Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2664
Revised: March 14, 2024
Accepted: April 9, 2024
Published online: May 26, 2024
Processing time: 89 Days and 3.8 Hours
Traumatic internal carotid artery (ICA) occlusion is a rare complication of skull base fractures, characterized by high mortality and disability rates, and poor prognosis. Therefore, timely discovery and correct management are crucial for saving the lives of such patients and improving their prognosis. This article retrospectively analyzed the imaging and clinical data of three patients, to explore the imaging characteristics and treatment strategies for carotid artery occlusion, combined with severe skull base fractures.
This case included three patients, all male, aged 21, 63, and 16 years. They underwent plain film skull computed tomography (CT) examination at the onset of their illnesses, which revealed fractures at the bases of their skulls. Ultimately, these cases were definitively diagnosed through CT angiography (CTA) examinations. The first patient did not receive surgical treatment, only anticoagulation therapy, and recovered smoothly with no residual limb dysfunction (Case 1). The other two patients both developed intracranial hypertension and underwent decompressive craniectomy. One of these patients had high intracranial pressure and significant brain swelling postoperatively, leading the family to choose to take him home (Case 2). The other patient also underwent decompressive craniectomy and recovered well postoperatively with only mild limb motor dysfunction (Case 3). We retrieved literature from PubMed on skull base fractures causing ICA occlusion to determine the imaging characteristics and treatment strategies for this type of disease.
For patients with cranial trauma combined with skull base fractures, it is essential to complete a CTA examination as soon as possible, to screen for blunt cerebro
Core Tip: This article emphasizes the importance of rapid computed tomography angiography examination for patients with skull base fractures, to detect potential carotid artery occlusions. This condition significantly complicates the prognosis due to its rarity, high mortality, and severe disability rates. Through the analysis of three distinct cases, this study showcases varied imaging characteristics and underscores the critical role of early diagnosis and tailored treatment strategies, including anticoagulation and decompressive surgery, in managing this severe complication effectively.
- Citation: Shangguan PX, Zhou KC. Imaging characteristics and treatment strategies for carotid artery occlusion caused by skull base fracture: Three case reports. World J Clin Cases 2024; 12(15): 2664-2671
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2664.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2664
The carotid canal is an important structure through which the internal carotid artery (ICA) enters the skull base. Located at the base of the skull within the temporal bone, it begins in the petrous part of the temporal bone and serves as the primary passage for the ICA to enter the cranial cavity. Once inside the skull, the artery extends upwards along the sphenoid bone's sinus part. While damage to the carotid canal and sphenoid bone is relatively common in skull base fractures, the progression to ICA occlusion is quite rare. ICA occlusion can lead to severe cerebrovascular ischemic injuries with high mortality and disability rates, and poor prognosis[1]. Therefore, early diagnosis and treatment are crucial for improving outcomes. Typically, patients with cranial injuries only undergo head computed tomography (CT) scans, which often result in a delayed assessment of the damage to the ICA. Therefore, conducting a comprehensive CT angiography (CTA) examination early on is particularly important. This paper reviews the cases of three patients hospitalized between January 2013 and December 2023 and analyzes their imaging characteristics and treatment strategies.
This study retrospectively collected data from three cases of skull base fractures combined with traumatic ICA occlusion, that were treated in Lishui Municipal Central Hospital from January 2013 to December 2023. The clinical research and the publication of this paper and its related images were approved by the Clinical Research Ethics Committee of Lishui Municipal Central Hospital. The retrospective analysis included clinical information from hospital records, such as age, sex, causative factors, symptoms, disease progression, imaging examinations, related injuries, and diagnoses. Detailed information about these three cases is presented in Table 1.
| Characteristics | Case 1 | Case 2 | Case 3 |
| Gender | Male | Male | Male |
| Age (yr) | 21 | 63 | 16 |
| Cause of injury | Fall from height | Struck by heavy object | Traffic accident |
| Admission GCS score | 15 | 5 | 7 |
| Time to detection of arterial occlusion | 7 d | 6 h | 6 d |
| Skull base fracture location | Sphenoid bone | Petrous part of the temporal bone | Sphenoid bone |
| Treatment | Anticoagulation | Surgery | Surgery |
| Hospital stay (d) | 46 | 10 | 76 |
| MRS score | 0 | 6 | 2 |
Case 1: A 21-year-old male was admitted to the hospital due to decreased movement in the left limbs 1 d, occurring 7 d post liver rupture surgery.
Case 2: The patient was brought to our hospital with a change in the level of consciousness 6 h after being struck on the head with a heavy object.
Case 3: This patient was admitted to our hospital 6 h after a vehicular accident which caused a change in consciousness.
Case 1: Following a high fall, the patient had experienced transient consciousness disturbances and was taken to a local hospital, where he was intubated and placed on mechanical ventilation. After thorough examination, he was diagnosed with liver rupture, cranial trauma, and a skull fracture. Therefore, he underwent surgery for liver laceration repair. On day 7 post-surgery, decreased movement in his left limbs was observed, and a head CT scan indicated a brain infarction, leading to his transfer to our intensive care unit.
Case 2: Approximately 6 h prior to admission, the patient suffered a head injury from a heavy object, leading to a transient disturbance in consciousness and nasal bleeding. En route to our hospital, the patient experienced another episode of loss of consciousness. Upon arrival, emergency tracheal intubation and mechanical ventilation were administered.
Case 3: The patient experienced a change in consciousness and bleeding from the nose and mouth 6 h prior, following a motorcycle accident. Upon arrival at the hospital, emergency tracheal intubation and mechanical ventilation were administered.
Case 1: The patient was previously healthy until he had sustained injuries from a high fall 7 d prior. This resulted in liver rupture, cranial trauma, and a skull fracture.
Case 2: The patient was previously healthy.
Case 3: The patient was previously healthy.
The patients and their family denied any history of cerebrovascular diseases.
Case 1: Upon admission, the patient had a Glasgow Coma Scale (GCS) score of 15, was intubated and receiving mechanical ventilation support. He had a body temperature of 39.3 °C, a respiratory rate of 29 breaths per minute (on mechanical ventilation), a heart rate of 145 beats per minute, and a blood pressure of 107/68 mmHg. The left pupil was 3 mm in diameter with sensitivity to light. The right pupil was 5 mm in diameter with no light reflex observed. Cardiopulmonary auscultation was generally normal, the abdomen was soft, and there was no shifting dullness detected.
Case 2: Upon admission, the patient's GCS score was 5, body temperature was 37.4 °C, respiratory rate was 10 breaths per minute, heart rate was 91 beats per minute, and blood pressure was 115/70 mmHg. The right pupil was 4.0 mm with no light reflex; the left pupil was approximately 2.5 mm with sluggish light reflex. Cardiopulmonary auscultation was largely normal, and the abdomen was soft.
Case 3: Upon admission, the patient's GCS score was 7, body temperature was 37.9 °C, respiratory rate was 15 breaths per minute, heart rate was 91 beats per minute, and blood pressure was 134/73 mmHg. The right pupil was 2.0 mm with no light reflex; the left pupil was approximately 3.0 mm, also with no light reflex. Cardiopulmonary auscultation was generally normal, and the abdomen was soft.
Case 1: Hemoglobin was 86 g/L, white blood cell count was 20.7 × 109/L, and platelet count was 268 × 109/L. Coagulation profile showed a prothrombin time of 13.4 s, and the international normalized ratio (INR) was 1.24.
Case 2: The patient’s hemoglobin was 146 g/L; white blood cell count 15.1 × 109/L; platelet count 167 × 109/L; prothrombin time 13.1 s; INR 1.21; and D-dimer, 34.70 mg/L.
Case 3: Blood tests showed a hemoglobin level of 137 g/L, white blood cell count was 5.5 × 109/L, and platelet count was 213 × 109/L. Coagulation tests indicated a prothrombin time of 20.6 s, INR of 1.03, and a D-dimer of 9.57 mg/L.
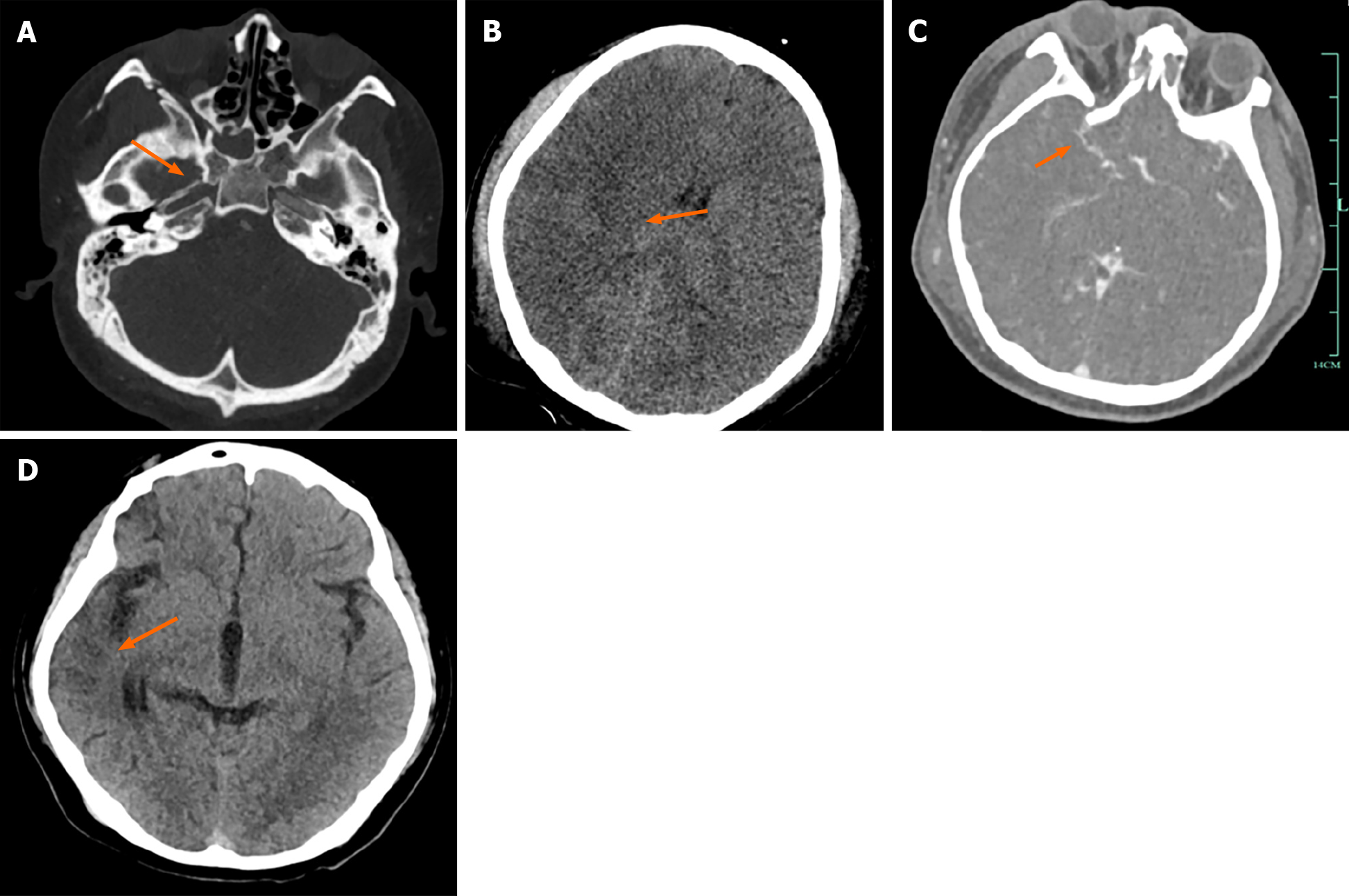
Case 1: Head CTA showed: (1) Brain contusion with brain swelling (predominantly on the right side), and occlusion of the right ICA; and (2) Multiple skull base fractures with sinus fluid accumulation, bilateral frontal soft tissue contusion (Figure 1A-C). After 46 d of treatment, a follow-up cranial CT scan revealed: Partial cerebral gyral atrophy in the right frontal and temporal lobes (Figure 1D).
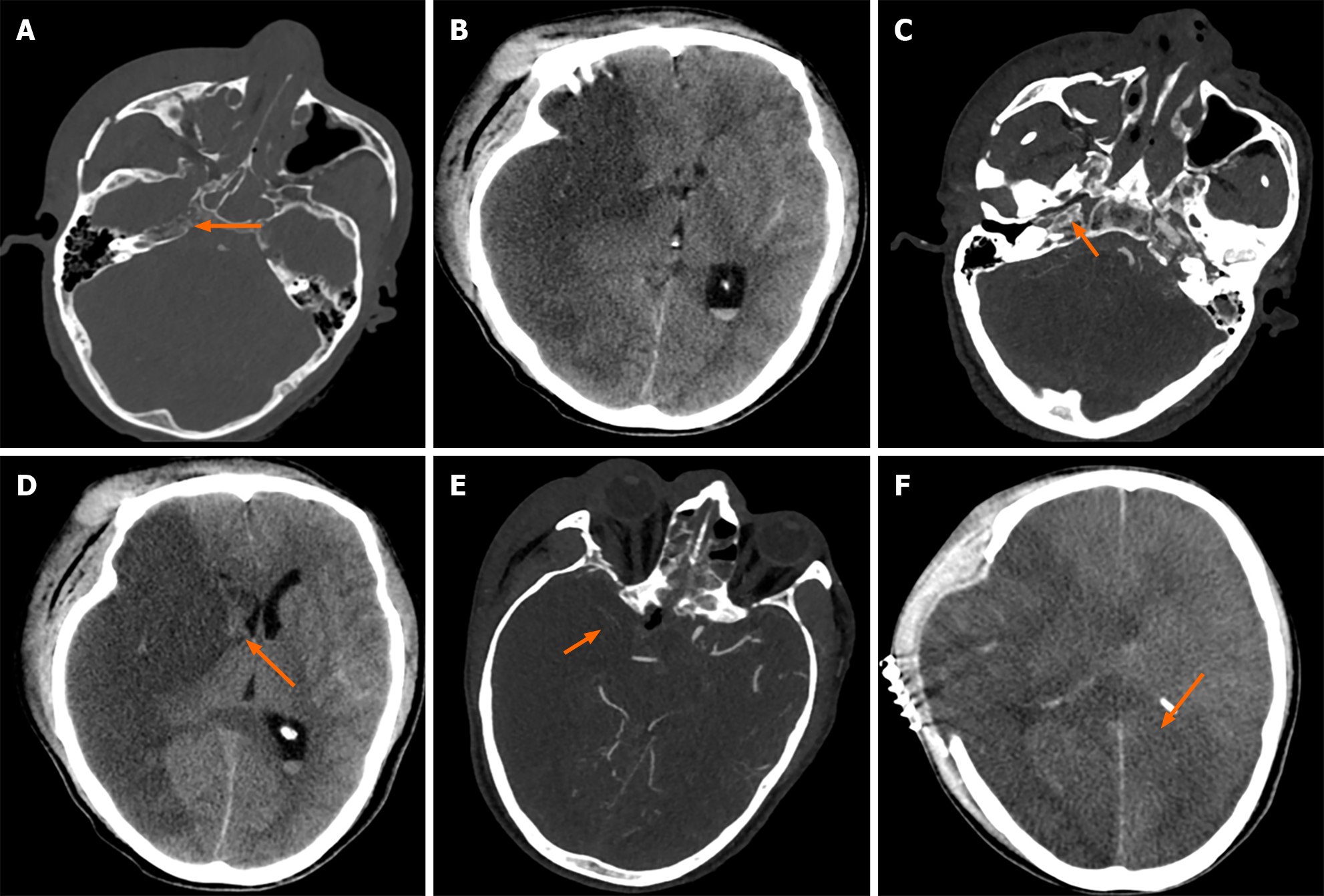
Case 2: A head CT scan showed low-density lesions in the right cerebral hemisphere, fractures of the right maxillary sinus wall, zygomatic arch, walls of the orbit, right ethmoid sinus, walls of both sphenoid sinuses, and nasal septum, with sinus fluid accumulation (Figure 2A and B). An emergency head and neck arterial CTA showed occlusion of the right ICA (Figure 2C). On the second day of admission, a cranial CTA revealed a large area of cerebral infarction in the right hemisphere and brain herniation (Figure 2D), with poor compensatory collateral circulation in the right intracranial vessels (Figure 2E). On the 10th day of admission, a cranial CT scan indicated: Post-craniotomy changes due to right hemisphere infarction, increased cerebral tissue swelling compared to before, and an expanded infarction area (Figure 2F).
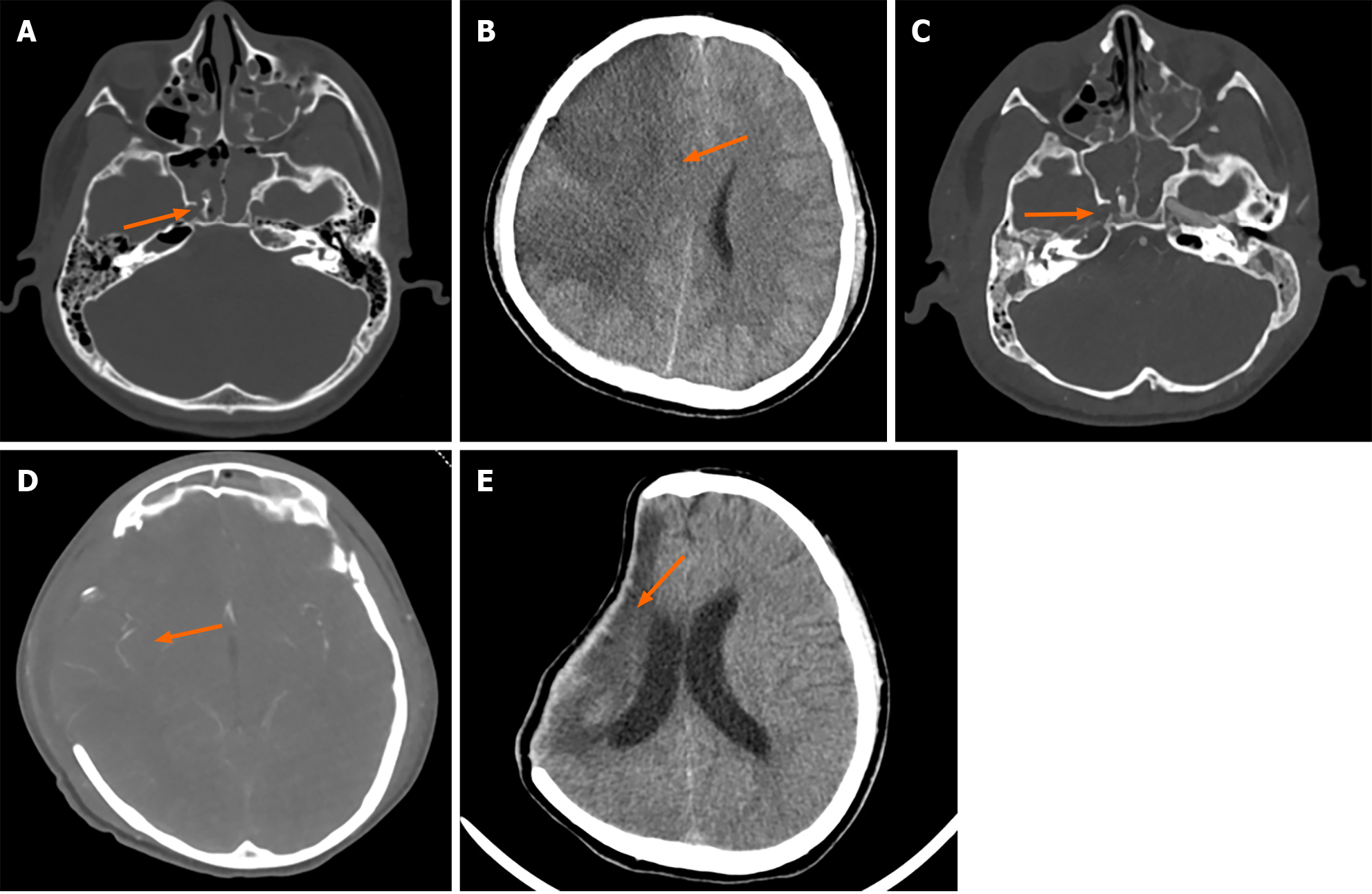
Case 3: Head and skull base CT scan revealed: (1) A right side frontal-temporal-parietal subdural hematoma, intraventricular hemorrhage, and pneumocephalus, widespread contusions in the facial and scalp soft tissues; and (2) Bilateral fractures of the frontal and temporal bones, top of the orbit, lateral wall of the sphenoid sinus, base of the middle cranial fossa, left side of the hard palate, with sinus and right mastoid effusion/blood accumulation (Figure 3A). On day 7 of hospitalization, the patient showed dilatation of the right pupil, and a CT scan indicated a large area of cerebral infarction and brain herniation on the right side (Figure 3B). The next day, a head CTA post-decompressive craniectomy showed right cerebral infarction and occlusion of the right ICA. Left side frontal-temporal subdural hematoma, intraventricular hemorrhage, and pneumocephalus; bilateral fractures of the frontal and temporal bones, top of the orbit, lateral wall of the sphenoid sinus, base of the middle cranial fossa, left side of the hard palate, with sinus and right mastoid effusion/blood accumulation were also confirmed (Figure 3C and D). After 76 d of treatment, a follow-up cranial CT scan indicated: Post-traumatic surgical changes, with a reduced lesion area in the right frontal, parietal, and temporal lobes compared to before (Figure 3E).
The final diagnosis was skull base fracture, right cerebral infarction, occlusion of the right ICA, and liver laceration.
The final diagnosis was skull base fracture, cerebral infarction, occlusion of the right ICA, and nasal bone fracture.
The final diagnosis was multiple skull base fractures, traumatic subarachnoid hemorrhage, occlusion of the right ICA, right cerebral infarction, subdural hematoma, and pulmonary contusion.
After multidisciplinary discussion, it was concluded that the patient's CTA showed abundant collateral circulation (Figure 1C) and no raised intracranial pressure, indicating no immediate need for emergency surgery. Considering the patient's recent abdominal surgery, multiple injuries, and fractures, along with a high risk of bleeding, it was decided to delay anticoagulation treatment. On the 7th day of hospital treatment, after fully assessing the bleeding risk, the patient received a low dose of low molecular weight heparin for anticoagulation (4000 U subcutaneous injection daily). During this period, coagulation factors were monitored, and no further bleeding was detected. On day 28 of hospitalization, the patient was switched to antiplatelet medication (aspirin 100 mg orally daily).
After multidisciplinary discussion, considering the high risk and poor outcome of surgery due to the occlusion of the ICA caused by compression from fractured bone fragments, the patient's family opted to decline surgery. The following day, the patient's right pupil dilated significantly, and an emergency CT scan showed a large area of cerebral infarction in the right brain and brain herniation (Figure 2D). An emergency decompressive craniectomy was performed, followed by symptomatic supportive treatment. Due to insufficient compensatory circulation in the brain (Figure 2E), the area of cerebral infarction further increased (Figure 2F).
The patient received routine mechanical ventilation, hypothermia to reduce brain swelling, and other symptomatic treatments after admission. On day 7, CT indicated cerebral infarction and brain herniation, prompting an emergency decompressive craniectomy. The next day, a head CTA confirmed right cerebral infarction and occlusion of the right ICA. After multidisciplinary discussion, it was concluded that, considering that the patient's collateral circulation was relatively functional (Figure 3D), emergency surgery was not immediately necessary. Therefore, a scheduled ICA bypass could be considered. Due to the high risk of bleeding and the family’s refusal of anticoagulation therapy, no anticoagulants were administered until discharge.
After 46 d of hospitalization, the patient improved and was discharged without any residual limb dysfunction. A follow-up head CT scan (Figure 1D) showed a significant reduction in the size of the cerebral infarction compared with that upon admission.
The patient's family therefore, requested discharge to take him home, where he died on the same day.
After 76 d of treatment, the patient was discharged in an improved condition: Muscle strength was Grade II in the left upper limb, Grade IV in the left lower limb, and Grade V in the right limbs. There was a slight decline in intelligence and speech. A follow-up head CT scan (Figure 3E) showed a reduction in the size of the right cerebral infarction lesion compared with previous images.
Falls from heights, traffic accidents, and crush injuries are common causes of fractures at the base of the skull, which can lead to blunt cerebrovascular injuries. These injuries have an incidence rate of less than 1%. They are often overlooked in clinical practice, eventually leading to severe traumatic ICA occlusion[2,3]. The mechanisms of these events are two-fold: Usually thrombotic, either due to thrombus formation at the site of intimal tear or by hemodynamic thrombus formation caused by arterial narrowing or obstruction, potentially leading to low-flow infarctions[4]. Due to their very low occurrence rate, these conditions often result in late diagnosis or misdiagnosis, with serious consequences. In the assessment of complex injuries, early detection and treatment of traumatic ICA occlusion are critical for saving the patient's life.
In diagnosing patients with fractures at the base of the skull, vigilance is required in the following aspects: (1) Understanding the etiology of such diseases, particularly in patients with severe fractures at the base of the skull, there is a need to be alert to the possibility of ICA injury. The study by Li et al[3] found that the incidence of ICA injury in patients with carotid canal fractures was more than four times higher than that in those without carotid canal fractures. All three patients mentioned in this study had carotid canal fractures; (2) Attention to clinical presentation: Patients with severe craniofacial trauma often exhibit varying degrees of altered consciousness. Clinical manifestations caused by traumatic occlusion of the ICA can be masked by the primary injury, leading to missed or incorrect diagnoses; and (3) Imaging with cranial CT scans: Although a plain cranial CT scan cannot diagnose ICA injuries, patients with carotid canal fractures, an initial GCS score of less than 9, and a history of severe head injury as indicated by the Abbreviated Injury Scale should be considered for early screening with CTA[5]. These points highlight the importance of a comprehensive and cautious approach to diagnosing and managing patients with skull base fractures, particularly regarding the potential for vascular injuries.
The current treatment approaches for traumatic ICA occlusion are not yet standardized, as there are no specific guidelines. Available treatments include anticoagulation, antiplatelet therapy, and open surgery: (1) Use and selection of anticoagulants: Recent studies indicate that there often exists a latent, asymptomatic period between the development of traumatic hemorrhage and ischemic stroke, during which established antithrombotic or anticoagulant therapy can improve neurological outcomes and mortality[6]. However, systemic anticoagulation poses a significant bleeding risk in trauma patients, especially after traumatic brain injury. Anticoagulation should not be initiated within the first 3 d post-injury for patients with mild or high-risk intracranial hemorrhage, as even prophylactic doses of heparin may lead to brain hemorrhage, with intracranial bleeding rates of 8%-16%[7,8]. Eastham[9] suggest using antithrombotic agents, such as heparin or aspirin, for mild cases of blunt cerebrovascular injury, but no specific anticoagulation regimen for traumatic ICA occlusion was mentioned. Moreover, the timing of initiating anticoagulant or antiplatelet therapy remains controversial. The first patient used anticoagulants without severe adverse reactions, offering some reference for future treatments; (2) Assessment of collateral circulation: Studies have shown that rapid assessment of collateral circulation using CTA, for patients with complete ICA occlusion may help determine future treatment approaches and patient prognosis. For patients with poor collateral circulation, treatment should focus on maintaining an elevated mean arterial pressure to prevent watershed ischemia[10]. Strong collateral circulation can reduce the extent of brain ischemia associated with these injuries. However, the patient's survival rate and prognosis can be directly affected if there is insufficient compensatory collateral circulation. In the mentioned cases, the prognosis of the second patient was worse than the first and third due to inadequate intracranial collateral compensation and older age; and (3) Open surgery: Extracranial-intracranial bypass surgery has been proven to be a useful method of vascular reconstruction. Currently, the preferred method for treating ICA injuries is the precise excision of the damaged arterial segment and delicate reconstruction of the ICA. This process aims to ensure the continuity and stability of intracranial blood supply as much as possible, effectively preventing cerebral ischemia and related complications[11].
Traumatic ICA occlusion is a rare and serious complication resulting from skull base fractures. To address this: (1) Rapid cranial CTA: If a cranial CT scan suggests a fracture of the carotid canal, it is crucial to promptly perform a cranial CTA to ascertain the extent of the ICA injury; (2) Consider use of anticoagulants: Anticoagulant therapy may be beneficial for the patient, but this requires a thorough evaluation of the overall health status and careful consideration of bleeding risks; and (3) Collateral circulation evaluation: Assessing collateral circulation is crucial for deciding on cerebral vascular reconstruction and predicting patient outcomes. Strong collateral circulation often leads to better outcomes and lower ischemic risk. These steps are essential for effectively managing this complication.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade A
P-Reviewer: Zhou Y, United States S-Editor: Zheng XM L-Editor: A P-Editor: Yu HG
| 1. | Nunnink L. Blunt carotid artery injury. Emerg Med (Fremantle). 2002;14:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Franz RW, Willette PA, Wood MJ, Wright ML, Hartman JF. A systematic review and meta-analysis of diagnostic screening criteria for blunt cerebrovascular injuries. J Am Coll Surg. 2012;214:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Li C, Green L, Wang J, Tully D, Woliansky J, Gumm K, Iseli C, Martin K, Read D. Factors associated with blunt internal carotid artery injury in petrous temporal bone fractures. Injury. 2022;53:2023-2027. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 4. | Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009;8:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 506] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 5. | Venkataraman SS, Herbert JP, Ravindra VM, Yu BN, Bollo RJ, Cox CS Jr, Gannon SR, Limbrick DD Jr, Naftel RP, Ugalde IT, Yorkgitis BK, Weiner HL, Shah MN. Multi-Center Validation of the McGovern Pediatric Blunt Cerebrovascular Injury Screening Score. J Neurotrauma. 2023;40:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE Jr, Johnson JL, Moore JB, Burch JM. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg. 2004;139:540-5; discussion 545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Eachempati SR, Vaslef SN, Sebastian MW, Reed RL 2nd. Blunt vascular injuries of the head and neck: is heparinization necessary? J Trauma. 1998;45:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, Croce MA. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51:279-85; discussion 285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 228] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Eastham S. Blunt cerebrovascular injuries in trauma. Int J Surg. 2016;33:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Morton RP, Hanak BW, Levitt MR, Fink KR, Peterson EC, Vilela MD, Kim LJ, Chesnut RM. Blunt traumatic occlusion of the internal carotid and vertebral arteries. J Neurosurg. 2014;120:1446-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Rostomily RC, Newell DW, Grady MS, Wallace S, Nicholls S, Winn HR. Gunshot wounds of the internal carotid artery at the skull base: management with vein bypass grafts and a review of the literature. J Trauma. 1997;42:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |