Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2499
Revised: April 6, 2024
Accepted: April 15, 2024
Published online: May 26, 2024
Processing time: 155 Days and 20.4 Hours
Bone metastasis has various negative impacts. Activities of daily living (ADL) and quality of life (QOL) can be significantly decreased, survival may be impacted, and medical expenses may increase. It is estimated that at least 5% cancer patients might be suffering from bone metastases. In 2016, we published the Comprehensive Guidelines for the Diagnosis and Treatment of Bone Metastasis. Since then, the therapeutic outcomes for patients have gradually improved. As life expectancy is a major determinant of surgical intervention, the strategy should be modified if the prolongation of survival is to be achieved.
To monitor how bone metastasis treatment has changed before and after launch of our guidelines for bone metastasis.
For advanced cancer patients with bone metastasis who visited the Department of Clinical Oncology at Akita University hospital between 2012 and 2023, parameters including the site and number of bone metastases, laboratory data, and survival time, were extracted from electronic medical records and the Katagiri score was calculated. The association with survival was determined for each factor.
Data from 136 patients were obtained. The 1-year survival rate for the poor prognosis group with a higher Katagiri score was 20.0% in this study, which was 6% and an apparent improvement from 2014 when the scoring system was developed. Other factors significantly affecting survival included five or more bone metastases than less (P = 0.0080), and treatment with chemotherapy (P < 0.001), bone modifying agents (P = 0.0175) and immune checkpoint inhibitors (P = 0.0128). In recent years, advances in various treatment methods have extended the survival period for patients with advanced cancer. It is necessary not only to simply extend survival time, but also to maintain ADL and improve QOL.
Various therapeutic interventions including surgical approach for bone metastasis, which is a disorder of locomotor organs, are increasingly required. Guidelines and scoring system for prognosis need to be revised promptly.
Core Tip: We investigated bone metastasis patients from 2012 to 2023 at Department of Clinical Oncology, Akita University Hospital to learn about recent trends in treatment methods and outcomes for patients with bone metastases. The prognosis of patients with bone metastases has improved recently. Now, there is a room for surgical interventions to the patients with bone metastases, which have been avoided due to their limited prognosis. These are expected to improve activities of daily living and quality of life and will benefit patients. Accordingly, guidelines and scoring system for prognosis need to be revised.
- Citation: Matsuda K, Shimazu K, Shinozaki H, Fukuda K, Yoshida T, Taguchi D, Nomura K, Shibata H. Recent trends in bone metastasis treatments: A historical comparison using the new Katagiri score system. World J Clin Cases 2024; 12(15): 2499-2505
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2499.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2499
Bone metastasis has various negative impacts. Activities of daily living (ADL) and quality of life (QOL) may be significantly decreased, survival may be impacted, and medical expenses may increase. Based on the SEER database, 113317 of 2470634 cancer patients in the United States from 2010 to 2016 experienced bone metastases[1].
Treatment of bone metastasis includes orthopedic surgery, radiotherapy, drug treatment including bone modifying agents (BMAs) and palliative care[2]. BMAs can suppress skeletal related events (SREs) composed of pathologic fracture, spinal cord compression, radiation or surgery to bone, and hypercalcemia of malignancy[2]. In recent years, the adjunctive treatments such as radiofrequency ablation are considered. Additionally, carrier-mediated drug delivery, bone substitutes to repair bone defects, and multifunctional scaffolds with bone tissue regeneration and antitumor properties are investigated[3]. In recent years, immune check point inhibitors (ICIs) have been used to treat various cancers[4]. There is a remarkable efficacy of ICIs in the treatment of tumors.
A large number of clinical trials reveal that ICIs have promising therapeutic effects, including overall survival (OS), objective effective rate and progression-free survival[4]. Despite the potential to improve cancer outcomes with use of ICIs, bone metastases remain may have different responses to ICIs than other disease sites[5]. The effects of ICI on bone metastasis should be understood, including elucidation of the bone microenvironment. Palliative care is given not only to relieve pain, but to provide holistic support, so that the QOL of patients can be improved[2].
Various scoring systems that determine the choice of intervention have also been developed[2]. The new Katagiri scoring system (NKSS), which was developed in 2014, is very good tool to predict the life expectancy of bone metastatic patients[6]. The NKSS consists of six items, including: (1) The characteristics of primary cancers; (2) The existence of visceral, brain, and peritoneal dissemination; (3) Laboratory data; (4) Eastern Cooperative Oncology Group performance status (PS); (5) History of chemotherapy; and (6) The existence of multiple bone metastasis. Scores range from 0 to 10[6]. In the NKSS, the survival rate was 27% at 6 months, and 6% at 1 year with an overall prognostic score of ≥ 7[6]. Based on the Japanese comprehensive guidelines for diagnosis and treatment of bone metastasis, published in 2016, surgery is not recommended when prognosis is predicted within 6 months. The prognosis is gradually improving, for example, the 5-year survival rate improved by 2% each year from 2015 to 2019 in the United States[7]. It is also necessary to consider the impact on the scoring system from changes in treatment regimens, such as approval of the ICIs. Therefore, we examined the factors impacting cancer patients with bone metastases from 2012 to 2023.
We retrospectively examined the clinical status of patients with bone metastases who underwent treatment at the Department of Clinical Oncology at Akita University hospital from December 2010 to March 2023.
Patient data included gender, age, site and number of bone metastases, site of primary tumor, metastasis other than bone, symptoms, opportunity for diagnosis, treatment history including radiotherapy, anticancer drugs, opioids, BMA and ICIs was extracted from their electronic medical records. Laboratory data at their visit included serum levels of C-reactive protein, lactate dehydrogenase, total bilirubin, calcium, and the number of platelets. Information regarding PS and ADL were also extracted from the medical records. Furthermore, the patient’s life and death were examined as well as their survival time from the day visiting our department, and a score was calculated by the NKSS.
Statistical analyses were performed using BellCureve for Excel Ver 4.04 (Social Survey Research Information Co., Ltd., Japan).
All procedures were conducted according to the Declaration of Helsinki.
For 12 years and 4 months, 136 patients were enrolled in this study (Table 1). This number includes 13% of the patients presenting to the Department of Clinical Oncology. Of these, 86 were male (63.2%). Ages ranged from 24 to 84 years, with a median age of 64 years. The primary sites of bone metastases are listed in Table 1. Cancer of unknown primary (CUP) [n = 31 (22.8%)] was the most common, followed by gastric cancer [n = 24 (17.6%)], colorectal cancer, [n = 21 (15.4%)] sarcoma [n = 10 (7.4%)], and pancreatic cancer [n = 9 (6.6%)]. The MST for all cancer patients with bone metastases was 8.6 months [95% confidence interval (95%CI): 6-10 months]. MSTs by primary sites were as follows: CUP (8.6 months, 95%CI: 1-16 months), gastric cancer (6.0 months, 95%CI: 5-7 months), colorectal cancer (8.5 months, 95%CI: 6-11 months), sarcoma (9.2 months, 95%CI: 0-24 months), and pancreatic cancer (9.3 months, 95%CI: 3-16 months). The relatively short survival period may reflect the nature of the Department of Clinical Oncology, which treats very advanced cancers.
| Primary site | n | Median survival time (95%CI, month) |
| CUP | 31 | 8.6 (1-16) |
| Gastric cancer | 24 | 6.0 (5-7) |
| Colorectal cancer | 21 | 8.5 (6-11) |
| Sarcoma | 10 | 9.2 (0-24) |
| Pancreatic cancer | 9 | 9.3 (3-16) |
| NET | 7 | 4.0 (2-7) |
| Esophageal cancer | 6 | 2.9 (1-4) |
| Skin cancer | 4 | 34.7 (4-66) |
| Thymic cancer | 3 | 14.0 (14-14) |
| Hematologic malignancy | 3 | Not reached |
| Urological cancer | 3 | 8.1 (0-17) |
| Others | 6 | 6.4 (3-10) |
| Overall | 136 | 8.1 (6-10) |
The frequency of bone metastases was as follows: single (n = 33, 24.3%), 2 or more and 4 or less (n = 44, 32.4%), and 5 or more (n = 58, 42.6%). The frequency of bone metastasis by site is shown in Supplementary Table 1. Metastases to the spine were the most frequent (n = 97, 71%) and the frequencies were as follows: cervical spine (n = 28, 21%), thoracic spine (n = 71, 52%), and lumbar spine (n = 64, 47%). Metastases to the pelvic bones, including the sacrum, ilium, and pubis were the next most frequent (n = 72, 53%). Metastases to other bones including ribs, scapula, and long bones, such as the femur, were third (n = 67, 49%).
Metastases were found in 96 cases by diagnostic imaging (70.5%) (Supplementary Table 2). Of the 136 patients, 16.9% had pain, and 5.1% had neurological symptoms, whereas 2.9% had bone symptoms, such as a fracture and 2.9% had elevated serum alkaline phosphatase levels (Supplementary Table 2). Of 136 cases, 70% were asymptomatic.
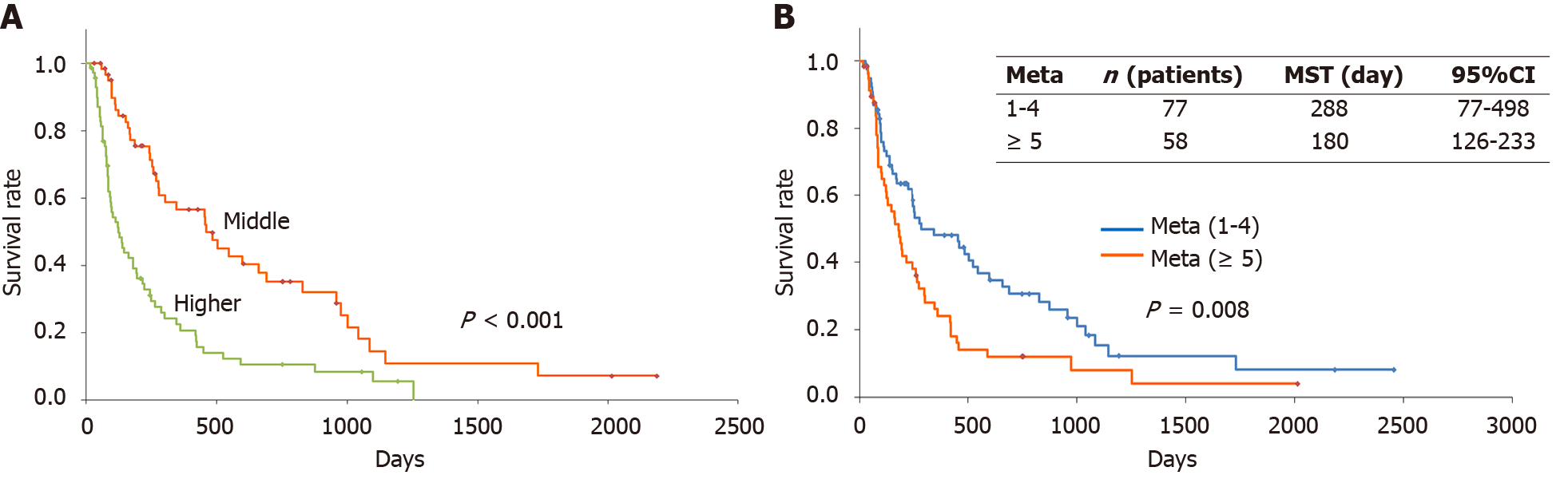
Calculation by NKSS calculations were conducted on the visiting day. Of the 136 patients, only 3 patients (2.2%) scored 0-3 (lower score), 62 patients (45.6%) scored 4-6 (middle score), and 71 patients (52.2%) scored 7-10 (higher score) (Figure 1A). The survival of the middle-score group (MSG) and the higher-score group (HSG) was examined. The 1-year survival rate was 56% for the MSG (Figure 1A and Table 2). This was similar to that of the original paper, which reported 49.3%. The 1-year survival rate was 20.0% in the HSG (Figure 1A and Table 2). This was greater than three times in the original paper, which was 6.0% (Figure 1A and Table 2). In this study, the MST of the MSG (461 d) was significantly longer compared with that of the HSG (124 d, P < 0.001).
| NKSS | n | 1-yr-survival rate in this study (2010-2023) (95%CI) | 1-yr-survival rate in the original study (2005-2008) (95%CI) |
| 0-3 | 3 | - | 91.4 (85.9–96.9) |
| 4-6 | 62 | 56 (43-70) | 49.3 (44.0-54.6) |
| 7-10 | 71 | 20 (10-31) | 6.0 (3.5–8.5) |
Survival with and without symptoms of bone metastasis were compared. The MST was 192 d with symptoms and 254 d without (Supplementary Figure 1). There was no significant difference (P = 0.4794). Bone metastases symptoms may not affect the survival of patients.
Next, we examined the effect of the number of bone metastases on survival. The MSTs for patients with ≤ 4 oligo-metastases were 288 d (Figure 1B). However, the MST of patients with at least 5 bone metastases was 180 d (Figure 1B). There was a significant survival difference between ≥ 5 multiple metastases and ≤ 4 oligo-metastases (P = 0.0080) (Figure 1B). This indicates that ≥ 5 bone metastases may negatively impact survival.
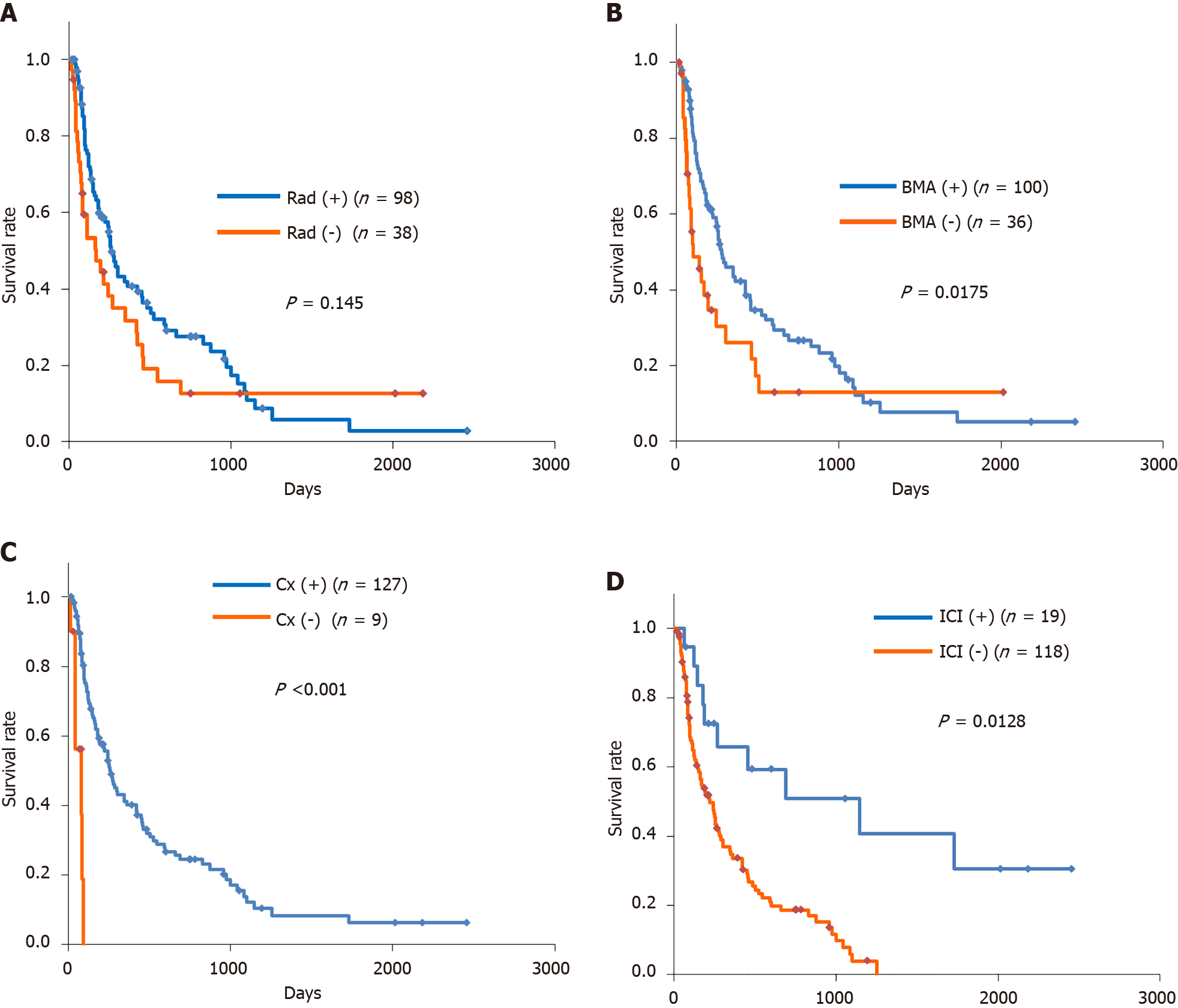
Of the 136 patients with bone metastases, 72.1% received radiotherapy. The MST was 263 d with radiotherapy and 168 d without (Figure 2A). There was no significant difference between the two groups (P = 0.145) (Figure 2A). The main purpose of radiotherapy is to relief pain, not necessarily to prolong survival. Of the 137 patients, 92.6% received chemotherapy. The MST was 263 d with chemotherapy and 82 d without (P < 0.001) (Figure 2B). Chemotherapy for advanced cancer, if the patient was acceptable, may represent longer survival. Of the 136 patients, 73.5% received BMA. The MST was 276 d with BMA and 99 d without (P = 0.0175) (Figure 2C). The purpose of using BMAs is to prevent the onset of SREs[2], and as a result, it is expected that the QOL and ADL of the patients with bone metastases will be maintained. Improvements in the QOL and ADL of patients may indirectly contribute to prolonging survival. Of the 136 patients, 14.0% received ICIs. The MST was 1146 d with ICIs and 222 d without (P = 0.0128) (Figure 2D). ICIs may have some survival benefits on the patients with bone metastases.
The 1-year survival rate for the MSG was almost the same as the original data, but it was not the same in the HSG, which was 6.0% and 20.0% in the original study and this study, respectively[6]. It is important to identify patients with bone metastasis and predict their survival rate. This information can guide the subsequent treatment and management. The predictive systems should be updated promptly. This study indicates the necessity of updating. Scoring system such as NKSS is used the most commonly, but in the future, machine learning might be preferred because of its innovation and accuracy[8]. It was suggested that chemotherapy, BMA and ICIs may contribute to prolonging survival. In this study, ICI was used in 8 cases (42.1%) of gastric cancer with bone metastases and 6 cases (31.6%) of lung cancer. Generally, patients with bone metastasis have a poor prognosis, so it is difficult to suggest the effectiveness of ICI. Combination therapy of nivolumab and ipilimumab for non-small cell lung cancer (NSCLC) has been shown to be more effective than platinum combination chemotherapy, regardless of the presence or absence of bone metastases[9]. The hazard ratio was higher for patients with bone metastases than those without bone metastases in spite the 95% confidential interval was slightly greater than 1.0[9]. On the other hand, the patients with NSCLC and bone metastases who received ICIs had a higher hazard of death[10]. In the pivotal trial of ICIs for gastric cancer, there is no description of bone metastasis[11]. The ICIs prolong survival in malignant tumors with bone metastasis in CheckMate 025, a randomized phase 3 trial of nivolumab vs everolimus in previously treated advanced renal cell carcinoma, in which a subgroup analysis revealed that median OS in patients with bone metastases was 18.5 months with nivolumab vs 13.8 months with everolimus[12]. Thus, ICIs might prolong the survival time of patients with bone metastasis, although it was not significant[12]. The effect on bone metastasis with ICIs is still debatable. The generalizability of our study might be limited as this study is retrospective from a single institute.
Currently, orthopedic interventions are largely inappropriate if the expected survival time is 6 months or less[2]. In this study, the 6-month survival rate of the HSG was 75.3% (Figure 1A). Much more patients in the HSG may have a chance of benefiting from orthopedic treatment. Recently, patient surveillance studies indicated that many patients desire survival with preserved ADL rather than simply prolonging survival[13]. It is very important to keep patients’ ADL with bone metastases by various interventions. This study is a retrospective survey at one facility and there were some limitations. It is necessary to expand the scale of such kinds of surveillance to verify the results in the future.
In recent years, advances in various treatment methods have extended the survival period for patients with advanced cancer. It is necessary not only to simply extend survival time, but also to maintain ADL and improve QOL. For this purpose, various therapeutic interventions including surgical approach for bone metastasis, which is a disorder of locomotor organs, are increasingly required. Scoring system for the individual patient prognosis should be updated promptly because this system is the starting point for the treatment of bone metastasis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Liu J, China S-Editor: Zheng XM L-Editor: A P-Editor: Li X
| 1. | Huang JF, Shen J, Li X, Rengan R, Silvestris N, Wang M, Derosa L, Zheng X, Belli A, Zhang XL, Li YM, Wu A. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann Transl Med. 2020;8:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Shibata H, Kato S, Sekine I, Abe K, Araki N, Iguchi H, Izumi T, Inaba Y, Osaka I, Kawai A, Kinuya S, Kodaira M, Kobayashi E, Kobayashi T, Sato J, Shinohara N, Takahashi S, Takamatsu Y, Takayama K, Tateishi U, Nagakura H, Hosaka M, Morioka H, Moriya T, Yuasa T, Yurikusa T, Yomiya K, Yoshida M. Diagnosis and treatment of bone metastasis: comprehensive guideline of the Japanese Society of Medical Oncology, Japanese Orthopedic Association, Japanese Urological Association, and Japanese Society for Radiation Oncology. ESMO Open. 2016;1:e000037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Bădilă AE, Rădulescu DM, Niculescu AG, Grumezescu AM, Rădulescu M, Rădulescu AR. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers (Basel). 2021;13:4229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L, Wang S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 2022;13:964442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 294] [Reference Citation Analysis (0)] |
| 5. | Wytiaz V, Van Poznak C. Checkpoint Inhibitors in Bone Metastatic Disease in Solid Tumors. Curr Osteoporos Rep. 2023;21:323-329. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Katagiri H, Okada R, Takagi T, Takahashi M, Murata H, Harada H, Nishimura T, Asakura H, Ogawa H. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11438] [Article Influence: 3812.7] [Reference Citation Analysis (4)] |
| 8. | Zhong X, Lin Y, Zhang W, Bi Q. Predicting diagnosis and survival of bone metastasis in breast cancer using machine learning. Sci Rep. 2023;13:18301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 9. | Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O'Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2019;381:2020-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1978] [Article Influence: 329.7] [Reference Citation Analysis (0)] |
| 10. | Qin A, Zhao S, Miah A, Wei L, Patel S, Johns A, Grogan M, Bertino EM, He K, Shields PG, Kalemkerian GP, Gadgeel SM, Ramnath N, Schneider BJ, Hassan KA, Szerlip N, Chopra Z, Journey S, Waninger J, Spakowicz D, Carbone DP, Presley CJ, Otterson GA, Green MD, Owen DH. Bone Metastases, Skeletal-Related Events, and Survival in Patients With Metastatic Non-Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. J Natl Compr Canc Netw. 2021;19:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1716] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 12. | Escudier B, Sharma P, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Gurney H, Donskov F, Peltola K, Wagstaff J, Gauler TC, Ueda T, Zhao H, Waxman IM, Motzer RJ; CheckMate 025 investigators. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab Versus Everolimus in Advanced Renal Cell Carcinoma. Eur Urol. 2017;72:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 13. | Hamano J, Hiratsuka Y, Morita T, Kizawa Y, Maeda I, Mori M. Preference of Japanese cancer patients for being informed about their prognosis. Ann Palliat Med. 2022;11:3426-3435. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |