Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2822
Revised: January 26, 2024
Accepted: March 13, 2024
Published online: June 6, 2024
Processing time: 196 Days and 21.3 Hours
Patients with giant intracranial aneurysms (GIAs) are at a high risk of rupture, morbidity, and mortality even after surgical or endovascular treatment. We de
A 72-year-old female patient complained of sudden pain in her right eye upon admission to our hospital. She had been diagnosed with a GIA [30 mm (axial) × 38 mm (coronal) × 28 mm (sagittal)] containing an aneurysmal thrombus located in the cavernous sinus segment of RICA diagnosed by magnetic resonance imaging (MRI), enhanced MRI, and magnetic resonance angiography more than 14 years ago. Later, with slow growth of the cavernous carotid GIA, aneurysmal throm
The risk of rupture of the cavernous carotid GIAs was relatively low and possibly further be reduced by the stasis flow and spontaneous occlusion of the parent artery internal carotid artery (ICA) induced by the mass effect of the cavernous carotid GIAs and the extremely rare aneurysmal calcification. However, nowa
Core Tip: A spontaneously occluded cavernous carotid giant intracranial aneurysm (GIA), which presented with sequential slow growth, continuous progression of aneurysmal thrombosis, aneurysmal calcification, spontaneous complete occlusion of the parent internal carotid artery, was not reported. Cavernous carotid GIAs have a low risk of rupture, but nowadays, we advocate for endovascular treatment to inhibit the growth of these aneurysms and prevent subsequent complications caused mainly by the mass effect of the cavernous carotid GIAs.
- Citation: Wang MX, Nie QB. Giant cavernous aneurysms occluded by aneurysmal thrombosis, calcification, parent artery occlusion: A case report and review of literature. World J Clin Cases 2024; 12(16): 2822-2830
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2822.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2822
The prevalence of giant intracranial aneurysms (GIAs, ≥ 25 mm in diameter) is approximately 5% (in Germany[1])-13.7% (in India[2]). GIAs have a high risk of rupture and can lead to higher morbidity and mortality rates, as well as a low complete occlusion rate in their natural progression[3-5] or even after being treated by surgical clipping[6] and a variety of endovascular treatment strategies[7-11]. Therefore, GIAs have no ideal treatment method in the past. However, we had a 72-year-old female patient with a GIA located in the cavernous sinus segment of right internal carotid artery (RICA) which had not been given any treatment early, but the cavernous carotid GIA was occluded spontaneously, accompanied by progressed aneurysmal thrombosis, aneurysmal calcification and complete occlusion of its parent internal carotid artery (ICA). To the best of our knowledge, this type of case has not been previously reported.
Our patient was a 72-year-old female with sudden pain in the right forehead, orbit, and eye, as well as skin numbness in the right cheek for 2 d.
The patient was diagnosed with a GIA in the cavernous sinus segment of the RICA since more than 14 years ago by other hospitals. She had not received surgical or endovascular treatments before being admitted to our hospital on October 4, 2018. Throughout her medical history, no subarachnoid hemorrhage (SAH) was found.
The patient had a medical history of hypertension for 26 years (since she was 46 years old), meningitis at 20 years old, systemic lupus erythematosus between the ages of 24 and 26 that was cured with steroids, acute pyelonephritis at 56 years old, allergies to alcoholic iodine solution at 37 years old and to cephalosporins at 61 years old, surgery for lumbar spine tuberculosis at 37 years old, and orthopedic surgery for a right femoral head fracture with metal implants at 70 years old, which prohibited magnetic resonance imaging (MRI) examinations later.
The patient did not have a history of smoking or alcohol consumption, and there were no reported family hereditary diseases.
Upon admission, the patient exhibited the following clinical signs in the right eye: vision loss, an enlarged and fixed pupil (4 mm in diameter), absence of direct and indirect light reflections, ptosis, extremely limited movement of the eye only towards the nasal side, and a present corneal reflex. These signs indicated functional loss of the right cranial nerve II (resulting in vision loss), palsy of the right cranial nerves III/oculomotor, IV/trochlear, and VI/abducens (resulting in loss of right eye movement), and functional impairment of the right cranial nerves V1 and V2/trigeminal [evidenced by decreased tactile sensation in the right forehead and facial regions innervated by the ophthalmic branch (V1) and ma
The laboratory tests showed that the patient had slightly elevated levels of total triglycerides (1.82, normal range 0.34-1.7 mmol/L), cholesterol (6.73, normal range 3.38-5.2 mmol/L), low-density lipoprotein cholesterol (3.89, normal range 1.82-3.64 mmol/L), glucose (7.79, normal range 3.9-6.1 mmol/L), calcium (2.96, normal range 2.1-2.7 mmol/L), urea (7.56, normal range 1.8-7.5 mmol/L), uric acid (463, normal range 102-417 μmol/L), β2-microglobulin (3.03, normal range 1.0-3.0 mg/L). The coagulation test results were within normal limits: Fibrinogen 3.665 (normal range 1.80-3.50 g/L), throm
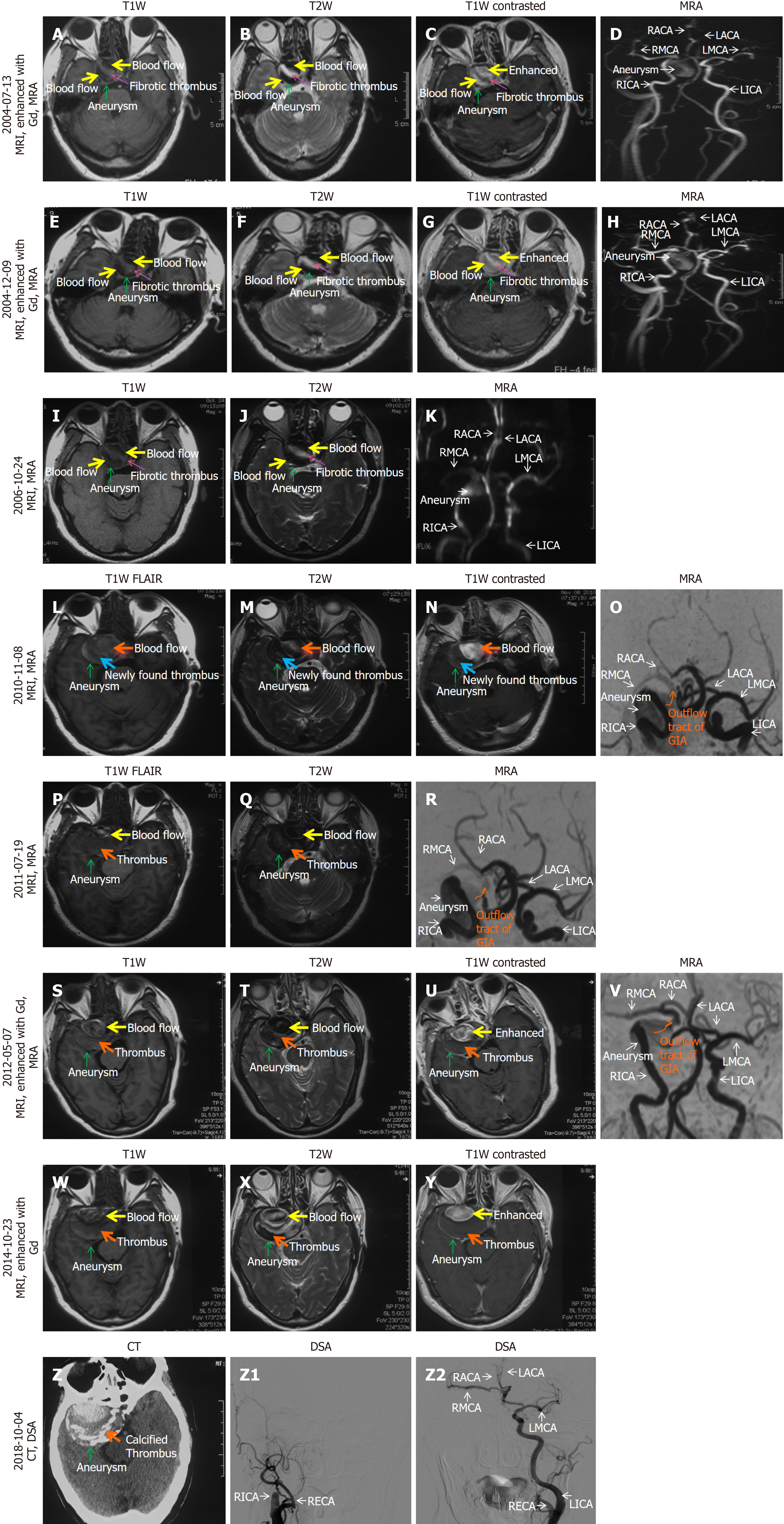
Due to gradually impaired vision and nearly complete loss of vision (with residual light perception) in her right eye, the patient underwent the first head MRI and enhanced MRI and magnetic resonance angiography (MRA) examinations on July 13, 2004. A GIA [30 mm (axial) × 38 mm (coronal) × 28 mm (sagittal)] in the cavernous sinus segment of the RICA was found [green arrow on MRI (Figure 1A-C), and MRA (Figure 1D)]. At this stage, the GIA contained mainly blood flow and thrombus. Later, on December 9, 2004 (Figure 1E-H) [31 mm (axial) × 39 mm (coronal) × 28 mm (sagittal)] and October 24, 2006 (Figure 1I-K) [34 mm (axial) × 33 mm (sagittal)], MRI and MRA did not show significant changes in the size of the GIA and the proportions of blood flow and thrombus inside the GIA.
On November 8, 2010 (Figure 1L-O) [37 mm (axial) × 39 mm (sagittal)], MRI showed that the cavernous carotid GIA had grown, and the thrombus content was predominant over the blood flow inside the GIA. The blood supply to the RICA distal to the GIA [right middle cerebral artery (RMCA) and right anterior cerebral artery (RACA)] was reduced due to the stretching and compression exerted by the GIA. The entire RICA was still unobstructed at this stage.
On July 19, 2011 (Figure 1P-R) [39 mm (axial) × 40 mm (coronal) × 39 mm (sagittal)], May 7, 2012 (Figure 1S-V) [56 mm (axial) × 52 mm (coronal) × 44 mm (sagittal) ] and October 23, 2014 (Figure 1W-Y) [56 mm (axial) × 52 mm (coronal) × 46 mm (sagittal)], MRI and MRA scans revealed a significant enlargement of the GIA and an increased proportion of thrombus compared to blood flow inside the GIA. MRA images (Figure 1R and V) showed that the entire RICA remained unobstructed on July 19, 2011 and May 7, 2012.
On October 4, 2018 (Figure 1Z-Z2), the thrombus inside the GIA became concentric calcification, as shown on computed tomography (CT) (Figure 1Z). The entire GIA, including the distal (RMCA, RACA) and proximal parts of RICA, was completely occluded, as they were not visible on digital subtraction angiography (DSA) (Figure 1Z1), RMCA and RACA were supplied by LICA via the anterior communicating artery (Figure 1Z2).
Between May 7, 2012, and October 4, 2018, before her first admission to our hospital on October 4, 2018, no MRA, angiography, such as DSA, or CT angiography was performed. Therefore, we could not determine when the complete occlusion of the whole RICA and its branches (RMCA, LACA, and ophthalmic artery) occurred.
In summary, over the course of more than 14 years, the cavernous carotid aneurysm was initially discovered to have a small amount of thrombus. As the aneurysm continued to grow chronically, the thrombosis inside it progressed. Even
The final diagnosis of the presented case was a spontaneously occluded cavernous carotid GIA secondary to continuous growth of the GIAs, progressing aneurysmal thrombosis, complete occlusion of the parent artery-RICA and aneurysmal calcification.
Upon the patient's admission to our hospital, it was observed that the mass effect of the cavernous carotid GIA had led to the functional impairment of the right cranial nerves II, III, IV, V1/V2, and VI, as well as complete occlusion of the RICA. Unfortunately, she had missed the optimal time window for endovascular therapy, which could have prevented the vision loss and ophthalmoplegia on the right and occlusion of the RICA caused mainly by the mass effect of the GIA. However, no other severe or new neurological deficits occurred due to adequate blood supply from the contralateral ICA, and she was managed conservatively.
During her 5-year telephone follow-up, we found out that the clinical symptoms mentioned above had not worsened over time.
Cavernous carotid aneurysms represent approximately 5% (40 cavernous carotid aneurysms in 35 consecutive patients out of 802 intracranial aneurysms in 711 consecutive patients diagnosed between January 1989 and April 2007 in Brazil[12]) to 8.3% (337 patients with cavernous carotid aneurysms out of 4060 patients with intracranial aneurysms diagnosed between 1991 and 1998 in the United States, Canada, and Europe[3]) of all intracranial aneurysms. The ratio of cavernous carotid GIAs in all GIAs is increased to 16% (21 cavernous carotid GIAs out of 129 GIAs in 125 consecutive patients treated between 1987 and 2007 in Finland)[13], and the ratio of the cavernous carotid GIAs in all cavernous carotid aneurysms is up to 50% (20 cavernous carotid GIAs out of 40 cavernous carotid aneurysms)[12]. It has been reported that aneurysmal thrombosis has a relatively high incidence rate, with 60% for partial thrombosis and 13%-20% for complete thrombosis, respectively[14]. Large and GIAs have a higher tendency to develop thrombosis[14]. However, simultaneous thrombosis of the cavernous carotid GIAs with occlusion of the parent ICA by ICA thrombosis is an exceedingly rare phenomenon. Until now, only a total of 22 cases have been reported (20 cases were summarized by Abousedu et al[15], 1 case was reported by Salih et al[16], and the present case; 50% (11/22) of them below 55 years old).
In this paper, we report a case of a spontaneously occluded cavernous carotid GIA presenting with gradual aneu
To better manage this type of patient, we conducted a literature review to understand the mechanisms underlying aneurysmal thrombosis, occlusion of the parent ICA by ICA thrombosis, occlusion of the cavernous carotid GIA, and aneurysmal calcification as well as their roles in affecting the rupture risk of intracranial aneurysms.
The mechanism of spontaneous aneurysmal thrombosis was thought to result from hemodynamic stress causing endothelial damage on aneurysmal wall. It was associated with an increased aneurysm dome-to-neck ratio and chronic aneurysms[15].
For the mechanisms of spontaneous occlusion of the parent ICA by ICA thrombosis in patients with cavernous carotid GIAs, the location of GIA in cavernous sinus is the vital contribution factor. The cavernous sinus is a narrow space, approximately 10 mm × 20 mm in size in a human adult, and has complex anatomical contents including cranial nerves III, IV, V, VI, ICA, dura mater, etc., and neighboring structures, such as like cranial nerve II[17]. Therefore, cavernous carotid GIAs can cause significant symptomatology through mass effect, such as headache, retroorbital pain, proptosis, hypopituitarism, and the clinical symptoms and signs related to injuries of cranial nerves II, III, IV, V1/V2, or VI (such as vision loss, ptosis, diplopia, or toothache, which were found in all 22 cases presenting simultaneous thrombosis of the cavernous carotid GIAs with occlusion of the parent ICA by ICA thrombosis[17,18], and flow stasis, progression of aneurysmal thrombosis into the parent ICA and subsequent ICA occlusion[12,15,18-20].
The spontaneous occlusion of the ICA typically progressed gradually with the slow growth of the cavernous carotid aneurysms, allowing enough time for the formation of adequate supply by contralateral circulation. This was present in 17 patients who underwent angiography examination[14,17,18]. So, ICA occlusion less frequently leads to severe acute complications, such as hemiplegia[21]. Of course, the growth and extension of aneurysmal thrombosis to the parent ICA caused by the ICA flow stasis could be very rapid (in 2 wk), resulting in retrograde ICA thrombosis (complicated by he
As mentioned above, in the over 14-year medical history of our case, her radiological images clearly displayed the promoting role of the chronic growth of the cavernous carotid GIA in inducing flow stasis within RICA and then exten
In our case, the mechanism of aneurysmal calcification may be related to aneurysmal thrombosis. Previous reports have indicated that wall calcification and mural thrombus often coexist in partially or completely thrombotic aneurysms, but are not observed in nonthrombotic aneurysms[23]. The early formation of aneurysmal thrombus prior to the calcifi
In brief, the location of the GIAs in the cavernous sinus, along with the chronically upgraded mass effect of the ca
When managing cavernous carotid GIAs, several aspects need to be comprehensively and dynamically evaluated.
The first issue to be mentioned is the risk of rupture in cavernous carotid GIAs. It is known that rupture has been associated with high morbidity and mortality[24]. Larger size of aneurysms was associated with a greater risk of rupture, especially for GIAs[3,13]. A multicenter study conducted by the International Study of Unruptured Intracranial Aneu
The second issue is that, it is unclear whether partial or complete aneurysmal thrombosis inhibits or promotes the rupture of GIAs. In human abdominal aortic aneurysms (AAAs), it is proposed that aneurysmal thrombosis has no protective effect for AAAs, but promotes AAA wall degeneration[25]. A follow-up (1 month to 12 years) of a consecutive series of 28 patients with GIAs without surgery revealed that thrombosed GIAs had a low rupture risk[26]. However, another study of 12 patients with spontaneously partially or completely thrombosed GIAs showed that massive aneury
In addition to the aforementioned factors, we believe that the stagnant flow in the ICAs compressed by the cavernous carotid GIAs further reduces the risk of rupture of the cavernous carotid GIAs.
In our case, aneurysmal calcification should also be considered as a factor that synergistically prevents rupture along with the factors mentioned above. It has been reported that aneurysmal wall calcification is associated with a low rupture rate[13]. Aneurysms with aneurysmal wall calcification and thrombus had an 11-fold lower rupture risk than those with
In summary, in the presence or absence of aneurysmal calcification, the location of the GIAs in the cavernous sinus and the mass effect of the cavernous carotid GIAs on the parent ICA also collectively reduce the risk of rupture of the ca
Therefore, the management approach for cavernous carotid aneurysms should shift from preventing aneurysm rupture to inhibiting aneurysm growth and the subsequent severe neurovascular complications, once patients present progressive cranial nerve deficits (III, IV, V1/V2, VI), visual symptoms (II), intractable retroorbital pain, or recurrent transient is
Currently, direct surgery for cavernous carotid aneurysms, such as direct clipping, is no longer recommended because it presents a great challenge to even a skilled neurosurgeon and is associated with potential excessive bleeding, cranial nerve injury, and mortality[24,29]. The newly improved endovascular therapy, such as, using a flow-diverting stent, is preferable due to its low incidences of periprocedural complications and mortality[29,30] and higher aneurysm lobliteration rate in long-term follow-up (12 months, 84%, 47/56)[30]. It also has the ability to reconstruct the ICA[29,31].
The limitation of this case report was that only one patient was included. No literature has reported this kind of GIA as in our case, and its management was not described in the Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms (American Heart Association/American Stroke Association)[32]. The adequate contralateral blood supply contributed to the absence of any recently occurred severe neurological complication of the spontaneous occlusion of the RICA in our patient, and the symptoms in her right eye could not be reversed. Her GIA was only given continuous monitoring, which should be treated at an early stage to prevent these mass effect-related complications.
Based on a clearly displayed evolution process of a spontaneously occluded cavernous carotid GIA presenting sequential slow growth, continuously progressing aneurysmal thrombosis, aneurysmal calcification and gradual occlusion of the parent ICA, we further analyzed their mechanisms in a series of cases with simultaneous aneurysmal thrombosis and spontaneous occlusion of the parent ICA by ICA thrombosis by conducting a literature review. The location of GIAs in the cavernous sinus and the mass effect of cavernous carotid GIAs played a major role in promoting spontaneous oc
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
| 1. | Regelsberger J, Groden C, Puchner MJ, Westphal M. [Diagnostic and therapeutical considerations in the treatment of giant aneurysms]. Zentralbl Neurochir. 2002;63:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (36)] |
| 2. | Sharma BS, Gupta A, Ahmad FU, Suri A, Mehta VS. Surgical management of giant intracranial aneurysms. Clin Neurol Neurosurg. 2008;110:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (33)] |
| 3. | Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O'Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2706] [Cited by in RCA: 2676] [Article Influence: 121.6] [Reference Citation Analysis (34)] |
| 4. | UCAS Japan Investigators; Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1076] [Article Influence: 82.8] [Reference Citation Analysis (36)] |
| 5. | Nanda A, Sonig A, Banerjee AD, Javalkar VK. Microsurgical management of giant intracranial aneurysms: a single surgeon experience from Louisiana State University, Shreveport. World Neurosurg. 2014;81:752-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (33)] |
| 6. | Nakase H, Shin Y, Kanemoto Y, Ohnishi H, Morimoto T, Sakaki T. Long-term outcome of unruptured giant cerebral aneurysms. Neurol Med Chir (Tokyo). 2006;46:379-84; discussion 384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (34)] |
| 7. | Jahromi BS, Mocco J, Bang JA, Gologorsky Y, Siddiqui AH, Horowitz MB, Hopkins LN, Levy EI. Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery. 2008;63:662-74; discussion 674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (15)] |
| 8. | Miyachi S, Hiramatsu R, Ohnishi H, Yagi R, Kuroiwa T. Usefulness of the Pipeline Embolic Device for Large and Giant Carotid Cavernous Aneurysms. Neurointervention. 2017;12:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (15)] |
| 9. | Oishi H, Teranishi K, Yatomi K, Fujii T, Yamamoto M, Arai H. Flow Diverter Therapy Using a Pipeline Embolization Device for 100 Unruptured Large and Giant Internal Carotid Artery Aneurysms in a Single Center in a Japanese Population. Neurol Med Chir (Tokyo). 2018;58:461-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (34)] |
| 10. | Yan P, Zhang Y, Liang F, Ma C, Liang S, Guo F, Jiang C. Comparison of Safety and Effectiveness of Endovascular Treatments for Unruptured Intracranial Large or Giant Aneurysms in Internal Carotid Artery. World Neurosurg. 2019;125:e385-e391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (36)] |
| 11. | Liang F, Zhang Y, Yan P, Ma C, Liang S, Jiang P, Jiang C. Predictors of Periprocedural Complications and Angiographic Outcomes of Endovascular Therapy for Large and Giant Intracranial Posterior Circulation Aneurysms. World Neurosurg. 2019;125:e378-e384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Vasconcellos LP, Flores JA, Conti ML, Veiga JC, Lancellotti CL. Spontaneous thrombosis of internal carotid artery: a natural history of giant carotid cavernous aneurysms. Arq Neuropsiquiatr. 2009;67:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Nurminen V, Lehecka M, Chakrabarty A, Kivisaari R, Lehto H, Niemelä M, Hernesniemi J. Anatomy and morphology of giant aneurysms--angiographic study of 125 consecutive cases. Acta Neurochir (Wien). 2014;156:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Das KK, Singh G, Pandey S, Bhaisora KS, Jaiswal A, Behari S. Completely Thrombosed Giant Intracranial Aneurysm with Spontaneous Thrombosis of the Parent Artery: Is It Nature's Divine Intervention and a Self-Cure? World Neurosurg. 2018;118:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Abousedu YAI, Saleem A, Alenezi S, Bosnjakovic P, Lazovic L, Alsheikh TM. Spontaneous thrombosis of a giant cavernous-carotid aneurysm with simultaneous ipsilateral complete parent artery occlusion: a rare phenomenon and review of the literature. Arch Clin Cases. 2023;10:21-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Salih M, Young M, Shutran M, Taussky P, Ogilvy CS. Spontaneous Thrombosis of a Giant Cavernous Internal Carotid Artery Aneurysm and Parent Vessel Occlusion in a Patient With Bilateral Cavernous Internal Carotid Artery Aneurysms. Cureus. 2023;15:e35231. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Balcerzak A, Tubbs RS, Zielinska N, Olewnik Ł. Clinical analysis of cavernous sinus anatomy, pathologies, diagnostics, surgical management and complications - Comprehensive review. Ann Anat. 2023;245:152004. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Sato K, Fujiwara S, Yoshimoto T, Onuma T. Two cases of spontaneous internal carotid artery occlusion due to giant intracranial carotid artery aneurysm. Stroke. 1990;21:1506-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Tsutsumi M, Kazekawa K, Tanaka A, Ueno Y, Nomoto Y. Spontaneous thrombosis of a giant intracavernous internal carotid artery aneurysm and ipsilateral internal carotid artery occlusion. Radiat Med. 2002;20:261-263. [PubMed] |
| 20. | Perrini P, Bortolotti C, Wang H, Fraser K, Lanzino G. Thrombosed giant intracavernous aneurysm with subsequent spontaneous ipsilateral carotid artery occlusion. Acta Neurochir (Wien). 2005;147:215-6; discussion 216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Gautier JC, Awada A, Majdalani A. Ophthalmoplegia with contralateral hemiplegia. Occlusion of the internal carotid artery due to thrombosis of an intracavernous aneurysm. Stroke. 1986;17:1321-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Yamagami K, Hatano T, Ando M, Chihara H, Ogura T, Suzuki K, Kondo D, Kamata T, Higashi E, Sakai S, Sakamoto H, Nagata I. Symptomatic Cavernous Internal Carotid Artery Aneurysm Complicated by Simultaneous Rapid Growth of the Intra-aneurysmal and Parent Artery Thromboses. NMC Case Rep J. 2021;8:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Pinto RS, Kricheff II, Butler AR, Murali R. Correlation of computed tomographic, angiographic, and neuropathological changes in giant cerebral aneurysms. Radiology. 1979;132:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Tawk RG, Hasan TF, D'Souza CE, Peel JB, Freeman WD. Diagnosis and Treatment of Unruptured Intracranial Aneurysms and Aneurysmal Subarachnoid Hemorrhage. Mayo Clin Proc. 2021;96:1970-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 25. | Boyd AJ. Intraluminal thrombus: Innocent bystander or factor in abdominal aortic aneurysm pathogenesis? JVS Vasc Sci. 2021;2:159-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Fujita K, Yamashita H, Masumura M, Nishizaki T, Tamaki N, Matsumoto S. [Natural history of giant intracranial aneurysms]. No Shinkei Geka. 1988;16:225-231. [PubMed] |
| 27. | Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982;45:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | dos Santos ML, Spotti AR, dos Santos RM, Borges MA, Ferrari AF, Colli BO, Tognola WA. Giant intracranial aneurysms: morphology and clinical presentation. Neurosurg Rev. 2013;36:117-22; discussion 122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Ambekar S, Madhugiri V, Sharma M, Cuellar H, Nanda A. Evolution of management strategies for cavernous carotid aneurysms: a review. World Neurosurg. 2014;82:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Guzzardi G, Stanca C, Cerini P, Del Sette B, Divenuto I, Malatesta E, Carriero A, Stecco A. Long-term follow-up in the endovascular treatment of intracranial aneurysms with flow-diverter stents: update of a single-centre experience. Radiol Med. 2018;123:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Walcott BP, Koch MJ, Stapleton CJ, Patel AB. Blood Flow Diversion as a Primary Treatment Method for Ruptured Brain Aneurysms-Concerns, Controversy, and Future Directions. Neurocrit Care. 2017;26:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Thompson BG, Brown RD Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES Jr, Duckwiler GR, Harris CC, Howard VJ, Johnston SC, Meyers PM, Molyneux A, Ogilvy CS, Ringer AJ, Torner J; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention; American Heart Association; American Stroke Association. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2368-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 696] [Article Influence: 69.6] [Reference Citation Analysis (0)] |