Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2606
Revised: March 6, 2024
Accepted: April 7, 2024
Published online: May 26, 2024
Processing time: 127 Days and 3.4 Hours
Ewing’s sarcoma (ES) is a neuroectodermal tumor that typically occurs in the bones and soft tissues of children and young adults. Primary renal ES is rare; only a few cases and a small case series have been documented, and only four cases involved primary renal ES in older people (> 65 years old).
Herein, we describe the radiological and pathological features of primary renal ES in an older person. A 76-year-old man complained of poor oral intake and was found to have a large cystic renal mass with indistinct margins on computed tomography. Ultrasound-guided biopsy revealed that the tumor contained small round blue cells. The patient underwent a right radical nephrectomy. The tumor cells showed diffuse membranous CD99, and nuclear friend leukemia integration 1 transcription factor and NK2 Homeobox 2. Fluorescence in situ hybridization revealed EWSR1 translocation. Postoperatively, 18F-fluorodeoxyglucose positron emission tomography revealed no evidence of metastasis. The patient was diagnosed with primary renal ES. Six months following the surgery, local recurrence and distant metastasis were observed. Primary renal ES is rare and often lethal in older individuals. The specific imaging findings are unknown, and treatment protocols have not been standardized.
This case report describes the radiological and pathological features of primary renal ES in an older person.
Core Tip: Primary renal Ewing’s sarcoma (ES) is extremely rare, whereas shown aggressive radiological features and poor outcome. It is important to consider primary renal ES in the differential diagnosis when renal mass shown indistinctive margin and necrosis/hemorrhage in older patients. Primary renal ES undergo surgical resection and receive adjuvant chemotherapy. Moreover, radiation therapy is efficient for local recurrence.
- Citation: Kim S, Park J, Ko YH, Kwon HJ. Primary Ewing sarcoma of the kidney mimicking cystic papillary renal cell carcinoma in an older patient: A case report. World J Clin Cases 2024; 12(15): 2606-2613
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2606.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2606
Ewing’s sarcoma (ES) is an aggressive malignant tumor that typically arises from the bones, and rarely from the retroperitoneum, head and neck, parameningeal, and genitourinary systems. ES mostly affects children and young adults, and is uncommon in older people. ES of the kidney was first described in 1975[1] and has a reported incidence of approximately 100 cases[1-3]. Among them, primary renal ES in older people (> 65 years) has been reported in only four patients in the English literature[4-7]. Primary renal ES typically remain asymptomatic initially, but when the tumor grows large enough, the tumor often manifest symptoms such as flank pain and hematuria.
Owing to its rarity, ES is sometimes misdiagnosed as other more common kidney cancers. ES lacks specific radiological findings; however, common features include large necrotic masses and intratumoral hemorrhage. Contrast-enhanced imaging reveal a mild and progressive enhancement pattern frequently associated with invasion of the perirenal fascia and renal sinuses[8]. Histological characteristics that confirm positive CD99 expression and the existence of a reciprocal translocation between the ES gene on chromosome 22 and members of the ETS family, most commonly friend leukemia integration 1 transcription factor (FLI1) or ERG, are typically used to confirm tissue diagnosis[6]. Primary renal ES has an aggressive clinical course and a poor prognosis. However, there is no consensus on optimal treatment because of its rarity.
This study reports a rare case of primary renal ES in an older man. Additionally, we reviewed the literature on primary renal ES in older patients.
Appetite loss for several months.
A 76-year-old male visited our hospital complaining of appetite loss for several months.
The patient had hypertension.
The patient had no familial medial history.
Tenderness and percussion pain in the left flank were observed during the physical examination.
The complete blood count, serum electrolyte values, and urine analysis results were all within the normal ranges. The patient showed normal kidney function test with blood urea nitrogen of 15.7 mg/dL, serum creatinin of 0.75 mg/dL, serum uric acid of 5.3 mg/dL and modification of diet in renal disease-estimated glomerular filtration rate of 100.99.
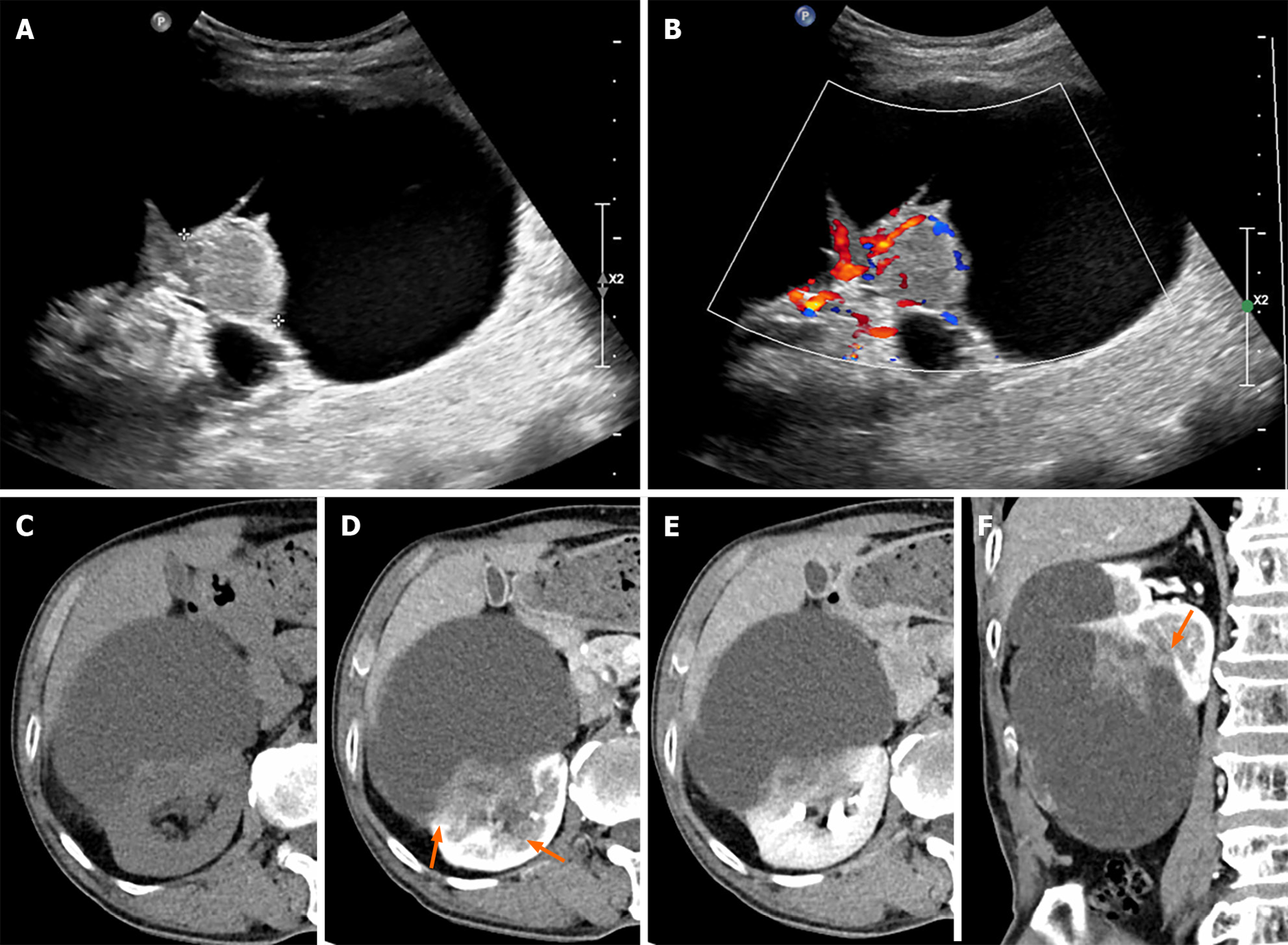
On renal ultrasonography, an 11 cm × 10 cm cystic lesion was identified in the right renal lower pole with a 4.4 cm × 1.8 cm solid component on the posterior aspect of the cystic lesion, which showed increased vascularity on color doppler mode (Figure 1A and B). On computed tomography (CT), a pre-contrast phase axial CT shows a cystic lesion with a mass at the posterior-lateral aspect of the right kidney interpolar region-lower pole (Figure 1C). A subtle high-attenuation area is seen in the cystic lesion, suggesting hemorrhage. Corticomedullary and excretory phase axial CT shows progressive enhancement of the mass (pre-contrast phase: 40 HU, corticomedullary phase: 70 HU, excretory phase: 80 HU) and a suspiciously indistinct margin and invasion of the sinus (Figure 1D and E). Corticomedullary phase coronal CT scan shows an irregular enhancing mass in a lesion with indistinct margins (Figure 1F).
Ultrasound guided, core needle biopsy: Under suspicion of cystic papillary renal cell carcinoma (RCC), an ultrasound-guided core needle biopsy was performed for a targeted intracystic solid mass. Histological evaluation revealed solid sheets of primitive, small, and uniform cells with hyperchromatic nuclei and scant cytoplasm. Immunohistochemical staining was positive for synaptophysin and CD99. Staining for desmin, FLI1, leukocyte common antigen (LCA), pan-cytokeratin, neuron-specific enolase, and S100 were negative. Fluorescence in situ hybridization (FISH) using formalin-fixed, paraffin-embedded tissues showed a t(11; 22) Ewing sarcoma region 1 (EWSR1) translocation (zytolight SPEC EWSR1 dual color break-apart probe, ZytoVision GmbH, Fischkai 127572 Bremerhaven, Germany). The mass was suggestive of ES.
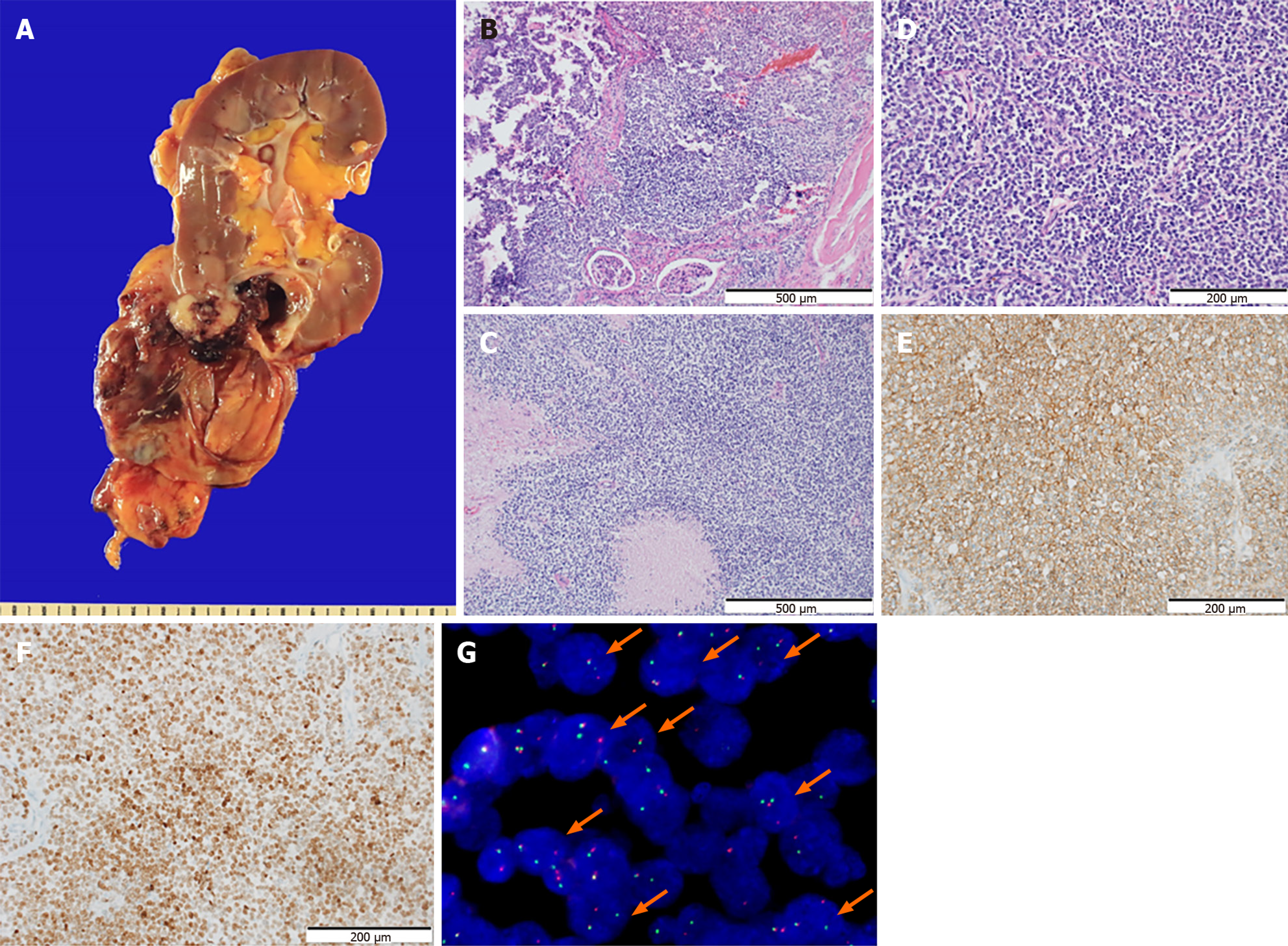
Surgical examination: The patient underwent a radical nephrectomy. The tumor was located at the lower pole of the right kidney. The large cystic tumor was well demarcated by the perirenal adipose tissue without rupture. Most tumors were cystic lesions, whereas the solid area showed gross infiltration of the renal parenchyma (Figure 2A). The border of the tumor infiltrating the renal parenchyma was confirmed using hematoxylin-eosin (HE) staining (Figure 2B). The tumor exhibited multifocal necrosis (Figure 2C). The solid part of the tumor in the specimen was composed of small round cells with scanty cytoplasm, similar to that in the biopsy specimen (Figure 2D). Ancillary tests for CD56, chromogranin A, FLI1, LCA, thyroid transcription factor-1, and cytokeratins (AE1/AE3) were negative. The tumor cells exhibited diffuse membranous staining for CD99 (Figure 2E) and NK2 Homeobox 2 (NKX2.2) (Figure 2F). FISH using formalin-fixed, paraffin-embedded tissues showed a t(11; 22) EWSR1 translocation (Figure 2G) (zytolight SPEC EWSR1 dual color break-apart probe, ZytoVision GmbH, Fischkai 127572 Bremerhaven, Germany).
The diagnosis was primary renal ES.
The patient underwent right radical nephrectomy. The patient did not undergo chemotherapy.
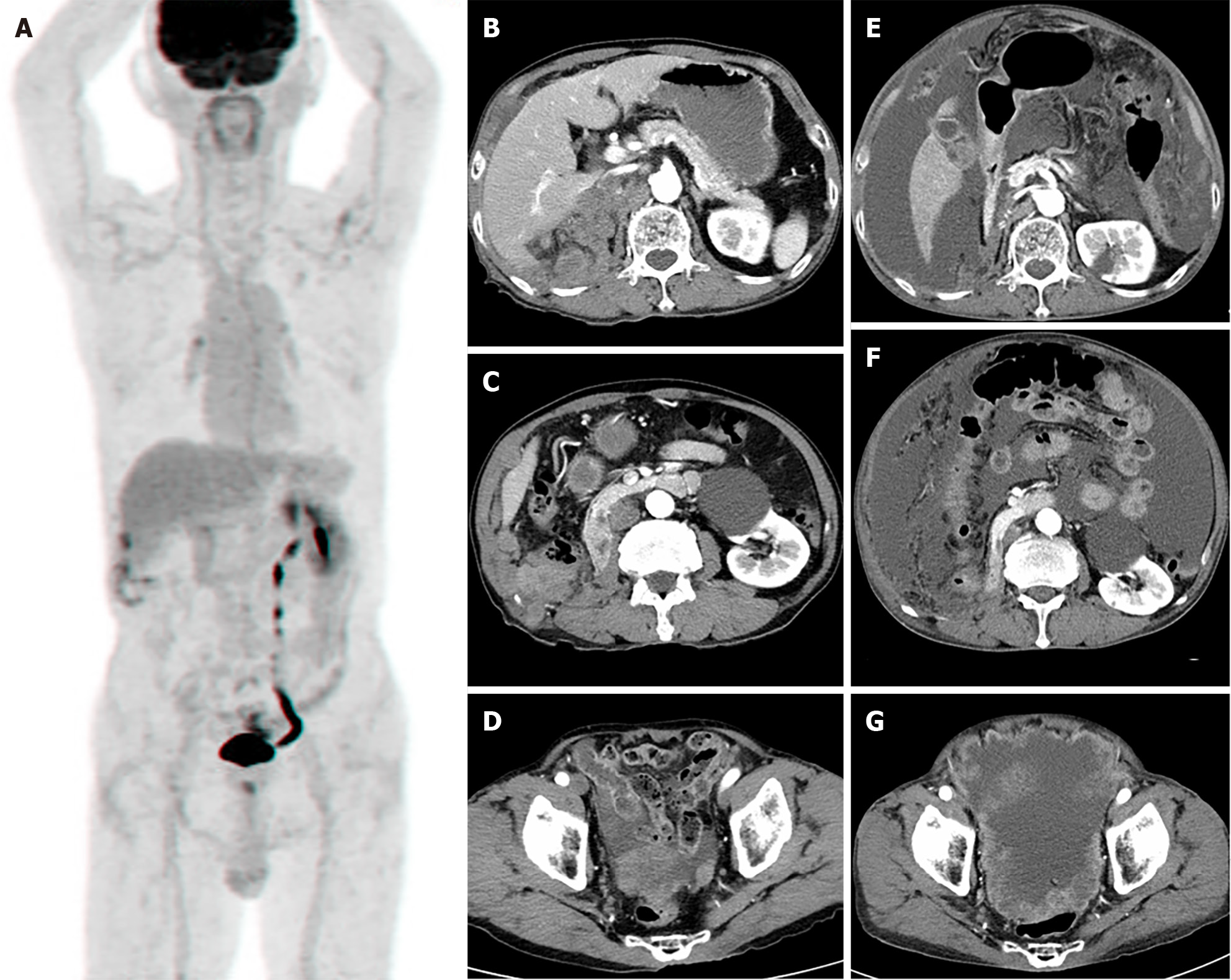
Postoperatively, torso positron emission tomography revealed no involvement of other organs (Figure 3A), and a whole-body bone scan revealed no abnormal uptake lesions. No recurrence was observed on abdominal or chest CT scans 3 months after surgery; however, CT performed 6 months after surgery showed local recurrence and peritoneal metastasis (Figure 3B-D). Thus, radiotherapy was administered for 10 cycles to control the pain. Eight months after surgery, abdominal CT revealed a locally controlled recurrent mass at the right nephrectomy site; however, multiple, more aggravated metastases were discovered (Figure 3E-G), and the patient died. The follow-up duration was 10 months.
The ES family of tumors comprises a group of high-grade small round cell tumors, including ES of the bone, extraskeletal ES, peripheral primitive neuroectodermal tumors (PNET), and thoracopulmonary PNET. The pathogenesis of ES is presently understood to involve the EWS/FLI oncoprotein, derived from reciprocal translocation between 11 and 22, t (11, 22) (q24; q12). This can be confirmed through FISH or reverse transcriptase polymerase chain reaction (RT-PCR).
Tumors are common in young children and adolescents. The average age at diagnosis was 30.7 years with a male to female ratio of 6:4[8]. Limited number of cases of primary renal ES with an aggressive clinical course has been reported in the English literature. Moreover, only four cases of ES in older people have been reported in the English literature (Table 1). Among a total of five older patients (aged 60 years or older) with primary renal ES, four died. The mean overall survival (OS) was 18.3 months, and the median OS was 16 months. This was shorter than the median OS of primary renal ES treated with radical nephrectomy of 27.4 months in a previous study[8]. This suggests that primary renal ES in older people has a worse prognosis, and that age is a significant prognostic factor for primary renal ES. Additionally, 60% of patients presented with metastasis at diagnosis. These results are similar to those of a previous study that reported metastasis in 37.5%–60% of the primary renal ES/PNET cases[2,6,9]. The high incidence of metastatic disease may be due to the aggressive nature of primary renal ES or the absence of specific clinical presentations of retroperitoneal tumors.
| Case | Age | Sex | Size | Side | Presentation | Surgery | Other therapy | Recurrence or metastasis | Follow-up (month) | Status |
| Current case | 76 | M | 11 | R | Incidentally found | Nephrectomy | RTx | Local recurrence, carcinomatosis | 10 | Dead |
| 1 | 69 | F | 5 | R | Abdominal pain | Nephrectomy | CTx | Lung, bone | 25 | Dead |
| 2 | 70 | F | NA | NA | NA | Nephrectomy | CTx + INF | Lung | 16 | Dead |
| 3 | 69 | F | NA | NA | NA | Nephrectomy | CTx | Lung | 22 | Dead |
| 4 | 73 | M | NA | R | Incidentally found | Nephrectomy | CTx | No | 7 | Alive |
Renal ES/PNET is not only a significant challenge for imaging diagnosis but also poses diagnostic challenges for pathology. Although there are no specific radiological findings for ES, the following are known: Large masses (60% of tumors > 10 cm) are heterogeneous in the non-contrast phase. Among the tumors, 95.9%, 32.7%, and 11.6% showed necrosis, intratumoral hemorrhage, and parenchymal calcification, respectively. In contrast, mild and progressive enhancement with invasion of the perirenal fascia (47.2%) and renal sinuses (67.9%) has been noted[8]. The radiological findings of PNET are similar to those of ES. PNET presents as a weakly enhanced large mass with multiple septate appearances, peripheral hemorrhage/necrosis, renal vein, or inferior vena cava thrombosis, accompanied by distant metastasis or renal vein thrombosis[10].
Our case showed an enhanced portion with extensive necrosis, mimicking cystic papillary RCC. Papillary RCC, a subtype of RCC constituting 10%-15% of RCCs cases, typically shows homogeneous enhancement on CT scans. However, approximately 15% of papillary RCC show heterogeneous enhancement with a large size (> 10 cm) and presence of a cystic portion or necrosis. Moreover, approximately 7% of these tumors may have intratumoral calcifications. The typical dynamic enhancement pattern of papillary RCC is characterized by mild (20 HU-40 HU) and persistent enhancement[11]. Approximately 98% of the tumors had a well-defined boundary that altered kidney contours and displaced adjacent structures; only 2% of papillary RCC showed an infiltrating margin, which commonly represented a high Fuhrman grade and had sarcomatoid features. Compared to the RCC, renal ES shares similar imaging findings with papillary RCC. In particular, ES/PNETs show an indistinctive infiltrative margin, which is a characteristic of ES. In the present case, the mass showed indistinctive margins with infiltration into the normal renal parenchyma. In addition, papillary RCC has lower incidence of renal vein invasion and lymph node metastasis; however, renal ES have highly incidence of renal vein invasion and lymph node metastasis.
Small round cell tumors present a diagnostic challenge for pathologists because of the poorly differentiated features of these high-grade tumors. Furthermore, ES masses often present with extensive hemorrhage and necrosis, and fine-needle biopsy is usually inadequate for diagnosis[12]. In many cases, round cell tumors require large immunohistochemical (IHC) panels and molecular testing. Light microscopy revealed the presence of small round tumor cells arranged in sheets, forming Homer-Wright rosettes. Tumor cells are positive for neuroectodermal IHC markers such as neuron-specific enolase and synaptophysin. The demonstration of reciprocal translocation t (11; 22) (q24; q12) by genetic studies is considered specific to PNET and ES[13].
According to EURO EWING 2012, treatment recommendations exist for primary ES[14]. Most patients undergo surgical resection and receive adjuvant chemotherapy. Effective chemotherapeutic agents include vincristine, doxorubicin, ifosfamide, etoposide, actinomycin D, and cyclophosphamide[15]. Molecular targeted therapies, including insulin-like growth factor 1 receptor antibodies, have shown promise for Ewing sarcoma family of tumours[16]. Our patient did not receive adjuvant chemotherapy; however, chemotherapy has been administered in other cases, leading to better outcomes. Radiotherapy is recommended for skeletal ES if unresectable tumors or unexpected positive margins are recommended for skeletal ES[17]. In the EURO EWING 2012 trial, patients with unresectable tumors, residual tumors, or metastases responded well to radiotherapy. Our patient showed local recurrence on abdominal CT, showing locally controlled recurrent tumors after radiotherapy.
ES should always be considered a differential diagnosis for aggressive renal tumors in older patients. While imaging diagnosis for renal ES still has limitations, when large-sized renal masses have necrosis, hemorrhage, and indistinctive margins with mild and persistent enhancement on dynamic contrast enhancement studies, although rare, considering primary ES is necessary, even in older patients, as a differential diagnosis. The final diagnosis is by histopathology with IHC and cytogenetic studies, such as FISH or RT-PCR. Early detection is crucial for the prompt initiation of chemo
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Wani I, India S-Editor: Che XX L-Editor: A P-Editor: Yu HG
| 1. | Seemayer TA, Thelmo WL, Bolande RP, Wiglesworth FW. Peripheral neuroectodermal tumors. Perspect Pediatr Pathol. 1975;2:151-172. [PubMed] |
| 2. | Rowe RG, Thomas DG, Schuetze SM, Hafez KS, Lawlor ER, Chugh R. Ewing sarcoma of the kidney: case series and literature review of an often overlooked entity in the diagnosis of primary renal tumors. Urology. 2013;81:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Zhang S, Li Y, Wang R, Song B. Ewing's sarcoma/primitive neuroectodermal tumor of the kidney: a case report and literature review. Transl Androl Urol. 2019;8:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Jimenez RE, Folpe AL, Lapham RL, Ro JY, O'Shea PA, Weiss SW, Amin MB. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney: a clinicopathologic and immunohistochemical analysis of 11 cases. Am J Surg Pathol. 2002;26:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Murugan P, Rao P, Tamboli P, Czerniak B, Guo CC. Primary Ewing Sarcoma / Primitive Neuroectodermal Tumor of the Kidney: A Clinicopathologic Study of 23 Cases. Pathol Oncol Res. 2018;24:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Tarek N, Said R, Andersen CR, Suki TS, Foglesong J, Herzog CE, Tannir NM, Patel S, Ratan R, Ludwig JA, Daw NC. Primary Ewing Sarcoma/Primitive Neuroectodermal Tumor of the Kidney: The MD Anderson Cancer Center Experience. Cancers (Basel). 2020;12:2927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Wedde TB, Lobmaier IV, Brennhovd B, Lohne F, Hall KS. Primary Ewing's Sarcoma of the Kidney in a 73-Year-Old Man. Sarcoma. 2011;2011:978319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Hu X, Li D, Cai J. Experience of CT diagnosis and management of primary renal Ewing's sarcoma: A retrospective analysis of 6 cases and a literature review. Medicine (Baltimore). 2022;101:e32189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Zöllner S, Dirksen U, Jürgens H, Ranft A. Renal Ewing tumors. Ann Oncol. 2013;24:2455-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Lee H, Cho JY, Kim SH, Jung DC, Kim JK, Choi HJ. Imaging findings of primitive neuroectodermal tumors of the kidney. J Comput Assist Tomogr. 2009;33:882-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Couvidat C, Eiss D, Verkarre V, Merran S, Corréas JM, Méjean A, Hélénon O. Renal papillary carcinoma: CT and MRI features. Diagn Interv Imaging. 2014;95:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Burchill SA. Ewing's sarcoma: diagnostic, prognostic, and therapeutic implications of molecular abnormalities. J Clin Pathol. 2003;56:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Takeuchi T, Iwasaki H, Ohjimi Y, Kaneko Y, Ishiguro M, Fujita C, Miura Y, Hiratsuka Y, Sakamoto K, Kikuchi M. Renal primitive neuroectodermal tumor: an immunohistochemical and cytogenetic analysis. Pathol Int. 1996;46:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Anderton J, Moroz V, Marec-Bérard P, Gaspar N, Laurence V, Martín-Broto J, Sastre A, Gelderblom H, Owens C, Kaiser S, Fernández-Pinto M, Fenwick N, Evans A, Strauss S, Whelan J, Wheatley K, Brennan B. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours - EURO EWING 2012 Protocol. Trials. 2020;21:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 915] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 16. | Javery O, Krajewski K, O'Regan K, Kis B, Giardino A, Jagannathan J, Ramaiya NH. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol. 2011;197:W1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | DuBois SG, Krailo MD, Gebhardt MC, Donaldson SS, Marcus KJ, Dormans J, Shamberger RC, Sailer S, Nicholas RW, Healey JH, Tarbell NJ, Randall RL, Devidas M, Meyer JS, Granowetter L, Womer RB, Bernstein M, Marina N, Grier HE. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children's Oncology Group. Cancer. 2015;121:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |