Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2529
Revised: February 21, 2024
Accepted: April 8, 2024
Published online: May 26, 2024
Processing time: 176 Days and 6 Hours
Lobulated intracranial aneurysm is a special type of aneurysm with at least one additional cyst in the neck or body of the aneurysm. Lobulated intracranial aneurysm is a complex aneurysm with complex morphology and structure and weak tumor wall, which is an independent risk factor for rupture and hemor
To investigate the efficacy and safety of endovascular treatment for ruptured lobulated anterior communicating artery aneurysm (ACoAA).
Patients with ruptured lobulated ACoAA received endovascular treatment in Sanming First Hospital Affiliated to Fujian Medical University from June 2020 to June 2022 were retrospectively included. Their demographic, clinical and imaging characteristics, endovascular treatment methods and follow-up results were collected.
A total of 24 patients with ruptured lobulated ACoAA were included, including 9 males (37.5%) and 15 females (62.5%). Their age was 56.2 ± 8.9 years old (range 39-74). The time from rupture to endovascular treatment was 10.9 ± 12.5 h. The maximum diameter of the aneurysms was 5.1 ± 1.0 mm and neck width were 3.0 ± 0.7 mm. Nineteen patients (79.2%) were double-lobed and 5 (20.8%) were multilobed. Fisher's grade: Grade 2 in 16 cases (66.7%), grade 3 in 6 cases (25%), and grade 4 in 2 cases (8.3%). Hunt-Hess grade: Grade 0-2 in 5 cases (20.8%), grade 3-5 in 19 cases (79.2%). Glasgow Coma Scale score: 9-12 in 14 cases (58.3%), 13-15 in 10 cases (41.7%). Immediately postprocedural Raymond-Roy grade: grade 1 in 23 cases (95. 8%), grade 2 in 1 case (4.2%). Raymond-Roy grade in imaging follow-up for 2 wk to 3 months: grade 1 in 23 cases (95.8%), grade 2 in 1 case (4.2%). Follow-up for 2 to 12 months showed that 21 patients (87.5%) had good functional outcomes (modified Rankin Scale score ≤ 2), and there were no deaths.
Endovascular treatment is a safe and effective treatment for ruptured lobulated AcoAA.
Core Tip: For ruptured lobulated anterior communicating artery aneurysm, preoperative angiography was used to fully evaluate the lobulated aneurysm, neck of the tumor and segment A and 1 vessels, to grasp reasonable operation timing, and to minimize the use of multiple or simplified systems for endovascular treatment during the operation, which was conducive to surgical safety and to reduce postoperative complications.
- Citation: Huang SX, Ai XP, Kang ZH, Chen ZY, Li RM, Wu ZC, Zhu F. Endovascular treatment of ruptured lobulated anterior communicating artery aneurysms: A retrospective study of 24 patients. World J Clin Cases 2024; 12(15): 2529-2541
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2529.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2529
Lobulated intracranial aneurysm is a special type of aneurysm with at least one additional cyst in the neck or body of the aneurysm[1]. Lobulated intracranial aneurysm is a complex aneurysm with complex morphology and structure and weak tumor wall, which is an independent risk factor for rupture and hemorrhage[2]. Lobular aneurysms located in the anterior communicating artery complex account for 36.9% of all intracranial lobular aneurysms[3]. Due to its special anatomical structure, both craniotomy and endovascular treatment are more difficult[4,5]. Compared with single-capsule aneurysms, craniotomy for lobular intracranial aneurysms has a higher risk and complication rate[2]. This is because it is difficult to ensure the complete occlusion of lobular aneurysms and the patency of the carrying artery, and more operations on the surrounding brain tissue are required to expose aneurysms, and the placement of aneurysm clips is more complicated. The prolonged use of fluorescein angiography and brain retractor during repeated operations will prolong the operative time and increase the probability of intraoperative brain injury. In view of the above reasons, the treatment of intracranial complex aneurysms has gradually shifted to intravascular therapy[6,7]. Cerebral infarction in the anterior cerebral artery region secondary to vasospasm after anterior communicating aneurysm rupture is a potentially significant adverse outcome[8]. We analyzed the safety and efficacy of ruptured anterior communicating artery aneurysm (ACoAA) treated intravascular at the Department of Neurosurgery, Sanming First Hospital Affiliated to Fujian Medical University.
Patients with ruptured lobulated ACoAA who received endovascular therapy at the First Affiliated Sanming Hospital of Fujian Medical University from June 2020 to June 2022 were retrospectively included. Inclusion criteria: (1) Subarachnoid hemorrhage was confirmed by computed tomography (CT) examination; and (2) Lobed ACoAA was indicated by conventional 320-slice CT angiography (CTA), which was confirmed by whole brain digital subtraction angiography (DSA). The evaluation criteria were as follows: Aneurysms located in the anterior communicating artery complex of the brain had ≥ 1 additional aneurysm sac from the common aneurysm neck or tumor body. When the diameter of the additional aneurysm sac was ≥ 63% of the diameter of the largest aneurysm sac, it was lobed; otherwise, it was ascus[1,9-10]; (3) Receive endovascular therapy; (4) Postoperative clinical follow-up time > 3 months. Exclusion criteria: (1) Multiple intracranial aneurysms; (2) Recurrence after previous intravascular therapy; (3) Other intracranial vascular diseases, such as arteriovenous malformation, moyamoya disease, arteriovenous fistula, etc.; (4) No interventional treatment was performed due to critical illness or combined with serious underlying diseases; and (5) Incomplete information. This study was approved by the Ethics Committee of the First Affiliated Sanming Hospital of Fujian Medical University (Ming Yilun[2020] 37).
Patients' age, sex, duration of rupture (i.e., onset of subarachnoid hemorrhage) to intravascular treatment, disease assessment (Fisher scale, Hunt-Hess scale, Glasgow Coma Scale, GCS score, hydrocephalus), basic aneurysm characteristics (maximum diameter, neck width, number of ascus), endovascular treatment (including extra- vascular drainage), and follow-up results.
Under general anesthesia, the right femoral artery was punctured by Seldinger technique, the 6F vascular sheath was inserted, and the whole body was heparinized. The intracranial blood vessels were investigated in detail for spasticity, arteriosclerosis, stenosis and collateral circulation compensation, and the location of the aneurysm and the carrier artery were determined. The direction of ACoAA, the angulation of the neck and the carrier artery, the shape of the true and lobulated tumors, and the vascular movement of the proximal aneurysms were mainly observed. The size of the neck and the tumor were measured, and the appropriate working Angle was selected based on the criterion that the overall shape of the aneurysm, the neck and the relationship between the neck and the carrier artery could be fully revealed. A long sheath plus an intermediate catheter was routinely used to establish access, and an 8 F long sheath was inserted through the 8 F artery sheath, and then an intermediate catheter was inserted through the long sheath to the C1 segment of the internal carotid artery. For small lobulated aneurysms (maximum diameter < 1 mm), a single microcatheter is guided into the mother tumor under the guidance of a micro-guide wire, and the spring coil is simply used to embolize the mother tumor according to the size of the tumor. In the process of embolization, when the spring ring overflows the bearing artery or is combined with the absolute wide neck, the stent can be used to assist embolization. For large lobular aneurysms, two microcatheters were guided by micro guides into each lobule, embolized with spring rings according to the size of the lobule, and then closed the common neck. If a relatively wide neck or an absolutely wide neck is expected to require stent-assisted treatment, the stent catheter is prepositioned to the distal end of the target vessel under the guidance of a micro-guide wire, and then the embolized microcatheter is placed in the lobules with high probability of rupture or the lobules with difficult placement through the stent mesh, and the aneurysm lobules are embolized one by one by adjusting the embolized microcatheter when the stent is half-released. Or the stent is completely released, and the pre-positioned embolization microcatheter embolizes one of the lobules, and then reshapes, perforates the stent mesh and embolizes the remaining lobules. Simplify multiple system operations as much as possible. For patients who were expected to require stent-assisted treatment during the operation, load dose dual therapy (aspirin 300 mg+ clopidogrel 300 mg) was administered 30 min before surgery. If emergency surgery was performed immediately after admission and stent assistance was required, Tirofiban was injected intravenously (10 μg/kg load) + intravenous [(0.1 μg/(kg min)] for 24 h, followed by dual antiplatelet therapy (aspirin 100 mg+ clopidogrel 75 mg), overlapping for 6 h. If acute thrombosis occurs during the operation, Tirofiban arterial injection plus postoperative intravenous pumping should be given immediately. In case of vasospasm, nimodipine 2 mg is administered slowly. All patients were treated with double antiplatelet agents for 3 months after stent placement, and then adjusted according to the results of angiography review. After the operation, conventional lumbar cisterna continuous drainage or lumbar puncture released bloody cerebrospinal fluid, anti-cerebrovascular spasm, water and electrolyte balance maintenance, dehydration to reduce cranial pressure and nutritional nerve and other symptomatic treatment.
The imaging results immediately after operation and during follow-up were evaluated according to Raymond-Roy occlusion classification[11] to evaluate the degree of embolism: Grade 1, complete occlusion; Grade 2, residual neck; Grade 3, residual tumor. The modified Rankin Scale (mRS) was used to evaluate the clinical outcome, and ≤ 2 was defined as good outcome.
SPSS 21.0 software was used for data processing. To conform to the normal distribution of measurement data to ± SD, comparison between the two groups use independent sample t-test; Measurement data with non-normal distribution were presented as median and interquartile range, and comparison between the two groups was analyzed using the Mann-Whitney U test. Count data were expressed as frequency and percentage, and comparison between groups was analyzed using the chi-square test or Fisher's exact test. Two-sided P < 0. 05 was considered statistically significant.
A total of 30 patients with ruptured lobulated ACoAA were treated during the study period. After excluding 3 patients who gave up treatment, 1 patient who chose craniotomy and 2 patients who were lost to follow-up, 24 patients were finally included in the analysis. There were 9 males (37.5%) and 15 females (62.5%). The average age was 56.2 ± 8.9 years (range 39-74 years). 19 cases (79.2%) were bilobed and 5 cases (20.8%) were poly lobulated. The clinical data of all patients are summarized in Tables 1 and 2.
| Case Number | Age | Sex | Time from rupture to endovascular treatment (h) | Maximum diameter of the aneurysms (mm) | Neck width (mm) | Lobed number | Fisher's grade | Hunt-Hess grade | Glasgow Coma Scale score | Hydrocephalus | Methods of Treatment | Immediately postprocedural Raymond-Roy grade | Complication | Imaging follow-up method | Follow-up Raymond-Roy grade |
| 1 | 39 | Female | 3 | 4.2 | 3 | 2 | 2 | 2 | 13 | No | Single microcatheter embolization | 1 | No | CT angiography | 1 |
| 2 | 42 | Female | 11 | 5.1 | 2.8 | 2 | 2 | 3 | 11 | No | Single microcatheter embolization | 1 | No | CT angiography | 1 |
| 3 | 63 | Female | 6 | 3.1 | 2.2 | 3 | 2 | 3 | 12 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 4 | 52 | Male | 7 | 5.8 | 4.1 | 2 | 2 | 3 | 12 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 5 | 43 | Female | 4 | 3.6 | 3.1 | 3 | 3 | 3 | 12 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 6 | 56 | Female | 6 | 3.7 | 2.7 | 2 | 3 | 4 | 11 | No | External ventricular drainage; Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 7 | 71 | Female | 48 | 3.6 | 3 | 2 | 2 | 1 | 14 | No | Single microcatheter embolization | 1 | No | CT angiography | 1 |
| 8 | 48 | Male | 7 | 5.5 | 3 | 3 | 2 | 2 | 13 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 9 | 61 | Female | 5 | 5.1 | 2.9 | 2 | 2 | 2 | 15 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 10 | 55 | Female | 7 | 5 | 2.6 | 2 | 2 | 3 | 13 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 11 | 65 | Female | 7 | 8.1 | 5.3 | 3 | 4 | 4 | 9 | Yes | External ventricular drainage; Auxiliary support coil embolization | 2 | Arterial thrombosis | Digital subtraction angiography | 2 |
| 12 | 58 | Male | 5 | 5.3 | 3 | 2 | 3 | 3 | 13 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 13 | 50 | Female | 10 | 4.6 | 2 | 2 | 2 | 3 | 13 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 14 | 63 | Female | 26 | 5.1 | 2.1 | 2 | 2 | 3 | 13 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 15 | 66 | Male | 6 | 5 | 3.2 | 2 | 4 | 4 | 9 | Yes | Double microcatheter embolization | 1 | Communicating hydrocephalus | CT angiography | 1 |
| 16 | 53 | Male | 8 | 6.1 | 3.9 | 2 | 3 | 3 | 12 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 17 | 74 | Female | 50 | 5.4 | 3.4 | 2 | 2 | 2 | 13 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 18 | 61 | Male | 4 | 4.3 | 2.3 | 2 | 2 | 3 | 12 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 19 | 55 | Male | 5 | 5.7 | 3 | 2 | 3 | 3 | 12 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 20 | 60 | Female | 5 | 5.7 | 3.8 | 3 | 2 | 3 | 12 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 21 | 48 | Female | 8 | 5.4 | 2.5 | 2 | 2 | 3 | 13 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 22 | 53 | Female | 12 | 4.9 | 2.3 | 2 | 2 | 4 | 12 | No | Double microcatheter embolization | 1 | No | CT angiography | 1 |
| 23 | 63 | Male | 7 | 6.1 | 2.9 | 2 | 3 | 4 | 11 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| 24 | 50 | Male | 6 | 5.5 | 2.8 | 2 | 2 | 4 | 11 | No | Auxiliary support coil embolization | 1 | No | Digital subtraction angiography | 1 |
| Double-lobed patients | Multilobed patients | |
| Age | 56.3 ± 9.0 | 55.8 ± 9.7 |
| Sex | 8 (42.1) | 1 (20) |
| Time from rupture to endovascular treatment (h) | 12.9 ± 13.8 | 5.8 ± 1.3 |
| Maximum diameter of the aneurysms (mm) | 5.0 ± 0.7 | 5.2 ± 2.0 |
| Neck width (mm) | 2.9 ± 0.5 | 3.5 ± 1.2 |
| Fisher's grade | ||
| Grade 2 | 13 (68.4) | 3 (60) |
| Grade 3 | 5 (26.3) | 1 (20) |
| Grade 4 | 1 (5.2) | 1 (20) |
| Hunt-Hess grade | ||
| Grade 0-2 | 4 (21.1) | 1 (20) |
| Grade 3-5 | 15 (78.9) | 4 (80) |
| Glasgow Coma Scale score | 12.2 ± 1.3 | 11.6 ± 1.5 |
| Hydrocephalus | 1 (5.2) | 1 (20) |
| Methods of treatment | ||
| Single microcatheter embolization | 3 (15.8) | 0 (0) |
| Double microcatheter embolization | 8 (42.1) | 2 (40) |
| Auxiliary support coil embolization | 7 (36.8) | 2 (40) |
| External ventricular drainage; Double microcatheter embolization | 1 (5.3) | 0 (0) |
| External ventricular drainage; Auxiliary support coil embolization | 0 (0) | 1 (20) |
| Immediately postprocedural Raymond-Roy grade | ||
| Grade 1 | 19 (100) | 4 (80) |
| Grade 2 | 0 (0) | 1 (20) |
| Complication | ||
| Arterial thrombosis | 0 (0) | 1 (20) |
| Communicating hydrocephalus | 1 (5.2) | 0 (0) |
| Follow-up Raymond-Roy grade | ||
| Grade 1 | 19 (100) | 4 (80) |
| Grade 2 | 0 (0) | 1 (20) |
Endovascular treatment was successfully performed in all patients. In 1 patient, the parent artery thrombosis was found by angiography after embolization of the aneurysm, and the blood flow was improved after intravenous infusion of tirofiban, but there was still significant neurological deficit (mRS Score 4) after the operation. One patient developed communicating hydrocephalus at 6 months follow-up, and the symptoms were improved after ventriculoperitoneal shunt (mRS Score 3). Immediate postoperative Raymond-Roy classification: 23 cases (95.8%) were grade 1 and 1 case (4.2%) was grade 2. Radiographic follow-up from 2 wk to 3 months showed that 23 cases (95.8%) were grade 1 and 1 case (4.2%) was grade 2. At 2-12 months follow-up, 21 patients (87.5%) had good outcome (mRS Score ≤ 2), 3 patients (12.5%) had poor outcome (mRS Score > 2), and there was no death.
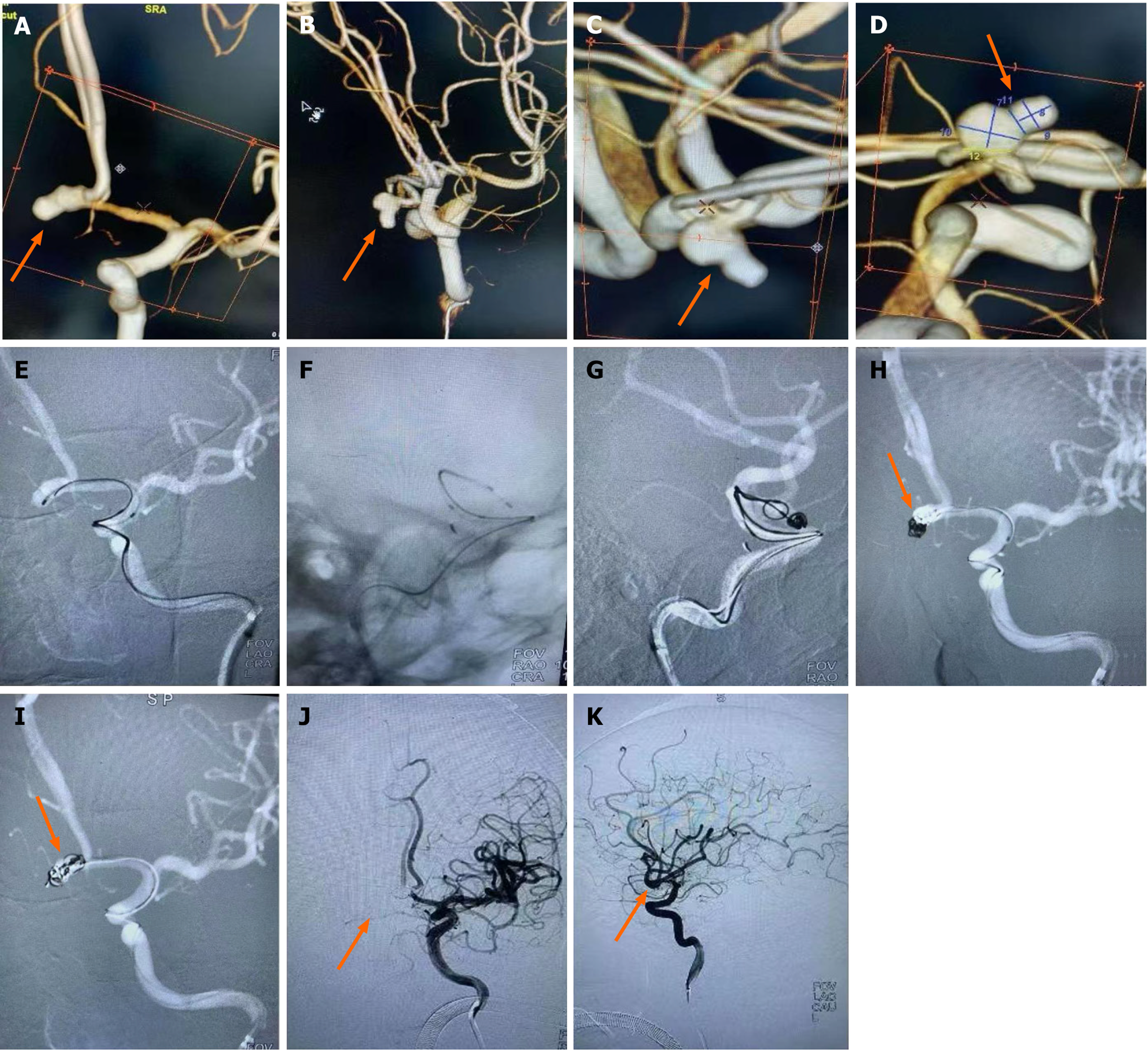
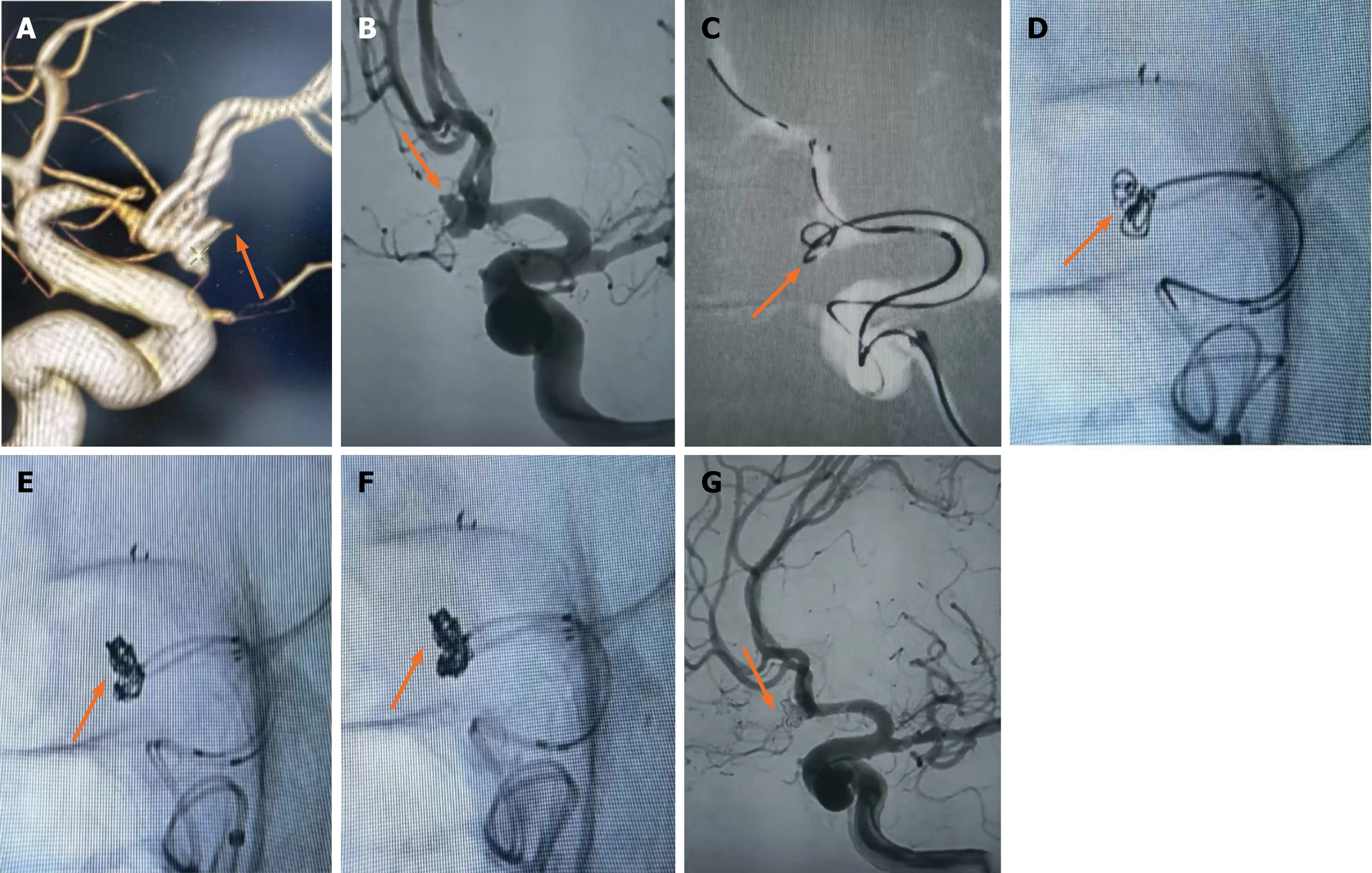
Case 9, a 61-year-old female, was admitted to hospital with "sudden severe headache for 5 h". Physical examination: lethargy, GCS score 15, bilateral pupil diameter 2.5 mm, sensitive light reflex, neck resistance, normal muscle strength and muscle tone in extremities, bilateral Babinski sign negative, Hunt-Hess grade 2. Emergency CT showed subarachnoid hemorrhage (Fisher grade 2), and CTA showed ACoAA. In the emergency department, endovascular treatment was performed under general anesthesia. DSA showed that A1 on the left side was divided into A2 on both sides, and ACoAA was bilobed, with sizes of 5.1 mm × 5.0 mm and 4.2 mm × 4.0 mm, respectively, and tumor neck 2.9 mm. Aneurysmal embolization was performed using dual microcatheter technique. Immediate postoperative angiography showed that the aneurysm was completely embolized and both sides were smooth. No aneurysm recurrence was observed in CTA reexamination 2 wk after surgery, and the patient recovered well and was completely asymptomatic at 3 months follow-up (Figure 1).
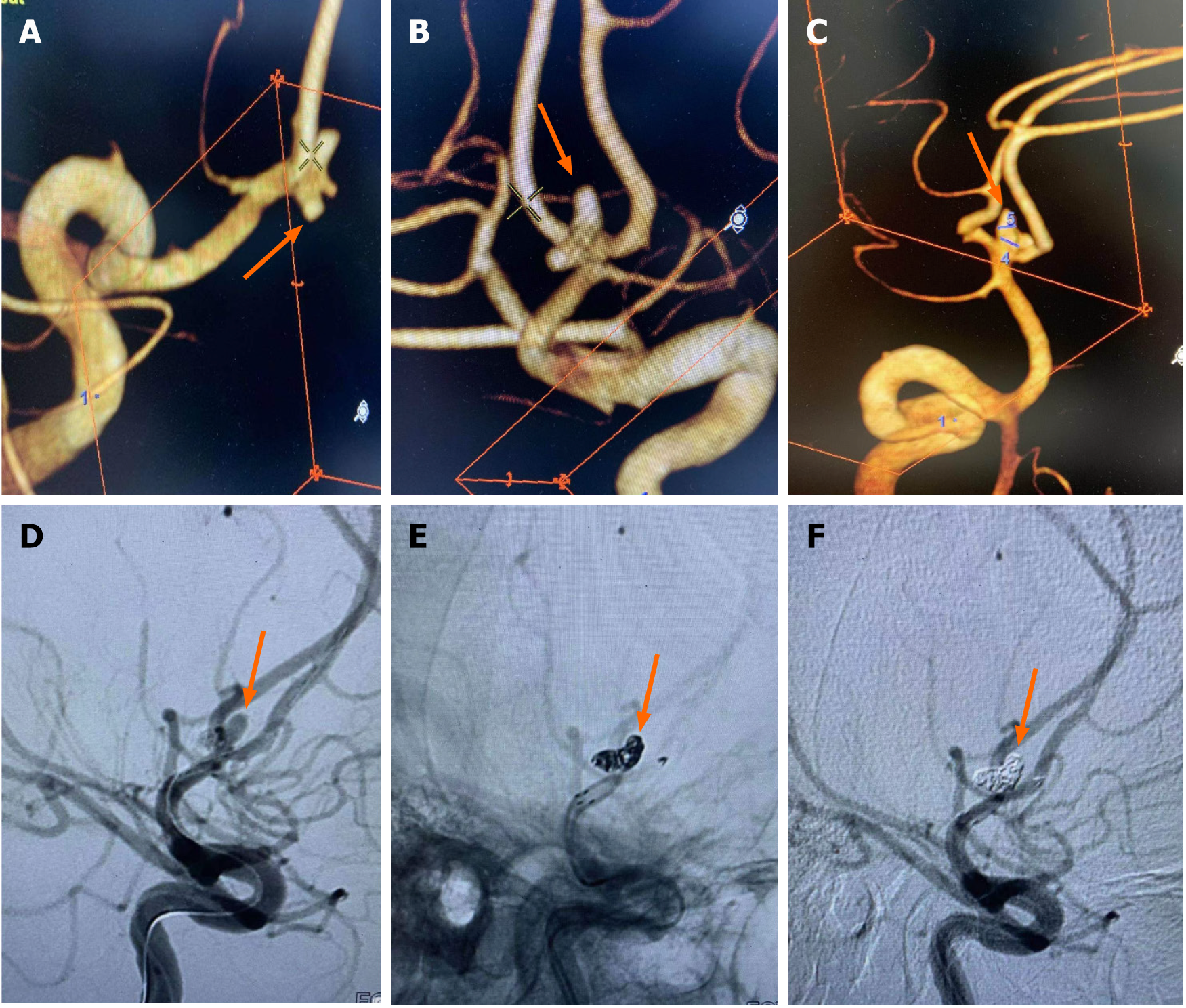
Case 5, a 43-year-old female, was admitted to hospital with "sudden severe headache for 4 h". Physical examination: Drowsiness, GCS score 12 points, bilateral pupil diameter 2.5 mm, sensitive light reflex, obvious neck resistance, normal muscle strength and muscle tone of extremities, bilateral Barbinsky sign negative, HuntHess grade 3. Subarachnoid hematoma was found on emergency CT (Fisher grade 3) and ACoAA was found on CTA. In the emergency department, endovascular treatment under general anesthesia was performed. DSA showed that A1 on the left side was separated from A2 on both sides, and ACoAA was tri-lobed, with sizes of 3.6 mm × 2.8 mm, 1.1 mm × 1.2 mm and 3.2 mm × 2.5 mm, respectively, and neck 3.1 mm. The stent assisted spring coil technique was used to embolize the aneurysm. Immediately after surgery, the angiography showed that the aneurysm was completely embolized and the bilateral A2 was smooth. Intraoperative Tirofiban arterial injection + continuous intravenous pump injection for 24 h, followed by dual antiplatelet therapy, overlapping for 6 h. Postoperative dual antiplatelet therapy lasted for 3 months. DSA review 3 months later did not show aneurysm recurrence, continue to take aspirin orally. At 12 months follow-up, the patient recovered well with some dizziness (Figure 2).
In this research, we fully evaluate the lobulated aneurysm, neck and A1 segment blood vessels, to grasp the reasonable operation time, and to minimize the use of multiple or simplified systems for endovascular treatment during the operation in order to ensure surgical safety and reduce postoperative complications.
As for the formation mechanism of lobulated intracranial aneurysms, the vascular self-protective response hypothesis suggests that in the weak part of the vascular wall of an aneurysm on the verge of rupture, it will bulge out like a balloon to reduce the local stress and achieve temporary equilibrium, thus leading to the formation of daughter tumors[12]. When the tumor continues to grow and exceeds a certain shape limit, this balance is disrupted, causing the aneurysm to rupture. The rationality of this hypothesis was confirmed by the numerical simulation of hemodynamics of intracranial lobular aneurysms conducted by researchers[13]. It can be seen that lobular aneurysms are formed on the basis of expansion of the tumor wall, which is originally weaker than the normal blood vessel wall, and are therefore more prone to rupture. Previous studies have shown that aneurysms located in anterior communicating artery, ruptured aneurysms, small aneurysms and irregular aneurysms combined with daughter tumors are independent risk factors for rupture during embolization of intracranial aneurysms[14-16]. Therefore, the endovascular treatment of ruptured lobulated ACoAA is more difficult.
In terms of surgical timing, lobular rupture of AcoAA is an irregular aneurysm with thinner wall, which is prone to re-rupture and bleeding. Therefore, etiological treatment should be carried out as soon as possible after medical treatment such as blood pressure control, sedation and anti-vasospasm. All patients were given intravascular therapy within 3 d. According to literature reports, the probability of rebleeding within the first 3 d after intracranial aneurysm rupture and hemorrhage is 14%, and cerebral vasospasm may occur after 3 d. Therefore, "3H" therapy can be started as soon as possible after endovascular treatment of aneurysms, which has a positive effect on the prevention and treatment of cerebral vasospasm[17]. In this group of cases, there was no adverse outcome caused by aneurysm re-rupture and hemorrhage and severe cerebral vasospasm.
In the interventional treatment of ruptured lobulated AcoAA, the embolization system often requires better support in path establishment because the aneurysm is located at a distant part of the anterior circulation, and some cases are complicated with aortic arch variation, arteriosclerosis or stenosis and tortuosity, resulting in difficulties in the placement of the access catheter. In this group of patients, all use long sheath + intermediate catheter to establish access. Long sheath can provide reliable support, and the distal end of the intermediate catheter is soft, which can reach a further position. The geometric shape and many anatomic variations of anterior cerebral artery A1 will affect the therapeutic effect of interventional surgery, and the dysplasia of segment A1 is an independent predictor of poor functional outcome after ACoAA endovascular therapy[18-20]. In this group, 22 cases were missing anterior cerebral artery A 1 segment on one side, and anterior cerebral artery A 1 segment on the other side was separated from bilateral A 2. Bilateral A1 was present in only 2 cases, but the bilateral A2 was asymmetrical, with one side being thinner. Once vasospasm was combined (4 cases in this group were combined with vasospasm of stage A 1, of which 1 did not improve after continuous nimodipine pumping treatment), multiple systems often could not be successfully put in place, or even could not accommodate multiple systems. In addition, when multiple systems are used, on the one hand, blood vessels may be stimulated and spasm may be aggravated, and prolonged surgical operation may affect distal blood flow; On the other hand, the interference of multiple systems can increase the difficulty and risk of surgery. Therefore, according to the number, size, shape, tip orientation and neck width of aneurysm lobes, this group of patients did not use multiple systems or simplified systems to perform operations, and the postoperative functional outcome rate was as high as 87.5%.
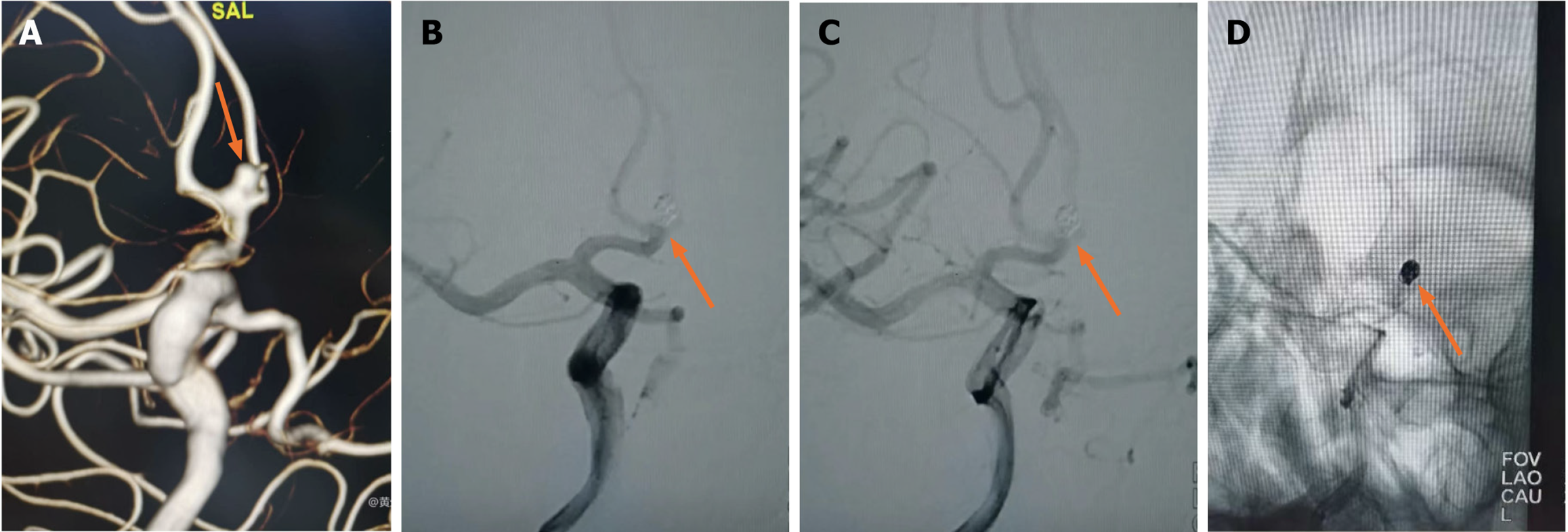
In this group of cases, when the ACoAA was accompanied by a daughter tumor on the top of the same long axis as the mother tumor, such as the daughter tumor < 1 mm (Figure 3), a single microcatheter was used to place in the mother tumor under the guidance of the guide wire, and the tension should be released at all times during the operation. The daughter tumor was generally the location of the aneurysm rupture, and the guide wire should not be touched. It is not necessary to deliberately plug the tumor during the filling process. If one or two spring rings are attached to the tumor, it is best, but the mother tumor and tumor neck must be packed tightly, and the tumor should not be visible during angiography. If the daughter tumor is larger than 1 mm (Figure 4), the embolization is performed using a single or double embolized microcatheter, but the microcatheter must reach the neck of the daughter tumor or enter the daughter tumor body under the guidance of a guide wire. A spring coil adapted to the size of the daughter tumor is selected for embolization. When the microcatheter placed in the daughter tumor retreated to the mother tumor due to "kicking tube", the mother tumor was further filled with dense.
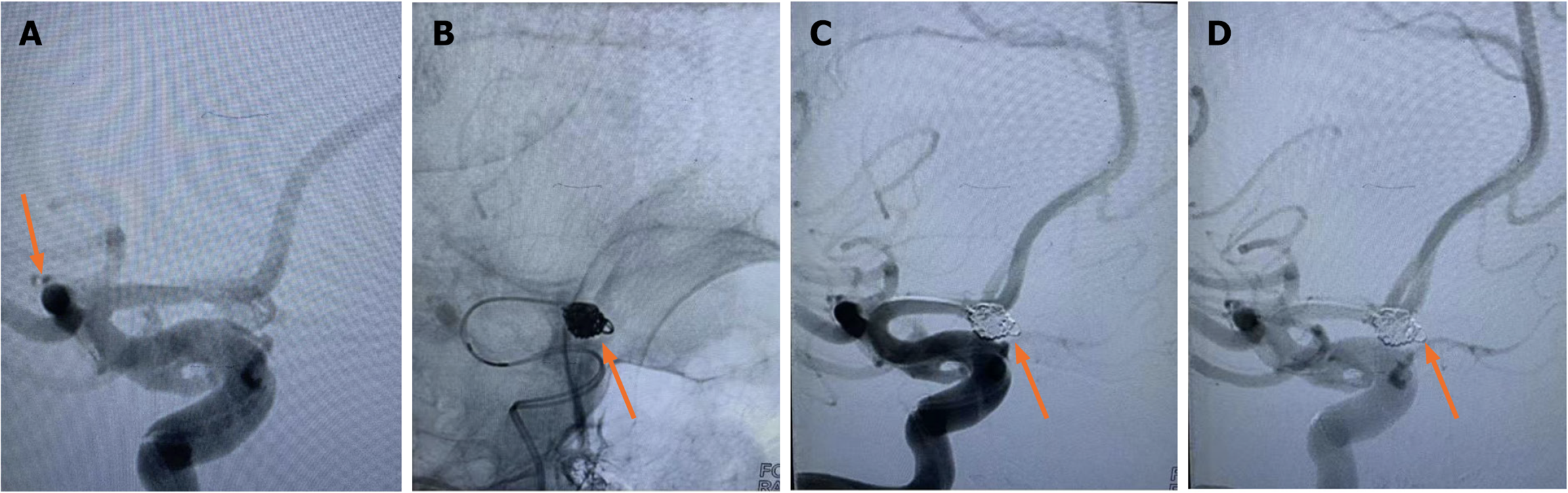
Although researchers[21] have reported the feasibility and safety of embolizing a variety of intracranial complex lobular ruptured aneurysms with a single microcatheter partition basket technique, the shaping of the microcatheter head is required to be accurate, and the switching position between each lobular aneurysm must be in place. Failure to meet the above requirements is likely to lead to surgical failure. Other researchers[22] have used double microcatheter technology to treat lobular aneurysms and achieved reliable efficacy. By reducing the utilization rate of stents, the use of postoperative dual antiplatelet drugs has been reduced, thus reducing the risk of rebleeding. In this group of cases, when the lobule and the mother tumor were similar in size and not in the same long axis (Figure 1), since the direction of each lobule could not complete the operation at the same working Angle, the double microcatheter was placed in place in different lobules under different working angles, and each lobule was alternately embolized in the double microtubule, and the embolization sequence was flexibly applied. When the embolization reached the common neck of the tumor between lobules, Due to the mutual winding and lateral extrusion between the spring rings, the stability of the spring rings can be achieved even in the relatively wide neck, and the use of the support is reduced. When the double-lobular (Figure 5) and triple-lobular (Figure 2) aneurysms needed stent assistance for embolization due to their wide neck, the stent catheter was placed at a distance of A2 under the guidance of the micro-guide wire. The embolized microcatheter was shaped according to the aneurysm loaves, guided by the micro-guide wire to one of the loaves, and the spring coil was released several times under the semi-release state of the stent. Then the stent is released completely, another embolization microcatheter is inserted through the stent mesh, and the lobules pointing forward are further filled, and finally the aneurysm is completely embolized. When using this technique, it is necessary to consider which lobe of the microcatheter is placed first. Progressive neurological impairment and limb weakness at stroke onset are independent predictors of very early death in patients with nontraumatic subarachnoid hemorrhage. These results further emphasize the need to establish early etiological diagnosis and active management of these patients, including early surgery in selected cases[23]. Our experience has been to put in place lobules with a high probability of rupture, such as special morphologic or combined daughter tumors; if it is difficult to determine which lobe is more likely to rupture, select a lobe that is not easy to get through the stent mesh in place.
The main limitation of this study is that the sample size is small and the retrospective analysis is difficult to avoid selection bias. In particular, there were no large aneurysms (diameter > 10 mm but ≤ 25 mm) or large aneurysms (diameter > 25 mm) in this group of patients, so whether this concept is safe and effective for intravascular therapy needs further study.
In our further experiments, we would include more patient cases and large aneurysms patients in our experiments to verify our present results. And more experiment on the onset mechanism and basic theories would contributes to the understanding of ACoAA.
In summary, for ruptured lobulated ACoAA, preoperative angiography was used to fully evaluate the lobulated aneurysm, neck of the tumor and segment A and 1 vessel, to grasp reasonable operation timing, and to minimize the use of multiple or simplified systems for endovascular treatment during the operation, which was conducive to surgical safety and to reduce postoperative complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neuroimaging
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade C
Scientific Significance: Grade B, Grade B,
P-Reviewer: Arboix A, Spain S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Forbes G, Fox AJ, Huston J 3rd, Wiebers DO, Torner J. Interobserver variability in angiographic measurement and morphologic characterization of intracranial aneurysms: a report from the International Study of Unruptured Intracranial Aneurysms. AJNR Am J Neuroradiol. 1996;17:1407-1415. [PubMed] |
| 2. | Goertz L, Kasuya H, Hamisch C, Kabbasch C, von Spreckelsen N, Ludyga D, Timmer M, Stavrinou P, Goldbrunner R, Brinker G, Krischek B. Impact of aneurysm shape on morbidity after clipping of unruptured intracranial aneurysms. Acta Neurochir (Wien). 2018;160:2169-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Goertz L, Hamisch C, Pflaeging M, Kabbasch C, Borggrefe J, Timmer M, Stravrinou P, Goldbrunner R, Brinker G, Mpotsaris A, Krischek B. Angiographic Characteristics of Lobulated Intracranial Aneurysms. World Neurosurg. 2019;131:e353-e361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Borggrefe J, Behme D, Mpotsaris A, Weber W. Complications Associated with Cerebral Aneurysm Morphology in Balloon-Assisted Coil Embolization of Ruptured and Unruptured Aneurysms-a Single-Center Analysis of 116 Consecutive Cases. World Neurosurg. 2016;91:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Matsukawa H, Kamiyama H, Miyazaki T, Kinoshita Y, Noda K, Ota N, Saito N, Takeda R, Tokuda S, Tanikawa R. Impacts of a Size Ratio on Outcome in Patients with Surgically Treated Unruptured Nondissecting Anterior Cerebral Artery Aneurysms. World Neurosurg. 2018;111:e250-e260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Bender MT, Wendt H, Monarch T, Lin LM, Jiang B, Huang J, Coon AL, Tamargo RJ, Colby GP. Shifting Treatment Paradigms for Ruptured Aneurysms from Open Surgery to Endovascular Therapy Over 25 Years. World Neurosurg. 2017;106:919-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Chiaradio JC, Guzman L, Padilla L, Chiaradio MP. Intravascular graft stent treatment of a ruptured fusiform dissecting aneurysm of the intracranial vertebral artery: technical case report. Neurosurgery. 2002;50:213-6; discussion 216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Arboix A, García-Eroles L, Sellarés N, Raga A, Oliveres M, Massons J. Infarction in the territory of the anterior cerebral artery: clinical study of 51 patients. BMC Neurol. 2009;9:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Abboud T, Rustom J, Bester M, Czorlich P, Vittorazzi E, Pinnschmidt HO, Westphal M, Regelsberger J. Morphology of Ruptured and Unruptured Intracranial Aneurysms. World Neurosurg. 2017;99:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Mayer TE. The unruptured intracranial aneurysm treatment score: A multidisciplinary consensus. Neurology. 2016;86:792-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, Lamoureux J, Chagnon M, Roy D. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1123] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 12. | Meng H, Feng Y, Woodward SH, Bendok BR, Hanel RA, Guterman LR, Hopkins LN. Mathematical model of the rupture mechanism of intracranial saccular aneurysms through daughter aneurysm formation and growth. Neurol Res. 2005;27:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Oliveira IL, Santos GB, Gasche JL, Militzer J, Baccin CE. Non-Newtonian Blood Modeling in Intracranial Aneurysm Hemodynamics: Impact on the Wall Shear Stress and Oscillatory Shear Index Metrics for Ruptured and Unruptured Cases. J Biomech Eng. 2021;143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Liu P, Lv X, Li Y, Lv M. Stent-assisted coiling of ruptured wide-necked intracranial aneurysms: A single-center experience of 218 consecutive patients. Neurol India. 2016;64 Suppl:S70-S77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Li L, Zhang X, Feng Z, Zhao R, Hong B, Xu Y, Huang Q, Liu J. Risk Factors for Intraprocedural Rupture in the Endovascular Treatment of Unruptured Intracranial Aneurysms: A Single-Center Experience with 1232 Procedures. World Neurosurg. 2019;123:e9-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Park YK, Yi HJ, Choi KS, Lee YJ, Chun HJ. Intraprocedural Rupture During Endovascular Treatment of Intracranial Aneurysm: Clinical Results and Literature Review. World Neurosurg. 2018;114:e605-e615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Egashira Y, Yoshimura S, Enomoto Y, Ishiguro M, Asano T, Iwama T. Ultra-early endovascular embolization of ruptured cerebral aneurysm and the increased risk of hematoma growth unrelated to aneurysmal rebleeding. J Neurosurg. 2013;118:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Ye J, Zheng P, Hassan M, Jiang S, Zheng J. Relationship of the angle between the A1 and A2 segments of the anterior cerebral artery with formation and rupture of anterior communicating artery aneurysm. J Neurol Sci. 2017;375:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Jabbarli R, Reinhard M, Roelz R, Kaier K, Weyerbrock A, Taschner C, Scheiwe C, Shah M. Clinical relevance of anterior cerebral artery asymmetry in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;127:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Yang F, Li H, Wu J, Li M, Chen X, Jiang P, Li Z, Cao Y, Wang S. Relationship of A1 Segment Hypoplasia with the Radiologic and Clinical Outcomes of Surgical Clipping of Anterior Communicating Artery Aneurysms. World Neurosurg. 2017;106:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Feng X, Peng F, Miao Z, Tong X, Niu H, Zhang B, Wang L, Guo E, Qi P, Lu J, Mo D, Wu Z, Wang D, Liu A. Procedural Complications and Factors Influencing Immediate Angiographic Results after Endovascular Treatment of Small (<5 mm) Ruptured Intracranial Aneurysms. J Stroke Cerebrovasc Dis. 2020;29:104624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Uchikawa Y, Mori K, Shiigai M, Konishi T, Hoshiai S, Ishigro T, Hiyama T, Nakai Y, Minami M. Double Coaxial Microcatheter Technique for Glue Embolization of Renal Arteriovenous Malformations. Cardiovasc Intervent Radiol. 2015;38:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Arboix A, Martí-Vilalta JL. Predictive clinical factors of very early in-hospital mortality in subarachnoid hemorrhage. Clin Neurol Neurosurg. 1999;101:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |