Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2445
Peer-review started: February 7, 2024
First decision: March 2, 2024
Revised: March 8, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: May 16, 2024
Processing time: 88 Days and 5.1 Hours
We report a rare case of primary clinical presentation featuring elevated creatine kinase (CK) levels in a neonate, which is associated with the LAMA2 gene. In this case, a heterozygous mutation in exon5 of the LAMA2 gene, c.715C>G (resulting in a change of nucleotide number 715 in the coding region from cytosine to gua
We analysed the case of a neonate presenting solely with elevated CK levels who was eventually discharged after supportive treatment. The chief complaint was identification of increased CK levels for 15 d and higher CK values for 1 d. Ad
Mutations in LAMA2 are associated with the clinical phenotype of increased neonatal CK levels, for which no specific treatment exists. Whole genome sequen
Core Tip: We analyzed the case of a neonate who appeared with only elevated creatine kinase (CK) and eventually was discharged after supportive treatment. The age of admission was 18 d, and the increased CK did not improve significantly after prolonged treatment with creatine and vitamin C. Whole exome sequencing identified the mutation of c.715C>G on LAMA2 in the newborn, which is associated with clinical phenotype.
- Citation: Yuan J, Yan XM. Detection of LAMA2 c.715C>G:p.R239G mutation in a newborn with raised creatine kinase: A case report. World J Clin Cases 2024; 12(14): 2445-2450
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2445.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2445
Creatine kinase (CK), a biomarker of muscle damage, fluctuates within a certain reference range, 40-320 IU/L and 25-200 IU/L for men and women, respectively. Increased CK may be related to muscle damage[1] and it may be asymptomatic[2]. A few similar reports have been found worldwide, and previous reports have shown that increased CK levels in newborns are related to genetic variations. In this article, we describe the clinical manifestations, diagnosis, and treatment of a newborn with increased CK values associated with LAMA2 gene mutation. The newborn was diagnosed in the term ward of the Children's Hospital of Soochow University. This study aimed to improve our understanding of this disease and advocated that neonatal clinicians should be aware of the serious potential problem of abnormally elevated CK in newborns shortly after birth.
Identification of increased CK levels for 15 d and higher CK values for 1 d.
The neonate was admitted to Changshu First People's Hospital for 8 d post-birth due to "perinatal infection". During this period, CK value were found to be elevated, peaking at 1348 U/L, with no specific treatment administered. After dis
The neonate, born at Changshu First People's Hospital on June 14, 2023, via eutocia at a gestational age 39 wk and mother being G2P2, had a birth weight 3700 g and recorded Apgar scores of 10 at both 1 min and 5 min post-birth. Amniotic fluid was clear, with a normal quantity, and no history of rescue suffocation was noted.
However, after birth, the neonate was hospitalised at Changshu First People's Hospital for 9 d due to perinatal infec
The mother was diagnosed with hypothyroidism and gestational hypertension at 11 and 26 wk gestation, respectively. Group B strep was a potential concern at 36 wk gestation, while the parents of the newborn had no significant medical history, were physically healthy, and were not close relatives.
Upon examination, the newborn displayed a body temperature of 36.5 °C, a pulse rate of 170 beats/min, a respiratory rate of 40 breaths/min, and an oxygen pulse of 99% under hood oxygen. Physical measurements included a body weight of 4.04 kg, a length of 52 cm, a head circumference of 35 cm, and a chest circumference of 34 cm. The newborn exhibited a full term appearance with a ruddy complexion, a flat anterior fontanel, measuring about 1.5 cm × 1.5 cm, and soft neck. The newborn experienced no shortness of breath, no obvious inspiratory trifossa sign, the lungs indicated rough brea
Peripheral blood examination revealed a CK value 2155.10 U/L. Myocardial enzyme levels indicated CK-MB at 7.70 ng/mL, troponin-T 42.81 ng/mL, myoglobin < 21 ng/mL. White blood cell count 9.77 × 109/L, red blood cell count 4.08 × 1012/L, hemoglobin 138 g/L, platelet distribution width 12.90%, and blood gas analysis showed Na+ 134 mmol/L, Ca2+ 1.15 mmol/L, Cl- 102 mmol/L, base excess 1.9 mmol/L, lactic acid 1.60 mmol/L, blood pH 7.496, oxyhemoglobin saturation 99.10%, oxygen partial pressure 119.0 mmHg, carbon dioxide partial pressure 31.70 mmHg, aspartic transaminase 42.30 U/L, alanine aminotransferase 44.10 U/L, total bilirubin 131.30 μmol/L, direct bilirubin 12.60 μmol/L, indirect bilirubin 118.70 μmol/L. Triiodothyronine, thyroxine, thyroid-stimulating hormone, free triiodothyronine, and free thyroxine levels showed no obvious abnormalities, and inflammatory markers showed no significant increases. Newborn screening results were unremarkable.
Chest and abdominal imaging demonstrated an increase and blurring of the lung texture alongside changes indicative of aright clavicle fracture. Echocardiography suggested atrial septal defect, categorised as a secundum type with a size of 3.1 mm.
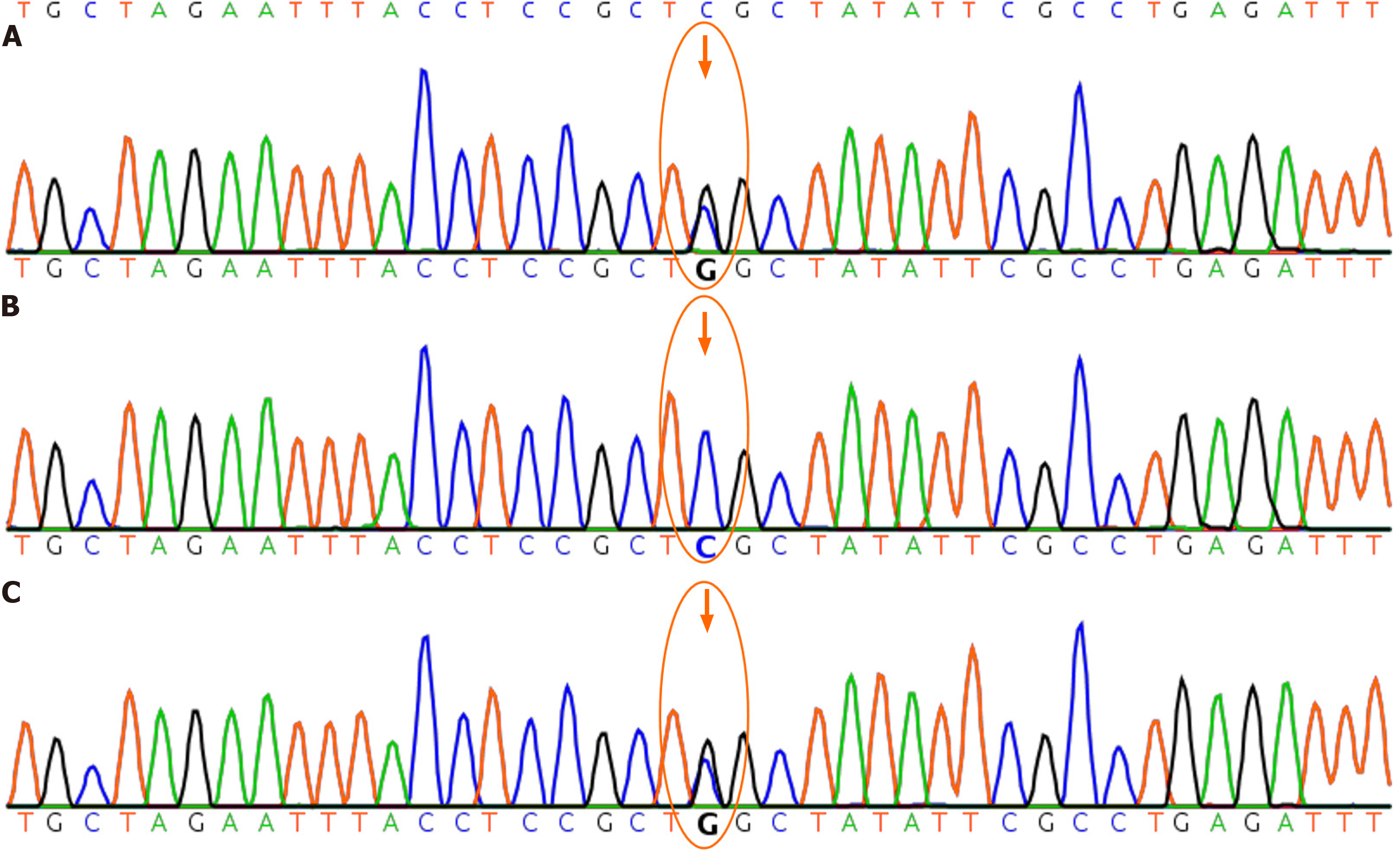
The patient (chr6:129465121) showed a heterozygous mutation of c.715C>G in the gene LAMA2; while the father (chr
Elevated CK levels; perinatal infection; fracture of the collarbone due to birth injury; atrial septal defect; neonatal pneumonia.
After the newborn’s hospitalisation, CK levels continuously increased, stabilising at approximately 2000 U/L. Upon active treatment with vitamin C and creatine phosphate sodium, the CK values decreased from 2155.1 U/L to 1803.1 U/L in 15 d, and remained significantly higher than the normal values. Therefore, an investigation into CK-related genes was undertaken, resulting in the diagnosis of elevated CK levels in the neonate. Throughout the hospitalisation period, apart from the increased CK levels, no notable clinical manifestations were observed. The liver function index and bilirubin levels did not exhibit significant increases. The patient did not experience breathing difficulties, digestive system ab
Gene mutation analysis revealed a heterozygous mutation in the exon5 region of the LAMA2 gene: c.715C>G (with nucleotide 715 in the coding region changing from cytosine to guanine), resulting in the amino acid change p.R239G (No. 239), characterised as a missense mutation[3]. This change led to replacement of amino acid arginine with glycine. In
Increased CK levels in neonates can be attributed to various factors, including both physiological and pathological factors. Physiologically, elevated CK levels may arise from compression injury of the skeletal muscle and transient hy
Monitoring increased CK levels in newborns can facilitate early detection of CMD before symptom onset[5]. Notably, a nonsense mutation, c.4048C>T (p.Arg1350*) in exon 27 may contribute to severe CMD[9]. A small percentage of indi
Increased CK caused by a heterozygous mutation of the LAMA2 gene has no specific treatment and can be self-limiting. Neonatal diseases can be diagnosed early through increasingly advanced genetic diagnostics. Monitoring and discovery of resymptomatic patients during the neonatal period will contribute to clinical research and measurable treatment procedures to observe therapeutic effects. In view of this case, neonatologists should observe an unexplained increase in CK and implement pathological analysis and whole-exome sequencing to make an early conclusive diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Oley MH, Indonesia S-Editor: Zheng XM L-Editor: A P-Editor: Xu ZH
| 1. | George MD, McGill NK, Baker JF. Creatine kinase in the U.S. population: Impact of demographics, comorbidities, and body composition on the normal range. Medicine (Baltimore). 2016;95:e4344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Wiley LK, Moretz JD, Denny JC, Peterson JF, Bush WS. Phenotyping Adverse Drug Reactions: Statin-Related Myotoxicity. AMIA Jt Summits Transl Sci Proc. 2015;2015:466-470. [PubMed] |
| 3. | Robinson LJ, Sudarshan D, Goold E, Comstock J, Klonoski J, Mao Q, Palmer CA, Davidson C. A novel, pathogenic COL4A1 missense variant involving thrombotic microangiopathic leukoencephalopathy in a neonate. J Neuropathol Exp Neurol. 2023;82:880-883. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Jarman M, Mathe N, Ramazani F, Pakseresht M, Robson PJ, Johnson ST, Bell RC; APrON and ENRICH study teams. Dietary Patterns Prior to Pregnancy and Associations with Pregnancy Complications. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Takeshita S, Saito Y, Oyama Y, Watanabe Y, Ikeda A, Iai M, Sato T, Ishigaki K, Ito SI. Infection-associated decrease of serum creatine kinase levels in Fukuyama congenital muscular dystrophy. Brain Dev. 2021;43:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Bellieni CV, Tomasini B, Bracciali C, Buonocore G. Normal values of creatine kinase and of MB-creatine kinase at birth in healthy babies. Minerva Pediatr (Torino). 2023;75:21-25. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Leverenz D, Zaha O, Crofford LJ, Chung CP. Causes of creatine kinase levels greater than 1000 IU/L in patients referred to rheumatology. Clin Rheumatol. 2016;35:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Park S, Maloney B, Caggana M, Tavakoli NP. Creatine kinase-MM concentration in dried blood spots from newborns and implications for newborn screening for Duchenne muscular dystrophy. Muscle Nerve. 2022;65:652-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Xiong H, Tan D, Wang S, Song S, Yang H, Gao K, Liu A, Jiao H, Mao B, Ding J, Chang X, Wang J, Wu Y, Yuan Y, Jiang Y, Zhang F, Wu H, Wu X. Genotype/phenotype analysis in Chinese laminin-α2 deficient congenital muscular dystrophy patients. Clin Genet. 2015;87:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Finsterer J, Scorza FA, Scorza CA. Significance of Asymptomatic Hyper Creatine-Kinase Emia. J Clin Neuromuscul Dis. 2019;21:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Reynolds TM, Tylee K, Booth K, Wierzbicki AS; PATHFINDER Project Collaboration group; Collaborators and research nurses as listed below. Identification of patients with Pompé disease using routine pathology results: PATHFINDER (creatine kinase) study. J Clin Pathol. 2019;72:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22357] [Article Influence: 2235.7] [Reference Citation Analysis (0)] |
| 13. | Davieson CD, Joyce KE, Sharma L, Shovlin CL. DNA variant classification-reconsidering "allele rarity" and "phenotype" criteria in ACMG/AMP guidelines. Eur J Med Genet. 2021;64:104312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Biesecker LG, Harrison SM; ClinGen Sequence Variant Interpretation Working Group. The ACMG/AMP reputable source criteria for the interpretation of sequence variants. Genet Med. 2018;20:1687-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 15. | Ghosh R, Harrison SM, Rehm HL, Plon SE, Biesecker LG; ClinGen Sequence Variant Interpretation Working Group. Updated recommendation for the benign stand-alone ACMG/AMP criterion. Hum Mutat. 2018;39:1525-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |