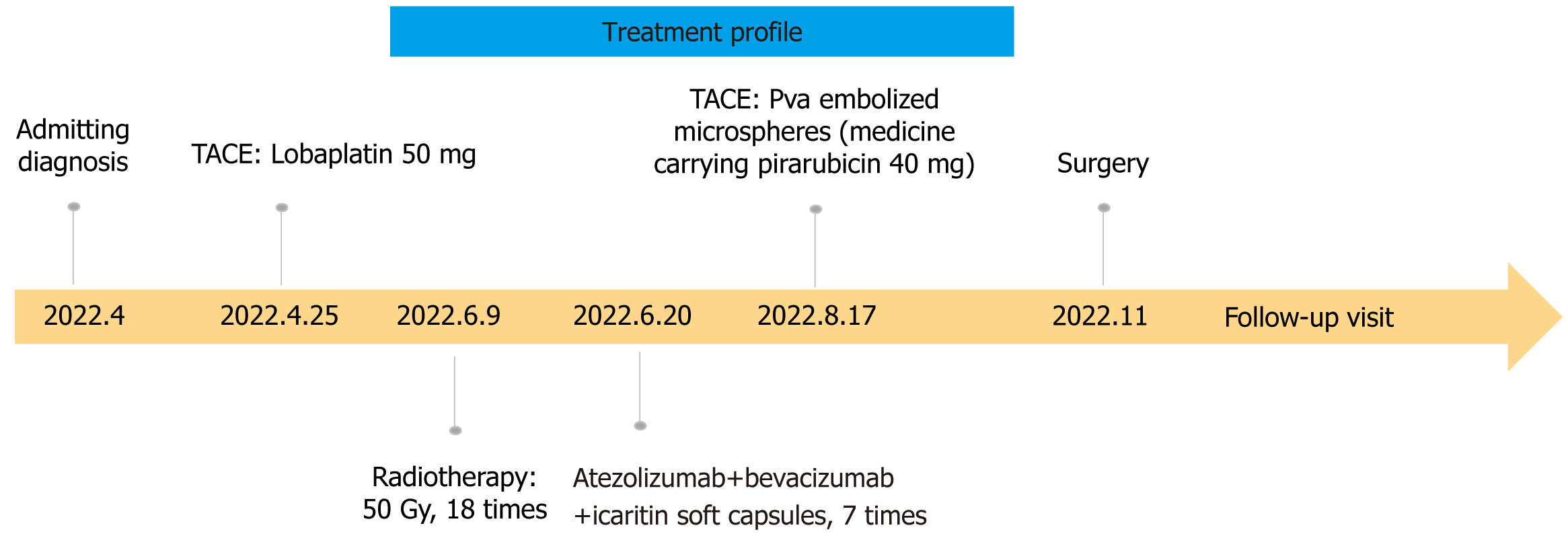
After puncture biopsy, it was confirmed as highly differentiated hepatocellular carcinoma and assessed as high risk for surgical resection. TACE treatment (Loblatin 50 mg infusion) was performed first, followed by radiotherapy (48 Gy for a total of 18 sessions) and 7 cycles of adjuvant treatment with atezolizumab (1200 mg) + bevacizumab (15 mg/kg) and icaritin soft capsule (3 tablets/per session, BID). On 2022.8.17, polyvinyl alcohol embolization (pirarubicin 40 mg) was performed again to consolidate the efficacy of treatment. Because of the high level of hepatitis B virus, the patient was given a full course of antiviral therapy with oral entecavir capsules one pill a day. During the whole course of treatment, the PIVKA-II decreased from 32004 to 31 ng/mL, and the AFP decreased from 110010 to 8.8 ng/mL (Figure 2). Significant shrinkage of the tumour can be seen by imaging changes over 7 months, with a significant reduction in the inferior vena cava from 56.6 mm to 27.9 mm (Figure 3). Transient transaminase elevation occurred during the treatment, and symptomatic liver-protective treatment was given.
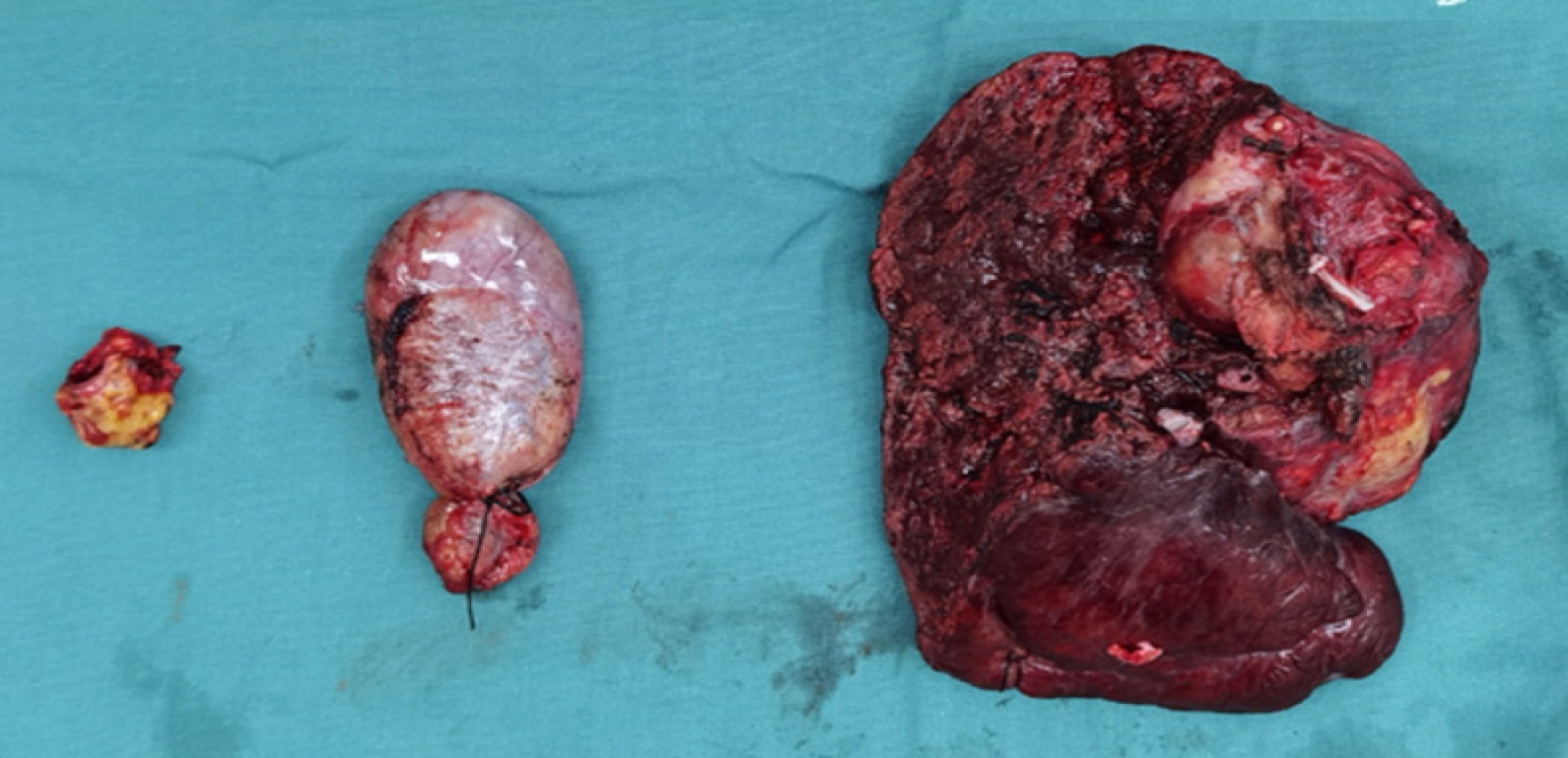
Imaging examination showed that the tumor gradually shrank, the portal vein tumor thrombus and the hepatic vein tumor thrombus gradually subsided, and the tumor markers AFP and PIVKA-II gradually decreased to normal levels. In November 2022, the review of the patient reached the criteria for radical surgery, and right hemihepatectomy and removal of vena cava tumor thrombus were performed. Extensive specimens were taken after surgery, the volume of the inferior vena cava tumor thrombus resection specimen was 2 cm × 1.5 cm × 1.5 cm, the cut surface was gray solid nature. The volume of the partial liver resection specimen was 13 cm × 10 cm × 5 cm, and a grey nodular mass was found in the gray-yellow cut surface, which locally seems to be necrotic, area: 6 cm × 4.5 cm, peripheral with visible several lower star diameter: 0.2-0.7 cm. Tumor bed, right branch of portal vein and inferior vena cava were seen in large amount of necrotic material, no active tumor cells were seen. Pathologic examination showed complete remission (Figure 4).
The patient had transient postoperative liver function abnormalities, including elevated bilirubin and transaminases, and was discharged from the hospital on the 8th postoperative day. Pathological results showed that no live hepatocellular carcinoma survived, and only necrotic tumor left. No recurrence was observed 7 months after hepatectomy, as it shown on imaging in Figure 5. The course of treatment is shown in Figure 6.
Written informed consent was obtained from the patient for the use of this case report and any accompanying images.
Hepatocellular carcinoma is the sixth most common malignant tumor in the world, with about 740000 new cases each year. Surgical treatment is an important means for patients with hepatocellular carcinoma to achieve long-term survival, but only a small number of patients have the opportunity for surgery or liver transplantation. For patients with advanced hepatocellular carcinoma, effective systemic treatment can help to reduce tumor burden, improve tumor-related symptoms, improve quality of life and prolong survival time. The prognosis of patients with hepatocellular carcinoma combined with hepatic vein/inferior vena cava tumor thrombus is extremely poor and progress rapidly. Most of them will suffer from hepatic failure in a short period of time or die of pulmonary embolism or cardiac tamponade due to tumor thrombus abscission. Without treatment, the median survival time of the patients is only 2.7 to 3 months[2]. Existing treatment modalities are ineffective in addressing this situation. Studies showed that advanced hepatocellular carcinoma has varying degrees of tumor shrinkage after downstage treatment. The 5-year survival rate after second-stage surgical resection or liver transplantation is 25%-57%[3]. Therefore, performing downstage/conversion surgery may be one of the important means to improve the prognosis of middle and advanced stage hepatocellular carcinoma.
Due to the biological characteristics of hepatocellular carcinoma and the anatomical features of liver, hepatocellular carcinoma cells are prone to invade the intrahepatic vascular system, especially the portal vein system, and form PVTT, with an incidence of 44%-62.2%. Once PVTT occurs in patients with liver cancer, the disease develops rapidly, and intrahepatic and extrahepatic metastases, portal hypertension, jaundice, ascites and so on may occur in a short period of time.
For the treatment of advanced hepatocellular carcinoma combined with portal vein tumor embolism, the results of a study of 6474 patients published by the Japanese Liver Cancer Study Group represent a higher level of treatment: Patients who did not undergo surgical treatment had a median overall survival of 0.48-1.1 years according to the different liver function scores (Child - Pugh, A, or B), and the 1-, 3-, and 5-year survival rates were 32.2%-53.1%, 13.0%-25.3%, and 7.9%-16.0%, respectively; for surgical patients, according to the tumor embolization classification (Japanese Society of Oncology Classification Vp 1-4) and the liver function score (Child - Pugh, A or B), the 90-d postoperative mortality rate was 2.4%-8.2%, the median recurrence-free survival rate was 0.38-1.23, and the median overall survival period was 1.44-2.87 years, and 1-, 3-, and 5-year survival rates of 61.3%-74.8%, 35.2%-49.1%, and 25.6%-39.1%, respectively[10].
There have been some findings suggesting the efficacy of selective internal radiotherapy for tumor shrinkage and its role in the translational treatment of hepatocellular carcinoma. Compared with external radiotherapy, selective internal radiotherapy has higher local dose and more accurate location for patients with portal vein tumor thrombus, and it also reduces radiation damage to normal liver tissue and has less impact on liver reserve function[11]. However, there is still relatively little clinical data in China, and more evidence is needed to verify its role. Japanese researchers compared the efficacy of sequential surgical treatment with radiotherapy and direct surgical treatment in a group of patients with tumor thrombus in the main trunk or first-grade branches of the portal vein. Radiotherapy was directed only to the tumor thrombus, with a dose of 30-36 Gy in 10-12 times, and the surgery was performed within 2 wk after radiotherapy. Postoperative pathological results showed that 5/6 (83.3%) patients in the radiotherapy sequential surgery group had complete necrosis of the thrombus in the main trunk of the portal vein. The 5-year survival rate was 34.8% in the radiotherapy sequential surgery group and 13.1% in the surgery alone group (P = 0.0359)[12]. For patients with technically resectable CNLC stage IIIa hepatocellular carcinoma, Wei et al[13] compared the efficacy of preoperative radiotherapy sequential surgical resection with that of surgical resection alone by RCT, and found that the portal vein tumor thrombus was downgraded from Cheng's type III to type II or from type II to type I in 20.7% of patients in the radiotherapy group, and that the survival of the patients was significantly improved by preoperative radiotherapy sequential surgical resection compared with surgical resection alone.
TACE is a commonly used treatment for unresectable hepatocellular carcinoma combined with PVTT, but whether it can be used in patients with PVTT type III/IV is still controversial, as it may lead to liver failure. Currently, it is considered that TACE can be consi
The role of TACE in conversion therapy has been explored and widely recognized. However, it should be noted that repeated TACE may lead to liver damage, which may affect the safety of hepatic resection after conversion. In the future, the success rate of conversion can be improved by improving the TACE treatment modality or combination therapy.
Inflammatory and non-inflammatory categories and genomic features of HCC are linked to immune checkpoint inhibitor response, but there are no validated biomarkers that can be used to guide clinical decision[16,17]. For patients with UR-HCC, sorafenib, a multi-kinase inhibitor, had been the only option of systemic therapy for a long time[18]; However, despite the widespread use of sorafenib in patients with UR-HCC, there were few reports of CS after sorafenib treatment. With the recent development of systemic therapy, a paradigm shift has occurred in the treatment of UR-HCC. In the phase 3 trial, several drugs were reported to be superior to sorafenib[19]. The REFLECT trial showed that lenva
In this case, after 7 cycles of treatment with atezolizumab combined with bevacizumab and icaritin soft capsules, it was shown that the tumor gradually shrank, the portal vein tumor thrombus and the hepatic vein tumor thrombus gradually regressed, and the activity decreased, which made surgical resection possible. In fact, several CS cases after Atez+Bev or lenvatinib have been reported. Hidaka et al[21] reported CS after treatment with Atez+Bev for HCC with portal vein tumor thrombus. Hoshino et al[22] reported the case of a HCCv15 cm in diameter occupying the right lobe with adrenal metastasis and invasion of the inferior vena cava, treated with 9 courses of Atez+Bev followed by CS. Shindoh et al[23] reported 16 cases of CS after lenvatinib among 107 cases that were initially UR-HCC. Thus, an increasing number of cases are being reported in initially UR-HCC can be resected following targeted therapies and immunotherapy, contributing to a prolonged prognosis. However, evidence-based data on the timing of surgery, surgical procedures, postoperative complications, postoperative recurrence time, and long-term prognosis in these patients are lacking. Although liver resection after conversion therapy is considered more difficult and more complicated than normal liver resection, CS can be performed safer with accurate preoperative evaluation (especially whether R0 can be achieved or not), precise intraoperative technique, and careful control of bleeding Further follow-up is needed for recurrence and long-term survival after CS[24]. Controlling adverse events through systemic therapy is also an extremely important factor in achieving conversion surgery. We should be pay attention to the use of ICIs in patients with autoimmune diseases, interstitial pneumonias, or a history of organ transplantation. In this case, only a transient transaminase elevation occurred throug
In this case, the patient used icaritin soft capsule which can directly inhibit the proliferative activity of tumor cells, inhibit cell proliferation and promote apoptosis[25,26]. Icaritin has anticancer effects both in vitro and in vivo. Its mechanism of action may be related to its antiangiogenic and antiproliferative effects in tumors[27]. Its antiangiogenic effect may mechanistically enhance the effect of targeted therapeutic agents. In vivo experiments in the present study demonstrated that icaritin could modulate immune cells in both bone marrow and peripheral blood[28]. Inhibition of programmed cell death protein 1 (PD-L1) expression by targeting protein IκB kinase α[28]. Icaritin inhibits PD-L1 expression by targeting protein IκB kinase α[29]. Icaritin reverses multidrug resistance in HepG2/adverse drug reaction human hepatocellular carcinoma cells by down-regulating the expression of MDR1 and P-glycoprotein[30].
We report a case of giant hepatocellular carcinoma combined with portal vein tumor thrombus and inferior vena cava tumor thrombus treated with TACE, radiotherapy and atezolizumab + bevacizumab + icaritin soft capsule followed by conversion surgery. The patient underwent R0 resection and the pathological results showed no residual viable tumor. The patient survived without recurrence for 7 months.
| 1. | Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: A retrospective study of 157 patients. Am J Clin Oncol. 1998;21:386-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 133] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Chinese Society of Liver Cancer; Chinese Medical Doctor Association. [Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus]. Linchuang Gandanbing Zazhi. 2019;35:737-743. [DOI] [Full Text] |
| 3. | [Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with hepatic vein or inferior vena cave tumor thrombus]. Zhongguo Shiyong Waike Zazhi. 2019;6:17-22. [DOI] [Full Text] |
| 4. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4627] [Article Influence: 925.4] [Reference Citation Analysis (2)] |
| 5. | Yamamura K, Beppu T, Miyata T, Okabe H, Nitta H, Imai K, Hayashi H, Akahoshi S. Conversion Surgery for Hepatocellular Carcinoma Following Molecular Therapy. Anticancer Res. 2022;42:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Song T, Lang M, Ren S, Gan L, Lu W. The past, present and future of conversion therapy for liver cancer. Am J Cancer Res. 2021;11:4711-4724. [PubMed] |
| 7. | Sun HC, Zhu XD. Downstaging Conversion Therapy in Patients With Initially Unresectable Advanced Hepatocellular Carcinoma: An Overview. Front Oncol. 2021;11:772195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Arita J, Ichida A, Nagata R, Mihara Y, Kawaguchi Y, Ishizawa T, Akamatsu N, Kaneko J, Hasegawa K. Conversion surgery after preoperative therapy for advanced hepatocellular carcinoma in the era of molecular targeted therapy and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci. 2022;29:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 939] [Article Influence: 313.0] [Reference Citation Analysis (0)] |
| 10. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O, Kaneko S, Kokudo N; Liver Cancer Study Group of Japan. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 11. | Kokabi N, Camacho JC, Xing M, El-Rayes BF, Spivey JR, Knechtle SJ, Kim HS. Open-label prospective study of the safety and efficacy of glass-based yttrium 90 radioembolization for infiltrative hepatocellular carcinoma with portal vein thrombosis. Cancer. 2015;121:2164-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Kamiyama T, Nakanishi K, Yokoo H, Tahara M, Nakagawa T, Kamachi H, Taguchi H, Shirato H, Matsushita M, Todo S. Efficacy of preoperative radiotherapy to portal vein tumor thrombus in the main trunk or first branch in patients with hepatocellular carcinoma. Int J Clin Oncol. 2007;12:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, Xing H, Xu Y, Shi J, Guo W, Zhou D, Zhang H, Sun H, Huang C, Lu C, Zheng Y, Meng Y, Huang B, Cong W, Lau WY, Cheng S. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. J Clin Oncol. 2019;37:2141-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 14. | Chung GE, Lee JH, Kim HY, Hwang SY, Kim JS, Chung JW, Yoon JH, Lee HS, Kim YJ. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Ajit Y, Sudarsan H, Saumya G, Abhishek A, Navneet R, Piyush R, Anil A, Arun G. Transarterial chemoembolization in unresectable hepatocellular carcinoma with portal vein thrombosis: a perspective on survival. Oman Med J. 2014;29:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1195] [Article Influence: 398.3] [Reference Citation Analysis (41)] |
| 17. | Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (1)] |
| 18. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10237] [Article Influence: 602.2] [Reference Citation Analysis (2)] |
| 19. | Kudo M. New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma. Int J Clin Oncol. 2022;27:1110-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3797] [Article Influence: 542.4] [Reference Citation Analysis (1)] |
| 21. | Hidaka Y, Tomita M, Desaki R, Hamanoue M, Takao S, Kirishima M, Ohtsuka T. Conversion surgery for hepatocellular carcinoma with portal vein tumor thrombus after successful atezolizumab plus bevacizumab therapy: a case report. World J Surg Oncol. 2022;20:228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Hoshino T, Naganuma A, Furusawa A, Suzuki Y, Hirai K, Sakamoto I, Ogawa T, Ogawa A, Hatanaka T, Kakizaki S. A case of conversion hepatectomy for huge hepatocellular carcinoma with adrenal metastasis and vascular invasion after atezolizumab-bevacizumab treatment. Clin J Gastroenterol. 2022;15:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Shindoh J, Kawamura Y, Kobayashi Y, Kobayashi M, Akuta N, Okubo S, Suzuki Y, Hashimoto M. Prognostic Impact of Surgical Intervention After Lenvatinib Treatment for Advanced Hepatocellular Carcinoma. Ann Surg Oncol. 2021;28:7663-7672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Luo L, He Y, Zhu G, Xiao Y, Song S, Ge X, Wang T, Xie J, Deng W, Hu Z, Shan R. Hepatectomy After Conversion Therapy for Initially Unresectable HCC: What is the Difference? J Hepatocell Carcinoma. 2022;9:1353-1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 999] [Article Influence: 333.0] [Reference Citation Analysis (2)] |
| 26. | Li H, Liu Y, Jiang W, Xue J, Cheng Y, Wang J, Yang R, Zhang X. Icaritin promotes apoptosis and inhibits proliferation by down-regulating AFP gene expression in hepatocellular carcinoma. BMC Cancer. 2021;21:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Cao Z, Cheng Y, Wang J, Liu Y, Yang R, Jiang W, Li H, Zhang X. HBP1-mediated transcriptional repression of AFP inhibits hepatoma progression. J Exp Clin Cancer Res. 2021;40:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Yang JX, Fichtner I, Becker M, Lemm M, Wang XM. Anti-proliferative efficacy of icariin on HepG2 hepatoma and its possible mechanism of action. Am J Chin Med. 2009;37:1153-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Mo D, Zhu H, Wang J, Hao H, Guo Y, Han X, Zou L, Li Z, Yao H, Zhu J, Zhou J, Peng Y, Li J, Meng K. Icaritin inhibits PD-L1 expression by Targeting Protein IκB Kinase α. Eur J Immunol. 2021;51:978-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Sun L, Chen W, Qu L, Wu J, Si J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and Pglycoprotein expression. Mol Med Rep. 2013;8:1883-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |