Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2729
Revised: April 8, 2024
Accepted: April 19, 2024
Published online: June 6, 2024
Processing time: 143 Days and 1.3 Hours
Paragangliomas (PG) are rare neoplasms of neuroendocrine origin that tend to be highly vascularized, slow-growing, and usually sporadic. To date, common treat
To evaluate the local control and effectiveness of exclusive fractionated stereo
We retrospectively evaluated patients with uPG (medically inoperable or refused SR) treated with FSRT with a Cyberknife System (Accuray Incorporated, Sunny
From May 2009 to January 2023, 6 patients with a median age of 68 (range 20-84) were treated with FSRT. The median delivered dose was 21 Gy (range 20-30 Gy) at a median isodose line of 75.5% (range 70%-76%) in 4 fractions (range 3-5 fractions). The median volume was 13.6 mL (range 12.4-65.24 mL). The median cumulative biological effective dose and equivalent dose in 2-Gy fractions were 70 Gy and 37.10 Gy respectively. Site of origin involved were the timpa-nojugular glomus (4/6), temporal bone, and cervical spine. In 1 of the 6 patients, the follow-up was insufficient; 5 of 6 patients showed a 5-year overall survival and 5-year progression-free survival of 100%. We observed negligible toxicities during and after RT. The majority of patients showed stable symptoms during follow-up. Only 1 patient developed spine metastases.
Our preliminary results on this small cohort of patients suggest that FSRT could be an effective and safe alternative to SR.
Core Tip: Paragangliomas (PGs) are rare tumors with neuroendocrine origin. These lesions tend to be highly vascular and embryologically arise from the extra-adrenal autonomic nervous system, located in the thoracic, abdominal, and head-neck regions. PGs are usually sporadic, except in a few cases that are genetically determined by gene mutations. The clinical signs and symptoms include pulsatile tinnitus, headache, hearing loss, vocal fold paresis, vertigo, lower cranial nerve palsies, and tachycardias. Radiographic studies are pathognomonic in diagnosis. Treatment with fractionated stereotactic radiation therapy can be an effective option for these lesions, especially in reserving facial nerve function.
- Citation: Pontoriero A, Critelli P, Zeppieri M, Angileri FF, Ius T. Treatment for paraganglioma with stereotactic radiotherapy. World J Clin Cases 2024; 12(16): 2729-2737
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2729
Paragangliomas (PGs) are rare non-epithelial neoplasms of neuroendocrine origin, also known as chemodectoma, highly vascular and represent 0.6% of all head-neck tumors[1,2]. They embryologically arise from the autonomic nervous system extra-adrenal, in the thoracic, abdominal, and head-neck regions. 1%-3% of head-neck PGs are associated with cate
According to updated classification, PGs are not defined as “benign” or “malignant” but tumors of undetermined bio-logic with metastatic potential (especially in lymph nodes, liver, and lung) and a tendency to local infiltration of surrounding tissue such as bones or vessels[5]. PGs are usually sporadic, except in a few cases that are genetically de
Depending on their location, size, and hormone activity, the clinical presentation may be extremely variable with pulsatile tinnitus, headache, hearing loss, vocal fold paresis, vertigo, lower cranial nerve palsies, tachycardia and la-bile blood pressure in catecholamine-secreting PGs. When the patient presents with classical symptoms related to cate
If the diagnosis of PGs is suspected, fine needle biopsy is not indicated, but radiographic studies are pathognomonic. Computed tomography (CT) with contrast enhances these highly vascular PGs and can be utilized to define bone erosion and any possible skull base involvement[12-14]. A complementary imaging modality is magnetic resonance imaging (MRI) with gadolinium contrast that better demonstrates the relation of the PGs to the adjacent vessels. The PGs are characterized by a “salt and pepper” pattern on T2-weighted MRI, due to the high-flow vascular voids within the vascular tumor[15].
Digital subtraction angiography and 3D time-of-flight magnetic resonance angiography can detect flow dynamics and vascular architecture with high sensitivity and specificity[16,17]. 18Ga-Dotatoc positron emission tomography (PET)-CT, fluorodeoxy-glucose PET/CT, and I-123-metaiodobenzylguanidine single photon emission CT/CT can also be used for detecting secreting PGs[18,19]. The classification of PGs is based on the extension of the tumor to surrounding anatomic structures (Table 1). Fisch classification, Glasscock-Jackson, and Shamblin classification are widely used for temporal PGs and carotid body tumors respectively[3,20-23].
| Glasscock-Jackson classification | Fisch classification | Shamblin classification | ||||
| TPG | JPG | JPG | Carotid PG | |||
| Type 1 | Small mass limited to the promontory | Involves jugular bulb, middle ear, mastoid process | Type A | Limited to middle ear cleft | Type 1 | Tumors are localized with minimal vascular attachments. These are easily amenable to complete resection with very little morbidity |
| Type 2 | Tumor completely filling the middle ear | Extends under canal, with or without intracranial extension | Type B | Limited to the tympanomastoid area; cortical bone over jugular bulb intact | Type 2 | Tumors partially surround the carotids. These are careful surgical excisions |
| Type 3 | Tumor filling middle ear and mastoid | Extends into petrous apex with or without intracranial extension | Type C (C1-C2-C3) | Involving the infralabyrinthine compartment and petrous apex of the temporal bone | Type 3 | Tumors encase the carotids. Surgical resection is difficult and may require major vessel reconstruction |
| Type 4 | Further extension through the EAC or anteriorly to the carotid artery | Extends beyond petrous apex into clivus or infratemporal fossa, with or without intracranial extension | Type D (D1-D2-D3) | Glomus jugular tumors with intracranial extensions | ||
For asymptomatic or elderly patients watch and wait approach is reasonable. For symptomatic patients, to date, common treatment options are surgical resection (SR), embolization with or without surgical resection, radiation therapy (RT), as definitive, adjuvant, or salvage therapy. SR represents the first-line treatment but, due to the proximity of the tumor to critical neurovascular structures, it is often complicated especially in larger tumors[24-26].
When PGs are considered unresectable (uPG), alternative treatments are represented by subtotal resection in association with RT (for residual, recurrent tumors or giant tumors) or RT alone. Several studies reported a significant local control with acceptable toxicities in patients who underwent subtotal resection followed by RT or treated with RT only[27-30]. RT treatment can be performed with a Cyberknife System (CK), Gamma Knife System (GKS), and linear accelerator (LINAC)-based stereotactic radiosurgery (SRS) with single or multi-fractions schedules [fractionated stereotactic RT (FSRT)][31-33].
SRS is usually performed when PGs measure less than 3 cm, whereas tumors that are larger or have a component of extracranial spread are suitable for conventionally fractionated radiotherapy [external beam RT (EBRT)][12-14]. The aim of our study is to evaluate the local control and effectiveness of exclusive FSRT treatment in uPG.
The study was conducted at the Department of Radiation Oncology of the University of Messina, Italy. Patients were retrospectively identified from the institutional register. Written consent was obtained from patients who were able to communicate or from their next of kin if the patient couldn't provide consent. The techniques described are standard and the data collection was retrospective, thus special Institutional Review Board approval was not required. This study was performed according to the ethical standards of our Institutional Review Board and in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The patients considered included those with PGs radiologically proven, age >18 years, and availability of complete pre- and postoperative clinical and radiological data (contrast- CT scan, MRI, and angiography) for localization of the tumors. The Glasscock-Jackson and Fisch classifications were used. To evaluate the facial nerve damage, the House-Brackmann grading system was used. All patients received exclusive FSRT.
The pretreatment imaging consisted of a thin-section multiplanar reconstruction-gradient echo volumetric study conducted on a Siemens Magnetom 1.5T MRI system (Siemens, Erlangen, Germany), performed with the following parameters: Repetition time 9.7 ms, echo time 4 ms, matrix 200 × 256, flip angle 1, orientation sagittal. A multislice CT was also performed using a multislice scanner, Siemens Sensation 16 (Siemens, Erlangen, Germany).
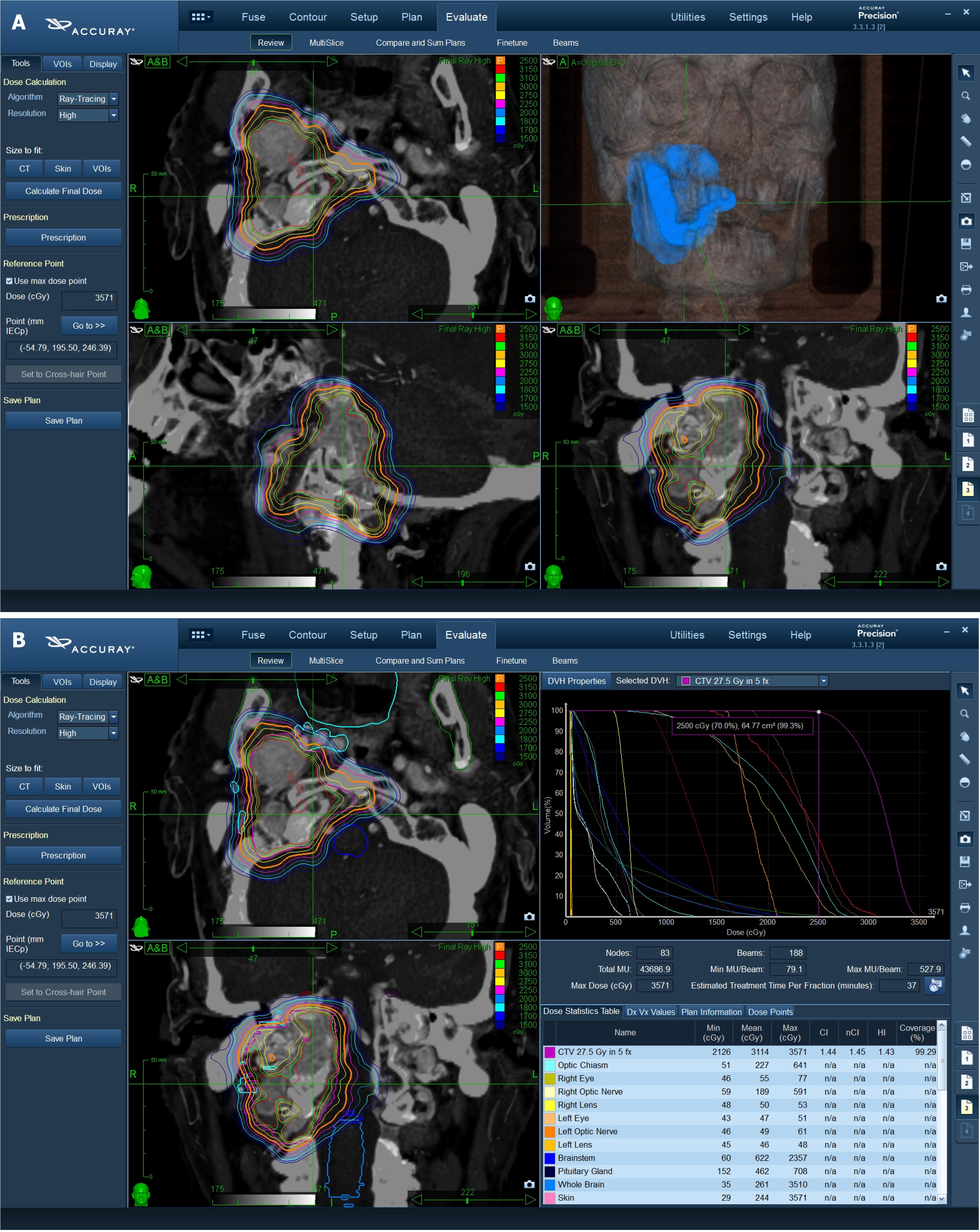
The Multiplan Treatment Planning System (Accuray Incorporated, Sunnyvale, California) was used for inverse treatment planning. The gross target volume (GTV) was contrast CT and MRI-based and was defined according to the radiological findings. Planning target volume (PTV) was created by defining a 1-mm margin to GTV.
The critical organs at risk (OARs) were outlined in the axial plane with a simultaneous display of contours on re-constructed orthogonal images. OARs included: Normal brain, optic chiasm, brain stem, hypophysis, bilateral eyes and lens, optic nerves, pituitary gland, and cochlea, cranial nerves, oral cavity, mandible, parotid gland, esophagus. The characteristics of treatment are reported in Figure 1.
Treatment was delivered using a CyberKnife System (Accuray Incorporated, Sunnyvale, California), an image-guided, frameless, LINAC-based, 6 MV radiosurgery system with Skull Tracking. The ray-tracing algorithm was routinely used for non-isocentric beam delivery.
Clinical and radiologic follow-up with contrast-enhanced MRI T1-T2 weighted, proton density, and fluid-attenuated in-version recovery sequences MRI, were obtained at three months and then every six months for two years followed by yearly evaluations. We included the latest available follow-up in this analysis.
Toxicity and initial efficacy were evaluated. The clinical status of the patients was classified using the Karnofsky Performance Status before treatment and at the last follow-up; new neurologic deficits and any neurological event were recorded separately. The revised NCI Common Terminology Criteria for Adverse Events, version 4.0 was applied to evaluate radiotherapy-related toxicity. RECIST v1.1 criteria were used to evaluate response to treatment.
From May 2009 to January 2023, 6 patients (4 females, 2 males) with a median age of 68 ± 24.4 interquartile range (IQR) (range 20-84) were treated with FSRT. Five of six patients had temporal PGs: In 4 patients, PGs originate from the timpano jugular glomus and one from the temporal bone. Based on Glasscock-Jackson classification, temporal PGs were type 3 (1/6), three jugular PGs were type 3 (3/6), and one type 2. According to Fish classification, 4 patients showed grade D and 1 grade C. One patient had cervical spine PG. At the time of presentation, symptoms included cervical mass with the displacement of critical structure and severe deficit of cranial nerves. Patients referred asymmetry of mouth (VII, facial nerve, House-Brackmann grade 4), hearing loss (VII acoustic nerve), dysphagia (IX, glossopharyngeal nerve), pulsatile tinnitus and swallowing difficulty (X, vagal nerve), and limited forehead movement mouth (XI, hypoglossal nerve). Only 1 patient showed SDHB mutation. The characteristics of patients are summarized in Table 2.
| Patients | Age (yr) | Site of origin | Fisch classification | Glasscock-Jackson classification | Surgery | Volume (mL) | Dose (Gy) | Isodose (%) | Fraction | Pathogenesis |
| 1 | 20 | TPGs | D | 3 | No | 12.4 | 21 | 75 | 3 | Sporadic |
| 2 | 60 | Cervical spine | \ | \ | No | 20.2 | 20 | 77 | 5 | SDHB mut |
| 3 | 68 | JPG | D | 3 | No | 12.7 | 20 | 73 | 3 | Sporadic |
| 4 | 44 | JPG | C | 2 | No | 14.5 | 21 | 76 | 3 | Sporadic |
| 5 | 84 | JPG | D | 3 | No | 64.24 | 25 | 70 | 5 | Sporadic |
| 6 | 74 | JPG | D | 3 | No | 10.99 | 30 | 82.5 | 5 | Sporadic |
The median delivered dose was 21 Gy ± 3.75 IQR (range 20-30 Gy) at a median isodose line 75.5% ± 3.25% IQR (range 70%-76%) in 4 fractions (range 3-5 fractions). The median volume was 13.6 mL ± 6.3 IQR (ranging 12.4-65.24 mL). The median cumulative biological effective dose (a/b 4.5) and equivalent dose in 2-Gy fractions were 70 Gy and 37.10 Gy ± 2.76 IQR respectively.
The median follow-up was 72 months ± 66.75 IQR. In 1 of the 6 patients, the follow-up was insufficient. The median overall survival (OS) was 72 months ± 111.75 IQR; the median progression-free survival (PFS) was 72 months ± 66.75 IQR 5 of 6 patients showed a 5-year OS and 5-year PFS of 100%. We observed negligible toxicities during and after RT. Five patients showed mild cranial nerve: 2 to the facial nerve (House-Brackmann grade 2), 1 to the vagal and hypoglossal cra
| Patients | OS (months) | PFS (months) | Response treatment |
| 1 | 180 | 144 | PD |
| 2 | 156 | 96 | PR |
| 3 | 72 | 72 | PR |
| 4 | 72 | 72 | PR |
| 5 | 5 | 5 | NA |
| 6 | 6 | 6 | PR |
The management of PGs is controversial. The first option is represented by surgical resection, but it is associated with a high complication rate as nerve damage, stroke, and bleeding, exacerbated by possible previous embolization[34-38]. RT was widely studied as an alternative to surgery and has shown favorable results[39]. Both FSRT and SRS have been investigated and historically considered for recurrent or residual disease[40,41] including PGs, after subtotal resection with high rates of local control[24-26].
SRS shows equal efficacy with lower toxicity rates than FSRT and is reasonable for PGs 3 cm or less in size[42-44]. Fatima et al[45], in their metanalysis, showed a pooled local control of 94.2% with no statistically significant difference in local control between different techniques of SRS (CK, GKS, LINAC). The analysis comprises patients who underwent both SR and embolization and subsequent RT, in a median volume of 8.4 mL and with a median dose of 15 Gy in 1-5 fractions[45].
Several studies showed an optimal local control with negligible toxicities delivering a single fraction in small lesions. Principal methods of SRS include GKS and LINAC radiosurgery[46]. Patel et al[47] evaluated the quality of life in 26 patients treated with RT performed with GKS, alone or in adjuvant modality. The median radiation dose was 16 Gy in PGs with a median tumor volume of 7090 mm3. The study showed better outcomes in patients who underwent primary SRS than adjuvant SRS[47].
Ehret et al[48] evaluated the efficacy of the SRS performed with CK in patients who had prior treatment, SR, or embolization. Ehret et al[48] using a median dose of 16.5 Gy, with a median prescription isodose line of 70% achieved a 5-year actuarial local control (LC) of 100% in PGs of 4.3 mL (median volume). Marchetti et al[31] delivered a median dose of 12 Gy (median isodose line was 78.6%) in PGs with a median volume of 3.6 mL with no radiological progression at the site of the treatment.
For greater lesions, FSRT or EBRT are generally considered. Marchetti et al[31], in patients with greater lesions ( median volume 16 mL), delivered a median dose of 25 Gy in 5 fractions at a median isodose line of 80%.
Tosun et al[49] delivered FSRT in 12 patients. The median dose of 24 Gy with a median isodose line of 75%. The median tumor volume was 35.5 mL (range 5.3-113.8 mL). Of them, 7 underwent SR and according to Fisch classification 6 were D2 and only 1 C1. Only 1 patient with D2 PGs had no SR and was treated with RT alone with a dose of 30 Gy in 10 frac
Another study reported a fractionated stereotactic treatment with CK in PGs with volume ranging from 0.84 to 69.3 cm3 (median volume of 4.64 cm3). Out of 36 patients, 12 with no prior treatment, received FSRT (volume ranged from 9.73 to 69.3 cm3). A patient with a volume of 69.3 cm3 was treated with 25 Gy in 5 fractions; another PG of 42 cm3 was divided into two portions (intracranial and cervical) that received 24 Gy in 3 fractions respectively[50].
In a retrospective study, 81 PGs were treated by conventional EBRT in 25 fractions with a median dose of 45 Gy (range 41.4-68 Gy), of whom 60 were treated with exclusive RT and 21 had prior surgery. The median GTV and PTV were 30 cm3. The 5-year and 10-year actuarial LC rates were 100% and 98.7% respectively. In this study, 5 patients showed grade 3 late toxicity and 2 patients developed secondary meningiomas[51].
There is no consensus to define PGs as “giant”. Main et al[30], in their case report, considered giant PG with a volume of 93.553 cm3 that was treated with only SR, whereas López-Arcas et al[52] reported a case of combination treatment in giant PG of a maximum diameter of 4 cm, removed surgically after embolization and treated with FSRT performed with a coverage dose of 14 Gy at an isodose of 83%. Other studies evaluated the efficacy of RT in unresectable or bulky diseases[53,54].
To the best of our knowledge, lack of data on the management of unresectable PGs due to high dimension or bone infiltration. This is the latest analysis of the efficacy and feasibility of exclusive FSRT in unresectable paraganglioma using a CK.
Here we report a case series of PG judged unresectable and treated with exclusive FSRT. The PGs volumes ranged from 12.4 to 65.24 mL. The treatment was delivered with a median dose of 21 Gy in 4 fractions using the CK. No acute and late toxicities were observed. All patients had stable or improved clinical status and LC rates were comparable with literature data. Characteristics of the analyzed studies are summarized in Table 4.
| Ref. | PGs Resectable | Embolization | Median dose (Gy) | Isodose (%) | Median fractions | Technique | Median volume (mL)/ size (cm) | Outcome |
| Fatima et al[45] | Yes (NA) | Yes (NA) | 15 | NA | 1-5 | CK-GKS-LINAC | 8.4 mL | 94.2% LC |
| Patel et al[47] | Yes (10/26) | Yes (1/26) | 16 | NA | 1 | GKS | 7090 mL | NA |
| Ehret et al[48] | Yes (20/53) | Yes (8/53) | 16.5 | 70 | 1 | CK | 4.3 mL | 100% 5-year LC |
| Marchetti et al[31] | Yes (NA) | No | 12.6 | 78.6 | 1 | CK | 3.6 mL | 33% LC (35 months) |
| 25 | 80 | 5 | 16.1 mL | |||||
| Tosun et al[49] | Yes (NA) | No | 24 | 75 | 3 | CK | 35.5 mL | 100% LC |
| Lieberson et al[50] | 24/12 | 20 | 80 | 1-5 | CK | 4.64 mL | 100% LC | |
| Dupin et al[51] | Yes (21/81) No (60/21) | Yes (1/81) | 25 | \ | 25 | 60Co machine/ LINAC | 30 mL | 100% 5-year LC; 98.7% 10-year LC |
| Main et al[30] | Yes (1/1) | Yes (1/1) | \ | \ | \ | \ | 93.553 mL | NA |
| López-Arcas et al[52] | Yes (1/1) | Yes (1/1) | 14 | 2 | CK | 4 cm | NA | |
| This study | No | No | 21 | 75.5 | 4 | CK | 13 mL | 100% |
The present study has several limitations; the small series of patients does not allow to perform powered statistical analyses and the minimum follow-up period is too short to evaluate long-term recurrent. Therefore, our results must be confirmed by studies with larger patient sizes and longer follow-ups.
In conclusion, despite the limitation of the study, our results suggest that exclusive FSRT could be an effective and safe alternative when SR is excluded.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade B
P-Reviewer: Ren G, China S-Editor: Che XX L-Editor: A P-Editor: Zhao S
| 1. | Taïeb D, Kaliski A, Boedeker CC, Martucci V, Fojo T, Adler JR Jr, Pacak K. Current approaches and recent developments in the management of head and neck paragangliomas. Endocr Rev. 2014;35:795-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Pellitteri PK, Rinaldo A, Myssiorek D, Gary Jackson C, Bradley PJ, Devaney KO, Shaha AR, Netterville JL, Manni JJ, Ferlito A. Paragangliomas of the head and neck. Oral Oncol. 2004;40:563-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 218] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Offergeld C, Brase C, Yaremchuk S, Mader I, Rischke HC, Gläsker S, Schmid KW, Wiech T, Preuss SF, Suárez C, Kopeć T, Patocs A, Wohllk N, Malekpour M, Boedeker CC, Neumann HP. Head and neck paragangliomas: clinical and molecular genetic classification. Clinics (Sao Paulo). 2012;67 Suppl 1:19-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Boedeker CC, Ridder GJ, Neumann HP, Maier W, Schipper J. [Diagnosis and management of cervical paragangliomas: the Freiburg experience]. Laryngorhinootologie. 2004;83:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Williams MD, Tischler AS. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Paragangliomas. Head Neck Pathol. 2017;11:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Burnichon N, Abermil N, Buffet A, Favier J, Gimenez-Roqueplo AP. The genetics of paragangliomas. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129:315-318. [PubMed] |
| 7. | Timmers HJ, Gimenez-Roqueplo AP, Mannelli M, Pacak K. Clinical aspects of SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2009;16:391-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Grufferman S, Gillman MW, Pasternak LR, Peterson CL, Young WG Jr. Familial carotid body tumors: case report and epidemiologic review. Cancer. 1980;46:2116-2122. [PubMed] [DOI] [Full Text] |
| 9. | Netterville JL, Jackson CG, Miller FR, Wanamaker JR, Glasscock ME. Vagal paraganglioma: a review of 46 patients treated during a 20-year period. Arch Otolaryngol Head Neck Surg. 1998;124:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 197] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Netterville JL, Reilly KM, Robertson D, Reiber ME, Armstrong WB, Childs P. Carotid body tumors: a review of 30 patients with 46 tumors. Laryngoscope. 1995;105:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 122] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Kiernan CM, Solórzano CC. Pheochromocytoma and Paraganglioma: Diagnosis, Genetics, and Treatment. Surg Oncol Clin N Am. 2016;25:119-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Hu K, Persky MS. Multidisciplinary management of paragangliomas of the head and neck, Part 1. Oncology (Williston Park). 2003;17:983-993. [PubMed] |
| 13. | Hu K, Persky MS. The multidisciplinary management of paragangliomas of the head and neck, Part 2. Oncology (Williston Park). 2003;17:1143-1153; discussion 1154, 1158, 1161. [PubMed] |
| 14. | Persky MS, Hu KS. Paragangliomas of the head and neck. In: Harrison LB, Hong WK, Sessions RB, editors. Head and Neck Cancer: A Multidisciplinary Approach. 3rd ed. Philadephia: Lippincott Williams & Wilkins; 2009: 655-687. |
| 15. | Olsen WL, Dillon WP, Kelly WM, Norman D, Brant-Zawadzki M, Newton TH. MR imaging of paragangliomas. AJR Am J Roentgenol. 1987;148:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 155] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Neves F, Huwart L, Jourdan G, Reizine D, Herman P, Vicaut E, Guichard JP. Head and neck paragangliomas: value of contrast-enhanced 3D MR angiography. AJNR Am J Neuroradiol. 2008;29:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | van den Berg R, Schepers A, de Bruïne FT, Liauw L, Mertens BJ, van der Mey AG, van Buchem MA. The value of MR angiography techniques in the detection of head and neck paragangliomas. Eur J Radiol. 2004;52:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Kong G, Schenberg T, Yates CJ, Trainer A, Sachithanandan N, Iravani A, Ravi Kumar A, Hofman MS, Akhurst T, Michael M, Hicks RJ. The Role of 68Ga-DOTA-Octreotate PET/CT in Follow-Up of SDH-Associated Pheochromocytoma and Paraganglioma. J Clin Endocrinol Metab. 2019;104:5091-5099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Chang CA, Pattison DA, Tothill RW, Kong G, Akhurst TJ, Hicks RJ, Hofman MS. (68)Ga-DOTATATE and (18)F-FDG PET/CT in Paraganglioma and Pheochromocytoma: utility, patterns and heterogeneity. Cancer Imaging. 2016;16:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Jackson CG, Glasscock ME 3rd, Harris PF. Glomus Tumors. Diagnosis, classification, and management of large lesions. Arch Otolaryngol. 1982;108:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 132] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Oldring D, Fisch U. Glomus tumors of the temporal region: surgical therapy. Am J Otol. 1979;1:7-18. [PubMed] |
| 22. | Fisch U. Infratemporal fossa approach for glomus tumors of the temporal bone. Ann Otol Rhinol Laryngol. 1982;91:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 152] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Shamblin WR, ReMine WH, Sheps SG, Harrison EG Jr. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 463] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Lim JY, Kim J, Kim SH, Lee S, Lim YC, Kim JW, Choi EC. Surgical treatment of carotid body paragangliomas: outcomes and complications according to the shamblin classification. Clin Exp Otorhinolaryngol. 2010;3:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Sanna M, Jain Y, De Donato G, Rohit, Lauda L, Taibah A. Management of jugular paragangliomas: the Gruppo Otologico experience. Otol Neurotol. 2004;25:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Ivan ME, Sughrue ME, Clark AJ, Kane AJ, Aranda D, Barani IJ, Parsa AT. A meta-analysis of tumor control rates and treatment-related morbidity for patients with glomus jugulare tumors. J Neurosurg. 2011;114:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Anderson JL, Khattab MH, Anderson C, Sherry AD, Luo G, Manzoor N, Attia A, Netterville J, Cmelak AJ. Long-term Outcomes for the Treatment of Paragangliomas in the Upfront, Adjuvant, and Salvage Settings With Stereotactic Radiosurgery and Intensity-modulated Radiotherapy. Otol Neurotol. 2020;41:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Papaspyrou K, Mewes T, Rossmann H, Fottner C, Schneider-Raetzke B, Bartsch O, Schreckenberger M, Lackner KJ, Amedee RG, Mann WJ. Head and neck paragangliomas: Report of 175 patients (1989-2010). Head Neck. 2012;34:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Künzel J, Iro H, Hornung J, Koch M, Brase C, Klautke G, Zenk J. Function-preserving therapy for jugulotympanic paragangliomas: a retrospective analysis from 2000 to 2010. Laryngoscope. 2012;122:1545-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Main AM, Benndorf G, Feldt-Rasmussen U, Fugleholm K, Kistorp T, Loya AC, Poulsgaard L, Rasmussen ÅK, Rossing M, Sølling C, Klose MC. Case Report: Giant Paraganglioma of the Skull Base With Two Somatic Mutations in SDHB and PTEN Genes. Front Endocrinol (Lausanne). 2022;13:857504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Marchetti M, Pinzi V, Tramacere I, Bianchi LC, Ghielmetti F, Fariselli L. Radiosurgery for Paragangliomas of the Head and Neck: Another Step for the Validation of a Treatment Paradigm. World Neurosurg. 2017;98:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Huo M, Sahgal A, Pryor D, Redmond K, Lo S, Foote M. Stereotactic spine radiosurgery: Review of safety and efficacy with respect to dose and fractionation. Surg Neurol Int. 2017;8:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Patel AK, Rodríguez-López JL, Hirsch BE, Burton SA, Flickinger JC, Clump DA. Long term outcomes with linear accelerator stereotactic radiosurgery for treatment of jugulotympanic paragangliomas. Head Neck. 2021;43:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Papaspyrou K, Mann WJ, Amedee RG. Management of head and neck paragangliomas: review of 120 patients. Head Neck. 2009;31:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Langerman A, Athavale SM, Rangarajan SV, Sinard RJ, Netterville JL. Natural history of cervical paragangliomas: outcomes of observation of 43 patients. Arch Otolaryngol Head Neck Surg. 2012;138:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Persky MS, Setton A, Niimi Y, Hartman J, Frank D, Berenstein A. Combined endovascular and surgical treatment of head and neck paragangliomas--a team approach. Head Neck. 2002;24:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Suárez C, Rodrigo JP, Bödeker CC, Llorente JL, Silver CE, Jansen JC, Takes RP, Strojan P, Pellitteri PK, Rinaldo A, Mendenhall WM, Ferlito A. Jugular and vagal paragangliomas: Systematic study of management with surgery and radiotherapy. Head Neck. 2013;35:1195-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Green JD Jr, Brackmann DE, Nguyen CD, Arriaga MA, Telischi FF, De la Cruz A. Surgical management of previously untreated glomus jugulare tumors. Laryngoscope. 1994;104:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Conti A, Pontoriero A, Iatì G, Cardali SM, Brogna A, Friso F, Rosetti V, Zoli M, Parisi S, Cacciola A, Lillo S, Pergolizzi S, Mazzatenta D. Image-Guided Multisession Radiosurgery of Skull Base Meningiomas. Cancers (Basel). 2020;12:3569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Pontoriero A, Critelli P, Conti A, Cardali S, Angileri FF, Germanò A, Lillo S, Carretta A, Brogna A, Santacaterina A, Parisi S, Pergolizzi S. The "Combo" radiotherapy treatment for high-risk grade 2 meningiomas: dose escalation and initial safety and efficacy analysis. J Neurooncol. 2023;161:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Parisi S, Ferini G, Lillo S, Brogna A, Chillari F, Ferrantelli G, Settineri N, Santacaterina A, Platania A, Leotta S, Casablanca G, Russo A, Pontoriero A, Adamo V, Minutoli F, Bottari A, Cacciola A, Pergolizzi S. Stereotactic boost on residual disease after external-beam irradiation in clinical stage III non-small cell lung cancer: mature results of stereotactic body radiation therapy post radiation therapy (SBRTpostRT) study. Radiol Med. 2023;128:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 42. | Hu K, Persky MS. Treatment of Head and Neck Paragangliomas. Cancer Control. 2016;23:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Pollock BE. Stereotactic radiosurgery in patients with glomus jugulare tumors. Neurosurg Focus. 2004;17:E10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Sahyouni R, Mahboubi H, Moshtaghi O, Goshtasbi K, Sahyouni S, Lin HW, Djalilian HR. Radiosurgery of Glomus Tumors of Temporal Bone: a Meta-analysis. Otol Neurotol. 2018;39:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Fatima N, Pollom E, Soltys S, Chang SD, Meola A. Stereotactic radiosurgery for head and neck paragangliomas: a systematic review and meta-analysis. Neurosurg Rev. 2021;44:741-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Dharnipragada R, Butterfield JT, Dhawan S, Adams ME, Venteicher AS. Modern Management of Complex Tympanojugular Paragangliomas: Systematic Review and Meta-Analysis. World Neurosurg. 2023;170:149-156.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Patel NS, Link MJ, Tombers NM, Pollock BE, Carlson ML. Quality of Life in Jugular Paraganglioma Following Radiosurgery. Otol Neurotol. 2019;40:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Ehret F, Kufeld M, Fürweger C, Haidenberger A, Schichor C, Tonn JC, Muacevic A, Hempel JM. Single-session image-guided robotic radiosurgery and quality of life for glomus jugulare tumors. Head Neck. 2020;42:2421-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Tosun İ, Atalar B, Şahin B, Güngör G, Aydin G, Yapici B, Özyar E. Robotic radiosurgery of head and neck paragangliomas: a single institution experience. Asia Pac J Clin Oncol. 2018;14:e3-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Lieberson RE, Adler JR, Soltys SG, Choi C, Gibbs IC, Chang SD. Stereotactic radiosurgery as the primary treatment for new and recurrent paragangliomas: is open surgical resection still the treatment of choice? World Neurosurg. 2012;77:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Dupin C, Lang P, Dessard-Diana B, Simon JM, Cuenca X, Mazeron JJ, Feuvret L. Treatment of head and neck paragangliomas with external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | López-Arcas JM, Colmenero CM, Martínez R, Martín-Hernán F, Ruiz-Sánchez B, Aragoneses JM. Giant carotid chemodectoma treated with a combination of surgery and CyberKnife radiotherapy: a case report and review of the literature. J Med Case Rep. 2022;16:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 53. | Parisi S, Ferini G, Cacciola A, Lillo S, Tamburella C, Santacaterina A, Bottari A, Brogna A, Ferrantelli G, Pontoriero A, Minutoli F, Pergolizzi S. A non-surgical COMBO-therapy approach for locally advanced unresectable pancreatic adenocarcinoma: preliminary results of a prospective study. Radiol Med. 2022;127:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 54. | Ferini G, Parisi S, Lillo S, Viola A, Minutoli F, Critelli P, Valenti V, Illari SI, Brogna A, Umana GE, Ferrantelli G, Lo Giudice G, Carrubba C, Zagardo V, Santacaterina A, Leotta S, Cacciola A, Pontoriero A, Pergolizzi S. Impressive Results after "Metabolism-Guided" Lattice Irradiation in Patients Submitted to Palliative Radiation Therapy: Preliminary Results of LATTICE_01 Multicenter Study. Cancers (Basel). 2022;14:3909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |