Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2642
Revised: March 8, 2024
Accepted: April 9, 2024
Published online: May 26, 2024
Processing time: 100 Days and 4.8 Hours
Tuberous sclerosis complex (TSC) and primary lymphedema (PLE) are both rare diseases, and it is even rarer for both to occur in the same patient. In this work, we have provided a detailed description of a patient's clinical presentation, imaging findings, and treatment. And a retrospective analysis was conducted on 14 published relevant case reports.
A 16-year-old male came to our hospital for treatment due to right lower limb swelling. This swelling is already present from birth. The patient’s memory had been progressively declining. Seizures had occurred 1 year prior at an unknown frequency. The patient was diagnosed with TSC combined with PLE through multimodal imaging examination: Computed tomography, magnetic resonance imaging, and lymphoscintigraphy. The patient underwent liposuction. The swelling of the patient's right lower limb significantly improved after surgery. Epilepsy did not occur.after taking antiepileptic drugs and sirolimus.
TSC with PLE is a rare and systemic disease. Imaging can detect lesions of this disease, which are important for diagnosis and treatment.
Core Tip: Tuberous sclerosis complex with primary lymphedema (TSC-PLE) is a rare, congenital and systemic disease closely related to gene mutations. The clinical manifestations of TSC-PLE are diverse. Multiple imaging methods can detect systemic organ and tissue lesions, which are important for clinical diagnosis and treatment. Rapamycin-targeted therapy is the main treatment for this disease and can simultaneously treat the symptoms of both TSC and PLE. Liposuction can effectively improve limb swelling in patients. This case is the 17th patient with TSC-PLE worldwide. And this case has comprehensive clinical, imaging, genetic testing and treatment.
- Citation: Li XP, Sun XL, Liu X, Wen Z, Jiang LH, Fu Y, Yue YL, Wang RG. Tuberous sclerosis complex combined with primary lymphedema: A case report. World J Clin Cases 2024; 12(15): 2642-2648
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2642.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2642
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic diseases characterized by multiple organ lesions and closely related to the TSC gene[1-3]. Lymphedema (LE) is the accumulation of protein rich lymphatic fluid in subcu
A 16-year-old male came to our hospital for treatment due to right lower limb swelling. This swelling is already present from birth without any obvious cause or reason. It worsened after activity and improved after rest.
Ten years prior, there had been swelling of the right scrotum and fluid accumulation in the right testicular sheath. Seven years prior, the patient had broken out with erysipelas. In recent years, the patient’s memory had been progressively declining. Seizures had occurred 1 year prior at an unknown frequency but improved after treatment with antiepileptic medication.
The skin became red and thick and exhibited poor elasticity, high temperature, and visible pigmentation. Restricted movement of joints was observed in the right lower limb.
Genetic testing revealed a mutation in the TSC2 gene. The heterozygous mutation of nucleotide number 2251 from cytosine C to thymine T (c.2251C>T) resulted in a mutation of amino acid number 751 from arginine to terminator (p.ARG751Ter). The patient's parents did not exhibit any TSC gene abnormalities.
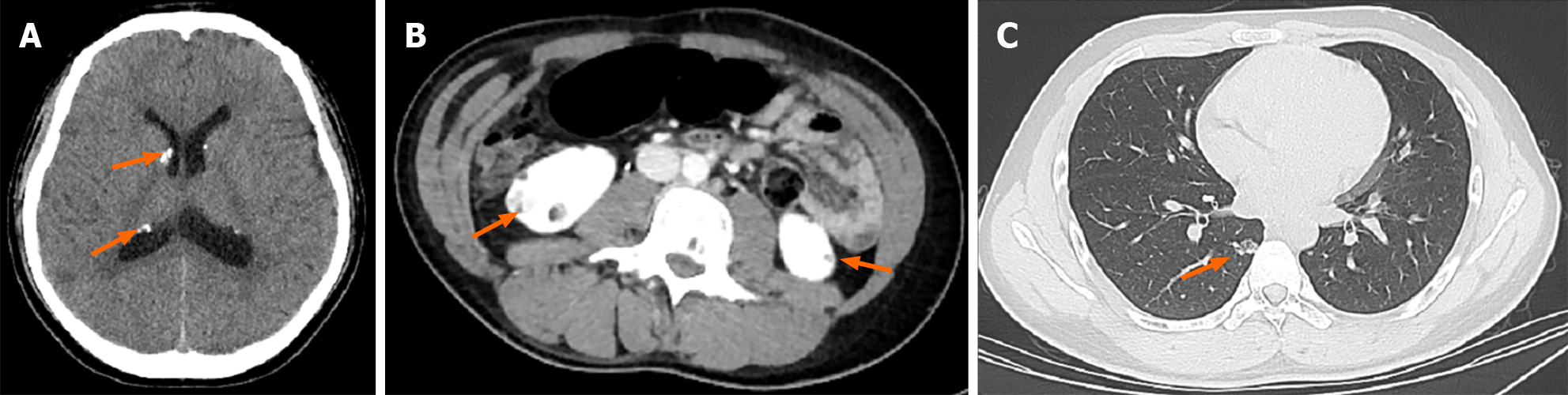
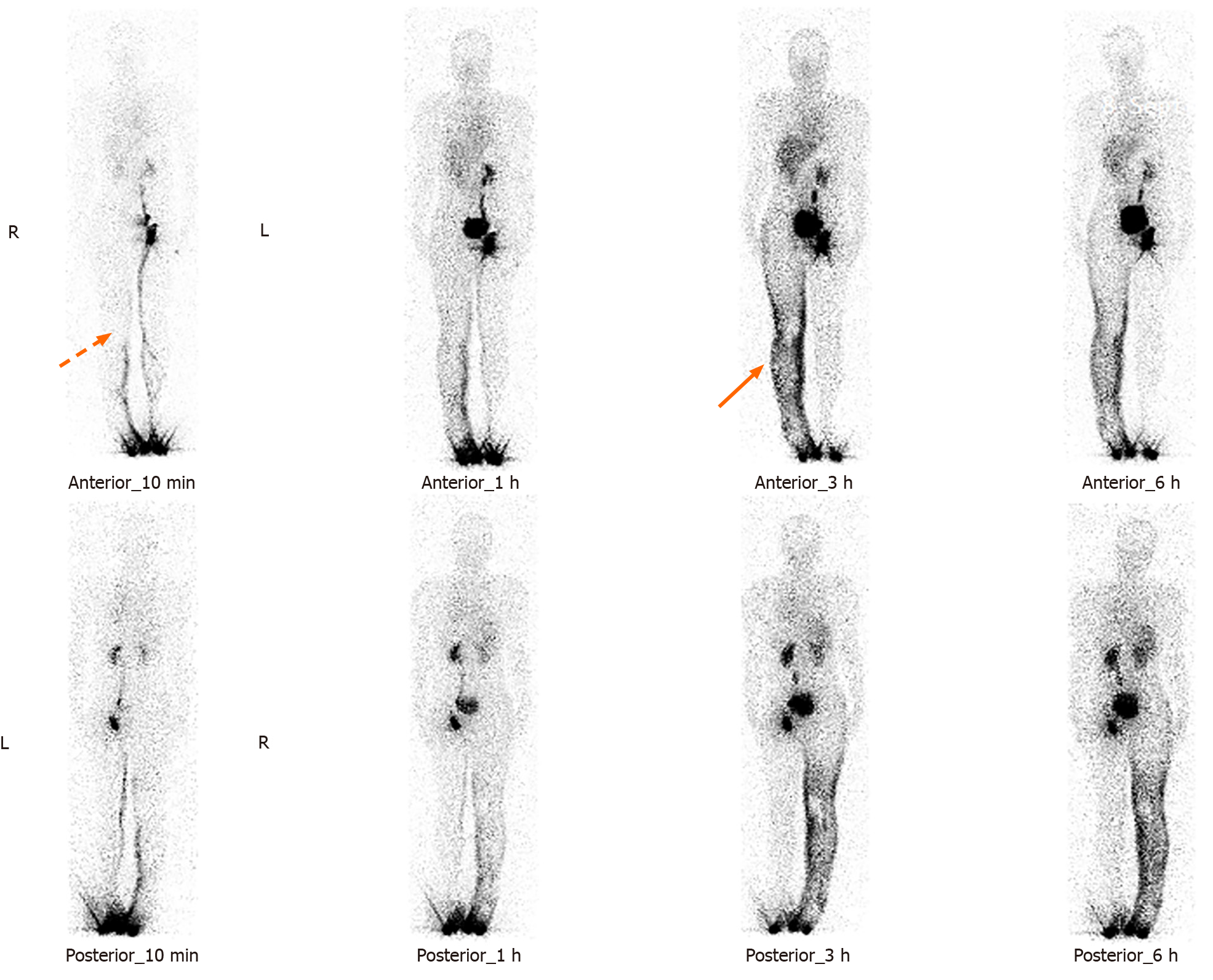
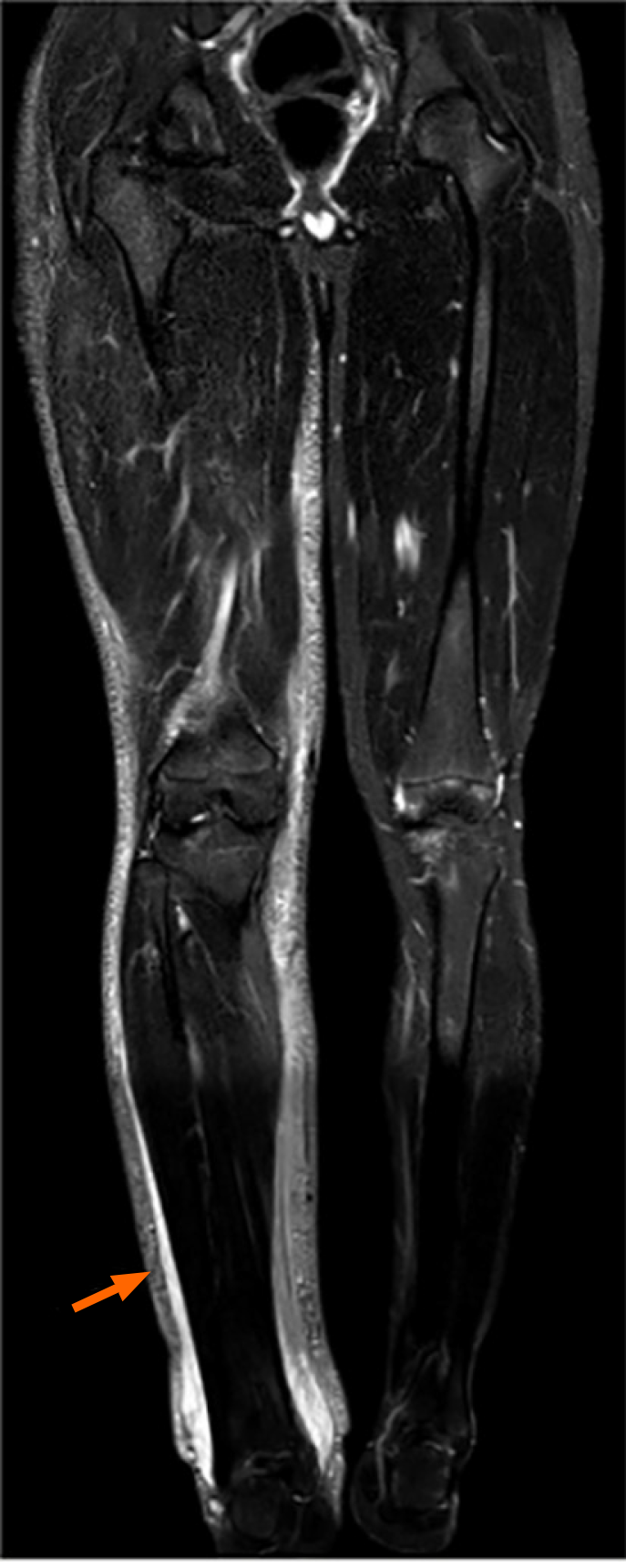
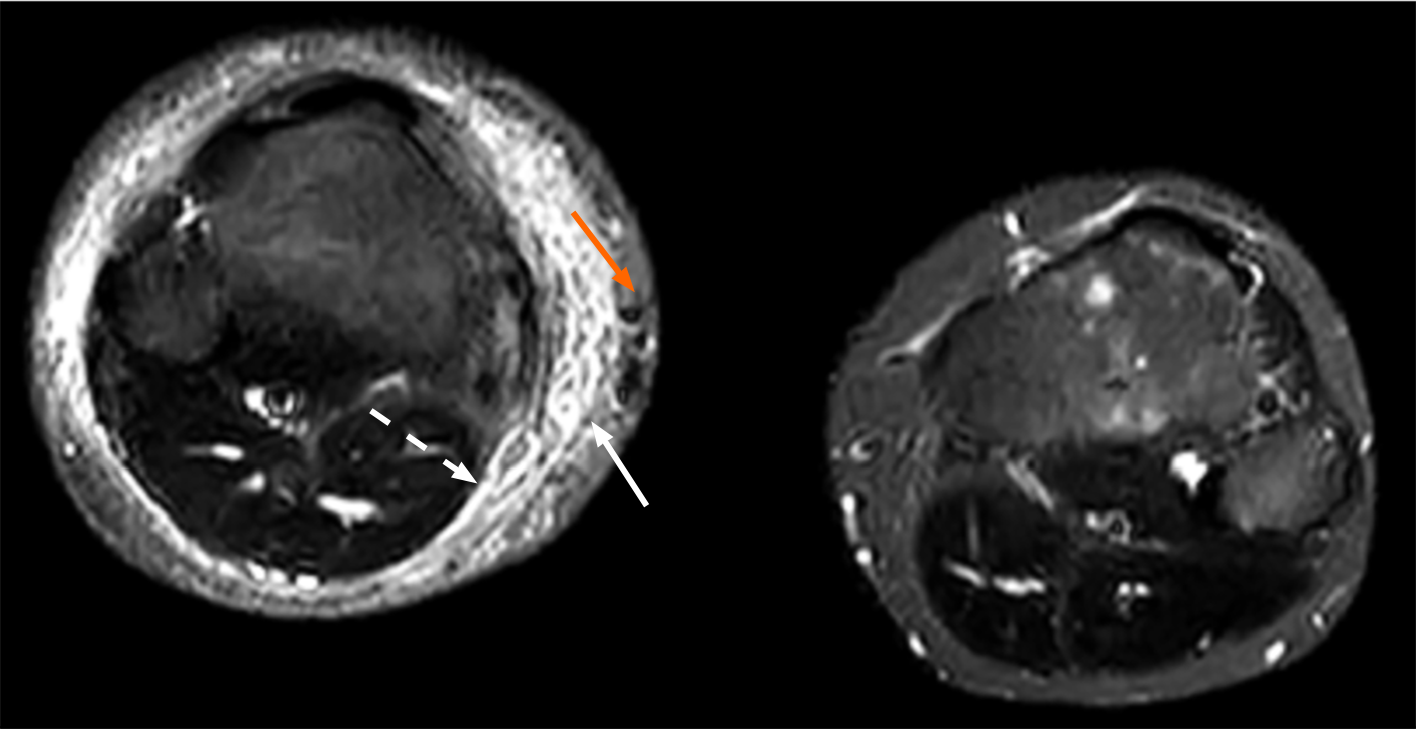
A computed tomography (CT) scan of the head revealed symmetrically distributed calcified nodules located beneath the ependymal membrane of both lateral ventricles (Figure 1A). An abdominal enhanced CT scan showed multiple, regular and well-defined nodules in both kidneys. The internal density of the nodule was uneven, with visible fat density and soft tissue density (Figure 1B). A chest CT scan showed multiple ground glass opacities in both lungs, mainly distributed under the pleura, with a diameter of 3-10 mm. However, no pulmonary cysts were found in either lung (Figure 1C). In addition, 99Tcm-DX-lymphoscintigraphy revealed thickening of the right lower limb, but lymphatic vessels in the right lower limb were not visualized. The right inguinal, iliac, and lumbar lymphatic vessels and lymph nodes were not visualized, and the imaging agent was diffusely and unevenly distributed in the right lower limb, waist, buttocks, and external genital area (Figure 2). The short time inversion recovery sequence of the lower limb magnetic resonance imaging (MRI) showed thickening of the skin and swelling of subcutaneous soft tissue in the right lower limb, with honeycomb, crescent, and band signs visible inside. Thickened, tortuous, and dilated veins were also observed (Figures 3 and 4).
Based on comprehensive clinical, imaging, and genetic testing, the male patient was diagnosed with TSC combined with PLE.
The patient was prescribed oral oxcarbazepine and sodium valproate to suppress seizures and sirolimus to treat the TSC. The patient underwent liposuction and volume reduction surgery to reduce lower limb edema.
Prognosis: The swelling of the patient's right lower limb significantly improved after surgery, and the morphology improved well. Epilepsy did not occur.
TSC is an autosomal dominant genetic disorder characterized by lesions in multiple organs. TSC1 or TSC2 gene mutations can lead to structural activation of the mechanical target of rapamycin pathway, dysregulation of cell proliferation, and obstruction of lymphangiogenesis. The incidence rate is approximately 1/6000-10000; these tumours can be familial or sporadic and are more likely to occur in young males. The typical clinical manifestations of the Vogt triad are epilepsy, intellectual disability, and facial sebaceous adenoma. Almost all organs in the body can be affected[1-3] (the skin, nervous system, heart, lungs, kidneys, bones, etc.). The main treatment method for TSC is the targeted drug rapamycin. The present patient was a male adolescent with clinical manifestations of epilepsy. The imaging findings included subependymal nodules, bilateral renal angiomyolipomas, and multiple ground glass opacity in both lungs on chest CT. Genetic testing revealed TSC2 gene mutations, consistent with previous literature reports[3].
PLE of the lower limbs is caused by congenital structural and/or functional deficiencies in the lymphatic system, leading to impaired lymphatic return and the accumulation of protein-rich fluid in the interstitial tissue in the skin and subcutaneous soft tissue of the lower limbs. Prolonged and progressive stimulation of adipose tissue ultimately leads to abnormal proliferation of fibrous connective tissue and irreversible fibrosis of adipose tissue. The most common clinical manifestation is obvious swelling of the skin of the lower limbs, with early onset of collapsible oedema. The skin gradually hardens over time, and there is late onset of abnormal thickening and segmental hypertrophy of the affected limb. In severe cases, this condition can lead to "elephantiasis" of the affected limb, bilateral limb asymmetry, and even limb deformities. The pathogenesis may be related to the following gene mutations[4-6]: Vascular endothelial growth factor receptor-3 (VEGFR-3), SRY-related high mobility group Box 18, and forkhead Box C2. The overall incidence rate of PLE is 1-2/100000, and PLE usually occurs in childhood, and there are more women affected than men[4]. The patient was diagnosed with primary lower limb LE through clinical and imaging examinations. The clinical manifestation was swelling of the right lower limb without obvious cause. A typical honeycomb-like pattern (honeycomb sign) was observed in the subcutaneous soft tissue of the right lower limb via MRI, and 99Tcm-DX-lymphoscintigraphy revealed the absence of lymphatic vessels and lymph nodes in the right lower limb accompanied by right lower limb skin lymphatic reflux, which is consistent with the imaging findings of LE reported by Liu et al[7] and Wen et al[8].
According to the literature, TSC with PLE (TSC-PLE) has only been observed in case reports. A summary of all relevant literature retrieved from PubMed from 1986 to 2023 is presented in Table 1. There were a total of 14 articles and 16 cases. Including this case report, there were a total of 17 patients, including 11 females (64.7%) and 6 males (35.3%). There were more women with TSC-PLE than men, with an age range of 0-28 years and an average age of 6.3 years. LE is more common in adolescents than in adults, and LE often occurs in the lower limbs; there was a total of 15 cases (88.2%), and all 15 cases of oedema occurred in one limb (two cases are unknown). There were 3 patients with TSC1 gene mutations, 7 patients with TSC2 gene mutations, and 7 patients with unclear gene mutations. Geffrey et al[9] reported that the incidence rate of PLE in TSC patients was 0.70% (2/286). However, the pathological and physiological mechanisms of TSC-PLE are not yet clear. Among the more than 20 genes involved in lymphatic system development, some are closely related to the mTOR pathway. Therefore, several researchers speculate that there may be five possible reasons for the occurrence of TSC-PLE: (1) The phosphoinositol 3-kinase signalling pathway plays an important role in the formation and remodelling of the lymphatic system. Due to TSC gene mutations, mTOR is activated, thereby inhibiting lymphatic vessel growth. Eventually, the affected limb's lymphatic system develops poorly[10]; (2) VEGFR-3 and VEGF-C are important regulatory factors that promote lymphatic vessel proliferation and cell migration. Studies have shown that VEGF-C can activate downstream mTOR/S6 kinase signalling pathways and that activated mTOR acts through p70S6 kinase[11]; (3) mTOR directly participates in the generation of lymphatic vessels: Studies have shown that the expression of mTOR in lymphatic endothelial cells is significantly increased and that mTOR is abnormally activated in lymphatic malformations[11]; (4) The reason why the incidence rate of female patients with TSC-PLE increases significantly may be related to oestrogen[9]; and (5) Smooth cell hypertrophy: Abnormal smooth cell hypertrophy within subcutaneous tissue compresses superficial lymphatic vessels, leading to impaired lymphatic reflux and ultimately resulting in LE[12].
| No | Ref. | Year | Number | Gender | Age (yr) | Edemasite | Gene | Treatment |
| 1 | Cottafava et al[16] | 1986 | 1 | Female | 7 | Left lower limb | Unknown | Anti convulsive drugs + anti ACTH drugs |
| 2 | Hirsch et al[17] | 1999 | 1 | Female | 28 | Left lower limb | Unknown | Subcutaneous lymphangiectomy + diuretic + elastic socks |
| 3 | Voudris et al[18] | 2003 | 1 | Female | 5 | Left lower limb | Unknown | Unknown |
| 4 | Lucas and Andrade[19] | 2011 | 1 | Female | 1 | Right lower limb | TSC1 | Unknown |
| 5 | Sukulal and Namboodiri[12] | 2012 | 1 | Female | 0 | Left lower limb | Unknown | Unknown |
| 6 | Navarre and Poitras[13] | 2014 | 1 | Male | 5 | Left lower limb | TSC2 | Fasectomy + toe amputation + antibiotics + enoxaparin + warfarin |
| 7 | Prato et al[20] | 2014 | 1 | Female | 4 | Left upper limb | TSC2 | Carbamazepine + topiramate + everolimus + amoxicillin-clavulanate potassium |
| 8 | Geffrey et al[9] | 2014 | 2 | Female/Female | 8/15 | Left lower limb/Left lower limb | TSC2/TSC2 | Rapamycin + lymphedema therapy |
| 9 | Hoshiai et al[21] | 2015 | 2 | Female/Female | 2/0 | Right lower limb/Right upper limb | Unknown | Unknown |
| 10 | Saffari et al[22] | 2019 | 1 | Male | 6 | lower limb but unknown left and right | TSC2 | Everolimus |
| 11 | Wiemer-Kruel et al[14] | 2020 | 1 | Male | 7 | Left lower limb | TSC2 | Levetiracetam + repair of aortic aneurysm + everolimus |
| 12 | Kaneshi et al[15] | 2020 | 1 | Male | 0 | lower limb but unknown left and right | Unknown | Ileostomy decompression + antibiotics + lymphedema therapy |
| 13 | Lin et al[11] | 2020 | 1 | Male | 3 | Left lower limb | TSC1 | Albumin + diuretics |
| 14 | Klinner et al[23] | 2020 | 1 | Female | 0 | Right lower limb | TSC1 | Aminohexenoic acid + lymphatic drainage + bandage compression |
| 15 | This case | 2023 | 1 | Male | 16 | Right lower limb | TSC2 | Sirolimus + oxcarbazepine + liposuction |
The main treatment for TSC-PLE patients is rapamycin, which can alleviate both TSC-related symptoms and lymphatic vessel abnormality-related symptoms (e.g., LE)[9]. Liposuction and volume reduction surgery can also be performed to reduce lower limb oedema and improve the appearance of the affected limb. In this case, antiepileptic drugs were used to suppress seizures. In addition, when TSC-PLE is combined with other critical signs, such as osteofascial compartment syndrome caused by severe LE, aortic aneurysm caused by severe vascular malformations, erysipelas and systemic infections caused by LE, and pulmonary embolism caused by deep vein thrombosis, timely symptomatic treatment should be administered[13-15].
TSC-PLE is a rare, congenital and systemic disease closely related to a variety of gene mutations and is most common among young women, with an overall incidence rate of approximately 0.7%. The clinical manifestations of TSC-PLE are diverse and include epilepsy, memory loss, intellectual disability, lower limb swelling, etc. Multiple imaging methods can detect systemic organ and tissue lesions, which are important for clinical diagnosis and treatment. Rapamycin-targeted therapy is the main treatment method for this disease and can simultaneously treat the symptoms of both TSC and PLE. Liposuction can effectively improve limb swelling in patients However, the specific pathophysiological mechanism of TSC-PLE is still unclear and requires further research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Kumar R, India S-Editor: Zheng XM L-Editor: A P-Editor: Yu HG
| 1. | Randle SC. Tuberous Sclerosis Complex: A Review. Pediatr Ann. 2017;46:e166-e171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Volpi A, Sala G, Lesma E, Labriola F, Righetti M, Alfano RM, Cozzolino M. Tuberous sclerosis complex: new insights into clinical and therapeutic approach. J Nephrol. 2019;32:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Gupta S, Kang HC, Faria SC, Choyke PL, Kundra V. Tuberous Sclerosis Complex (TSC): Renal and Extrarenal Imaging. Acad Radiol. 2022;29:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 4. | Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg. 2007;59:464-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 445] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 5. | Grada AA, Phillips TJ. Lymphedema: Pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77:1009-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (1)] |
| 6. | Ho B, Gordon K, Mortimer PS. A Genetic Approach to the Classification of Primary Lymphoedema and Lymphatic Malformations. Eur J Vasc Endovasc Surg. 2018;56:465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Liu M, Zhang Y, Li X, Hao Q, Li B, Wang R. MRI-based volume measurement methods for staging primary lower extremity lymphedema: a single-center study of asymmetric volume difference-a diagnostic study. BMC Musculoskelet Disord. 2023;24:810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Wen Z, Tong G, Liu Y. Potential Utilization of Lymphoscintigraphy in Patients With Klippel-Trenaunay Syndrome. Clin Nucl Med. 2021;46:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Geffrey AL, Shinnick JE, Staley BA, Boronat S, Thiele EA. Lymphedema in tuberous sclerosis complex. Am J Med Genet A. 2014;164A:1438-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Pollack SF, Geffrey AL, Thiele EA, Shah U. Primary intestinal lymphangiectasia treated with rapamycin in a child with tuberous sclerosis complex (TSC). Am J Med Genet A. 2015;167A:2209-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Lin WH, Zhang ZH, Wang HL, Ren L, Geng LL. Tuberous sclerosis complex presenting as primary intestinal lymphangiectasia: A case report. World J Clin Cases. 2020;8:1995-2000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Sukulal K, Namboodiri N. Congenital lymphedema: another unique and gender specific stigmata of tuberous sclerosis? Indian Pediatr. 2012;49:845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Navarre P, Poitras B. Lymphoedema in tuberous sclerosis: case report and review of the literature. J Pediatr Orthop. 2014;34:e27-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Wiemer-Kruel A, Mayer H, Ewert P, Martinoff S, Eckstein HH, Kriebel T, Bissler J, Franz D, Bast T. Congenital Lymphatic Malformation and Aortic Aneurysm in a Patient with TSC2 Mutation. Neuropediatrics. 2020;51:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Kaneshi Y, Shibasaki J, Aida N, Shimokaze T, Toyoshima K. Indocyanine green lymphography for congenital lymphatic dysplasia with tuberous sclerosis complex: A case report. Pediatr Int. 2020;62:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Cottafava F, Cosso D, Brida di Priò S, Grossi-Bianchi ML, Fedi M, Fontana F, Racugno A, Tosca P. [A case of Bourneville's tuberous sclerosis with elephantiasis (caused by lymphedema) of the left leg]. Minerva Pediatr. 1986;38:49-52. [PubMed] |
| 17. | Hirsch RJ, Silverberg NB, Laude T, Weinberg JM. Tuberous sclerosis associated with congenital lymphedema. Pediatr Dermatol. 1999;16:407-408. [PubMed] [DOI] [Full Text] |
| 18. | Voudris KA, Skardoutsou A, Vagiakou EA. Tuberous sclerosis and congenital lymphedema. Pediatr Dermatol. 2003;20:371-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Lucas M, Andrade Y. Congenital lymphedema with tuberous sclerosis and clinical Hirschsprung disease. Pediatr Dermatol. 2011;28:194-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Prato G, Mancardi MM, Baglietto MG, Janis S, Vercellino N, Rossi A, Consales A, Raso A, Garrè ML. Congenital segmental lymphedema in tuberous sclerosis complex with associated subependymal giant cell astrocytomas treated with Mammalian target of rapamycin inhibitors. J Child Neurol. 2014;29:NP54-NP57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Hoshiai S, Oguma E, Sato Y, Konishi T, Minami M. Congenital focal lymphedema as a diagnostic clue to tuberous sclerosis complex: report of two cases diagnosed by ultrasound. Skeletal Radiol. 2015;44:1165-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Saffari A, Brösse I, Wiemer-Kruel A, Wilken B, Kreuzaler P, Hahn A, Bernhard MK, van Tilburg CM, Hoffmann GF, Gorenflo M, Hethey S, Kaiser O, Kölker S, Wagner R, Witt O, Merkenschlager A, Möckel A, Roser T, Schlump JU, Serfling A, Spiegler J, Milde T, Ziegler A, Syrbe S. Safety and efficacy of mTOR inhibitor treatment in patients with tuberous sclerosis complex under 2 years of age - a multicenter retrospective study. Orphanet J Rare Dis. 2019;14:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Klinner J, Krüger M, Brunet T, Makowski C, Riedhammer KM, Mollweide A, Wagner M, Hoefele J. Congenital lymphedema as a rare and first symptom of tuberous sclerosis complex. Gene. 2020;753:144815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |