Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2431
Revised: March 14, 2024
Accepted: April 2, 2024
Published online: May 16, 2024
Processing time: 101 Days and 18.9 Hours
Cronkhite-Canada syndrome (CCS) is a rare disease of unknown etiology. The optimal treatment for CCS remains unknown. Treatment with corticosteroids is considered the mainstay treatment because of its high efficacy, but the therapeutic strategy for steroid-resistant CCS is not yet established.
This is the case of an 81-year-old woman who was diagnosed with CCS. Given her severe diarrhea, nausea, vomiting, and hypoproteinemia, hormone therapy (40 mg/d) was administered, and the symptoms improved within 1 wk. After 3 mo, the patient had no obvious symptoms. The polyps were significantly reduced on review gastroscopy and colonoscopy, thus hormone reduction gradually began. The hormone level was maintained at 10 mg/d after 6 mo. Despite the age of the patient and the side effects of hormones, the patient had no obvious discomfort. However, hormone drugs were discontinued, and mesalazine was administered orally at 3 g/d. The patient's symptoms continued to improve after a follow-up of 5 years.
Corticosteroids and mesalazine are potential treatment options for CCS.
Core Tip: Cronkhite-Canada syndrome (CCS) is a rare disease of unknown etiology. The optimal treatment for CCS remains unknown. Treatment with corticosteroids is considered the mainstay treatment because of its high efficacy, but the therapeutic strategy for steroid-resistant CCS is not yet established. The current report describes a case of CCS wherein after the initial therapy with corticosteroids, gastrointestinal symptoms were resolved and polyps were significantly reduced. This was followed by mesalazine monotherapy, which led to long-lasting remission. Thus, corticosteroids and mesalazine are potential treatment options for CCS.
- Citation: Chen YL, Wang RY, Mei L, Duan R. Sustained remission of Cronkhite-Canada syndrome after corticosteroid and mesalazine treatment: A case report. World J Clin Cases 2024; 12(14): 2431-2437
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2431.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2431
Cronkhite-Canada syndrome (CCS) is characterized by gastrointestinal polyposis and ectodermal changes (i.e., to the hair, skin, nails, and tongue)[1]. It was first reported in 1955[2]. Since then, more than 600 cases have been reported[3]. Due to the rarity of the syndrome, evidence-based therapies do not exist. Existing regimens combine multiple therapies, but none of them are consistently effective[4].
Although corticosteroids are routinely used as first-line therapy, many cases have been reported to be steroid-resistant[5]. Furthermore, associated side effects, such as sepsis and thrombosis, may occur after the induction of steroid therapy[6]. The current report describes a case of CCS wherein after the initial therapy with corticosteroids, gastrointestinal symptoms were solved and polyps were significantly reduced. This was followed by mesalazine monotherapy, which led to long-lasting remission.
An 81-year-old Chinese woman reported a 2-months history of watery diarrhea, severe nausea and vomiting, alopecia, and dysgeusia.
The patient visited a hospital in October 2018 due to a 2-months history of watery diarrhea, described as yellow watery stool, without pus or blood, occurring about 20 times a day. She had frequent nausea and vomiting, followed by reduced taste, poor appetite, and inability to eat. General weakness, bilateral limb edema, severe hair loss, and skin melanosis were also observed. She noted no obvious abdominal pain nor distension, acid reflux or heartburn, panic, or shortness of breath. However, she reported dysgeusia and weight loss of 10.0 kg in the past 2 months. The symptoms did not improve despite treatment with eprazole for acid inhibition, electrolyte correction, and probiotics.
The patient had hypertension and coronary heart disease.
The patient’s family history revealed no cases of gastrointestinal polyposis or colorectal malignancy. The patient had no smoking or drinking habits. She did not use special drugs, nor was she exposed to toxic substances.
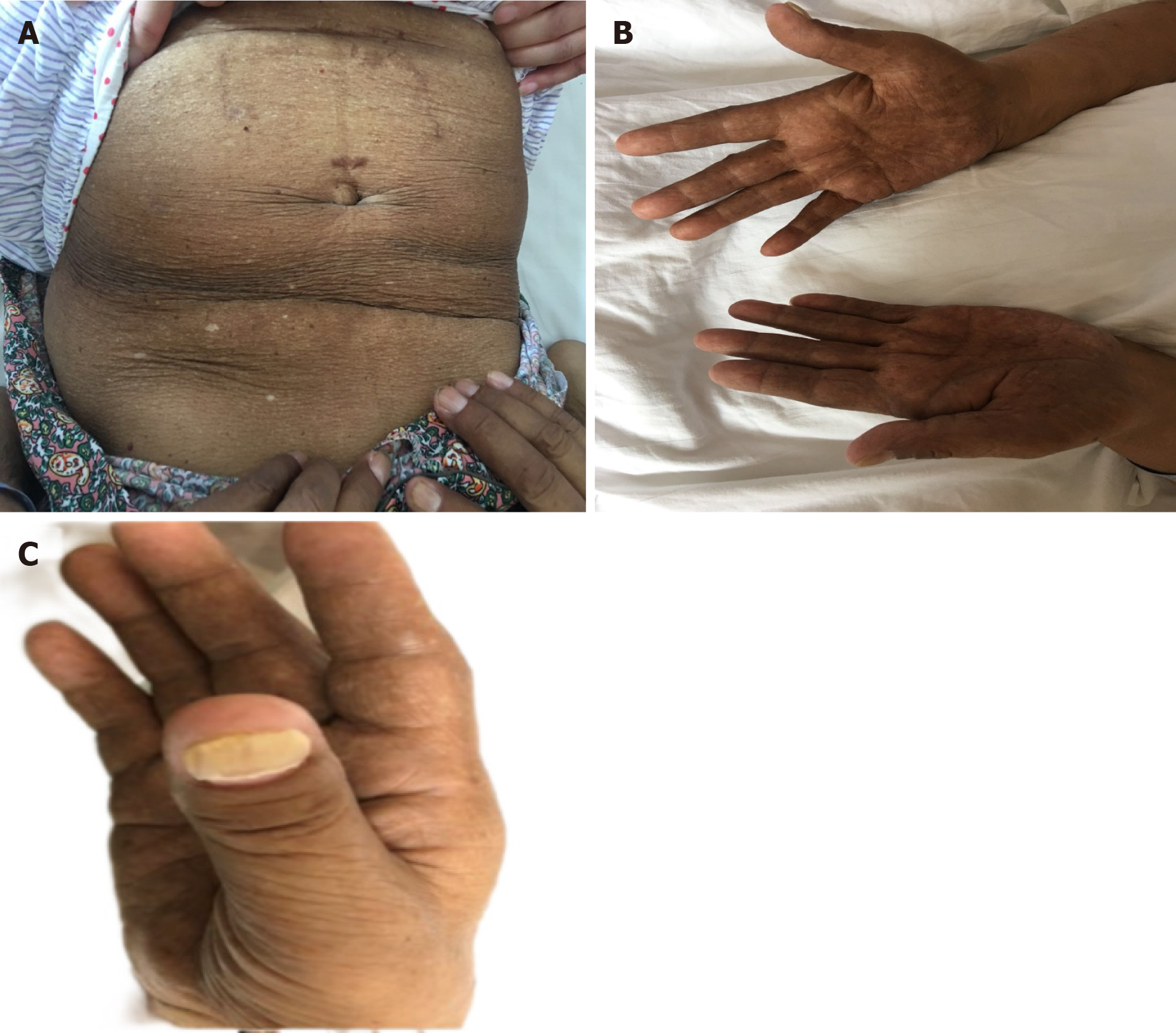
The patient’s vital signs were stable. Her hair and eyebrows were sparse, and skin pigmentation was noted around the palms, lips, and abdomen on both sides (Figure 1A and B). Her oral mucosa showed no ulcerations or leukoplakia. The cardiopulmonary examination showed no abnormalities. She had a flat abdomen, without gastrointestinal type, peristaltic wave, varicose veins, direct nor rebound tenderness, or muscle tension. The nails of both thumbs were yellow and thick (Figure 1C).
Laboratory data revealed hypoproteinemia (albumin level: 26 g/L). Routine blood and urine tests were normal. Stool examinations were positive for occult blood and three types of parasites. However, stool cultures showed no abnormalities. Potassium and calcium levels were 2.9 and 1.94 mmol/L, respectively. The erythrocyte sedimentation rate was elevated (73.0 mm/h), but the C-reactive protein level was normal. The routine kidney function tests were similarly normal. An immunological test revealed that the patient was negative for anti-nuclear antibodies, and thyroid function was normal. Epstein-Barr virus and cytomegalovirus were negative, and tumor markers were negative. Adrenocorticoids were within the normal range. A 13C breath test revealed that she was negative for Helicobacter pylori (H. pylori).
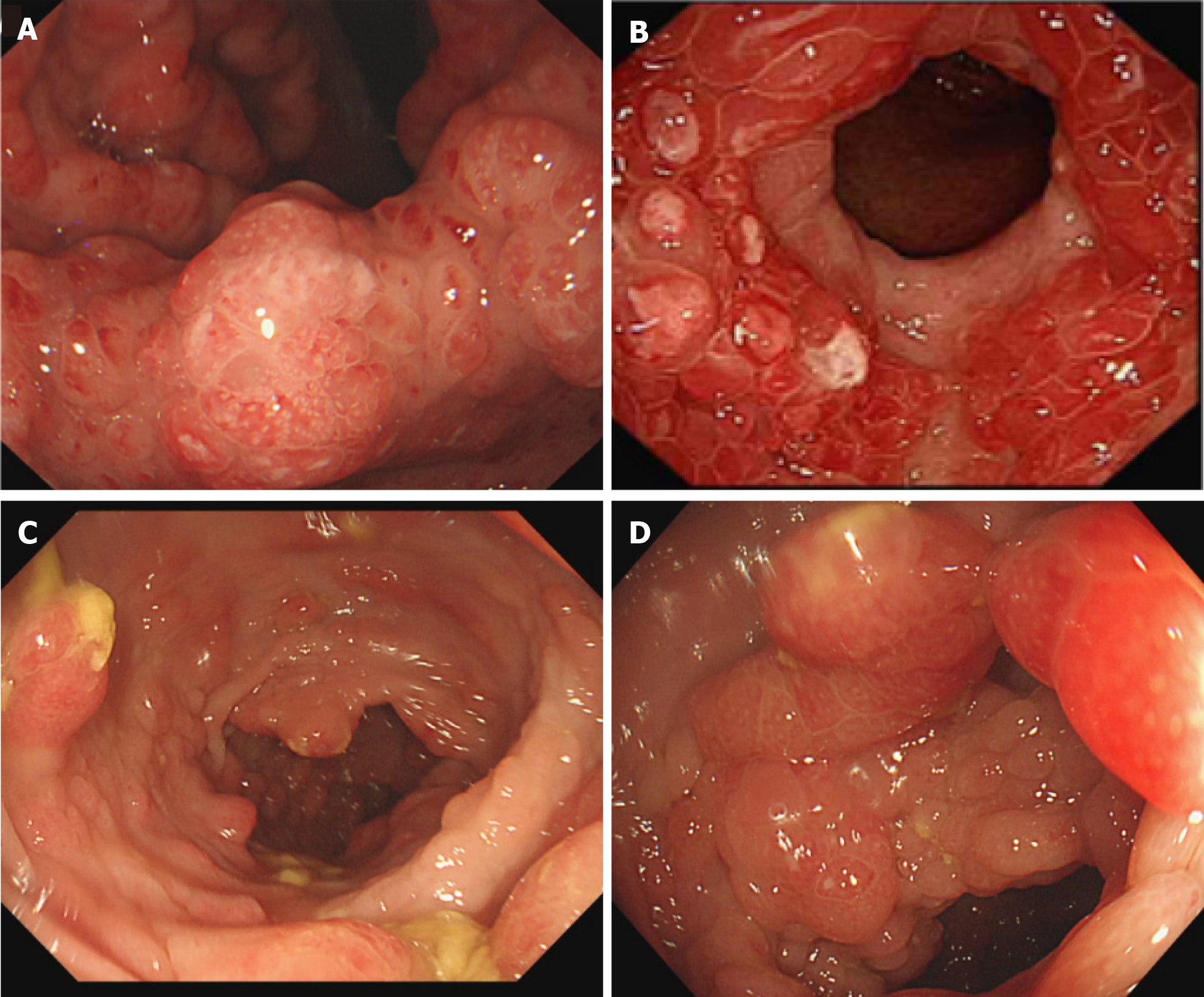
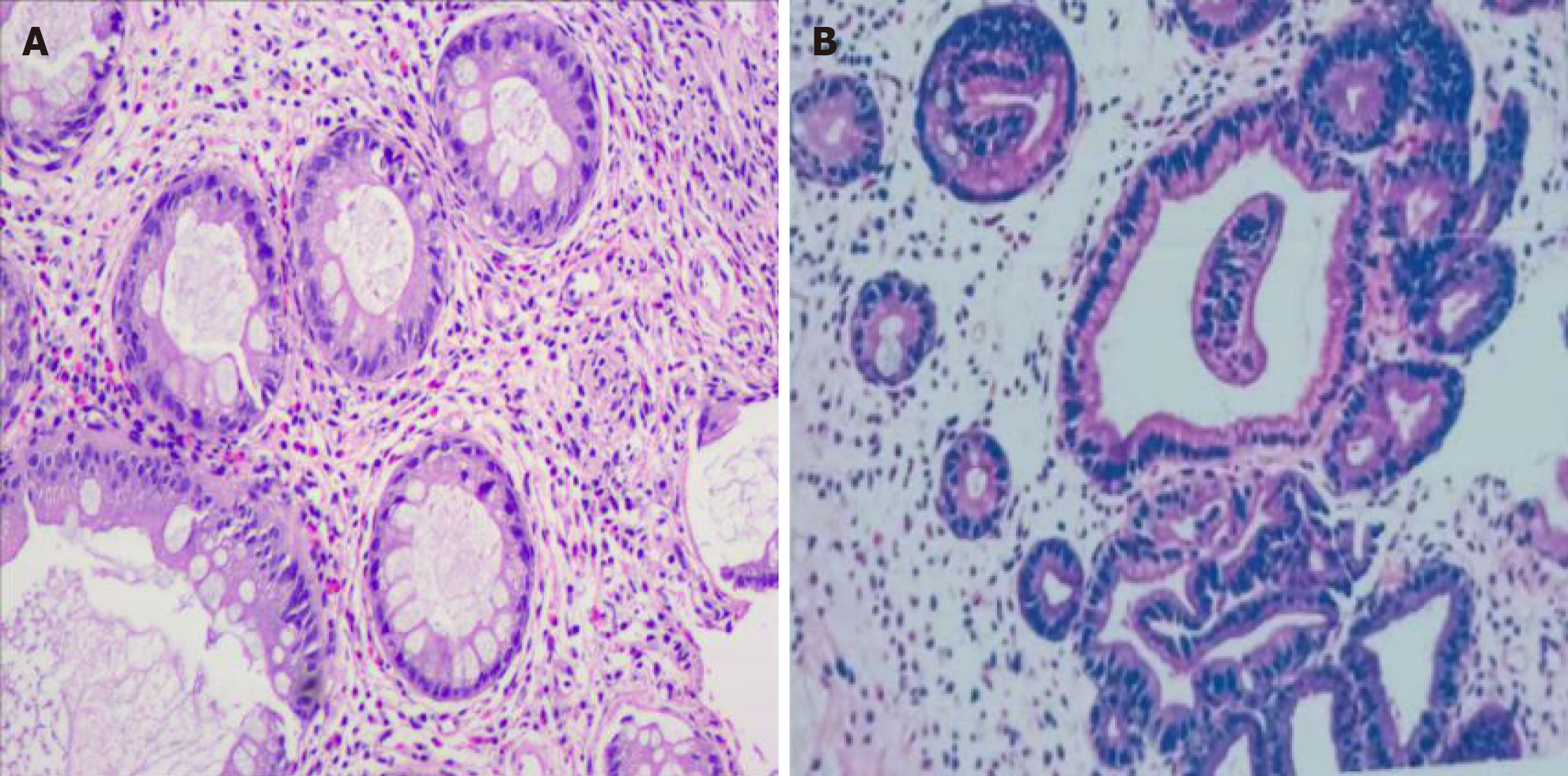
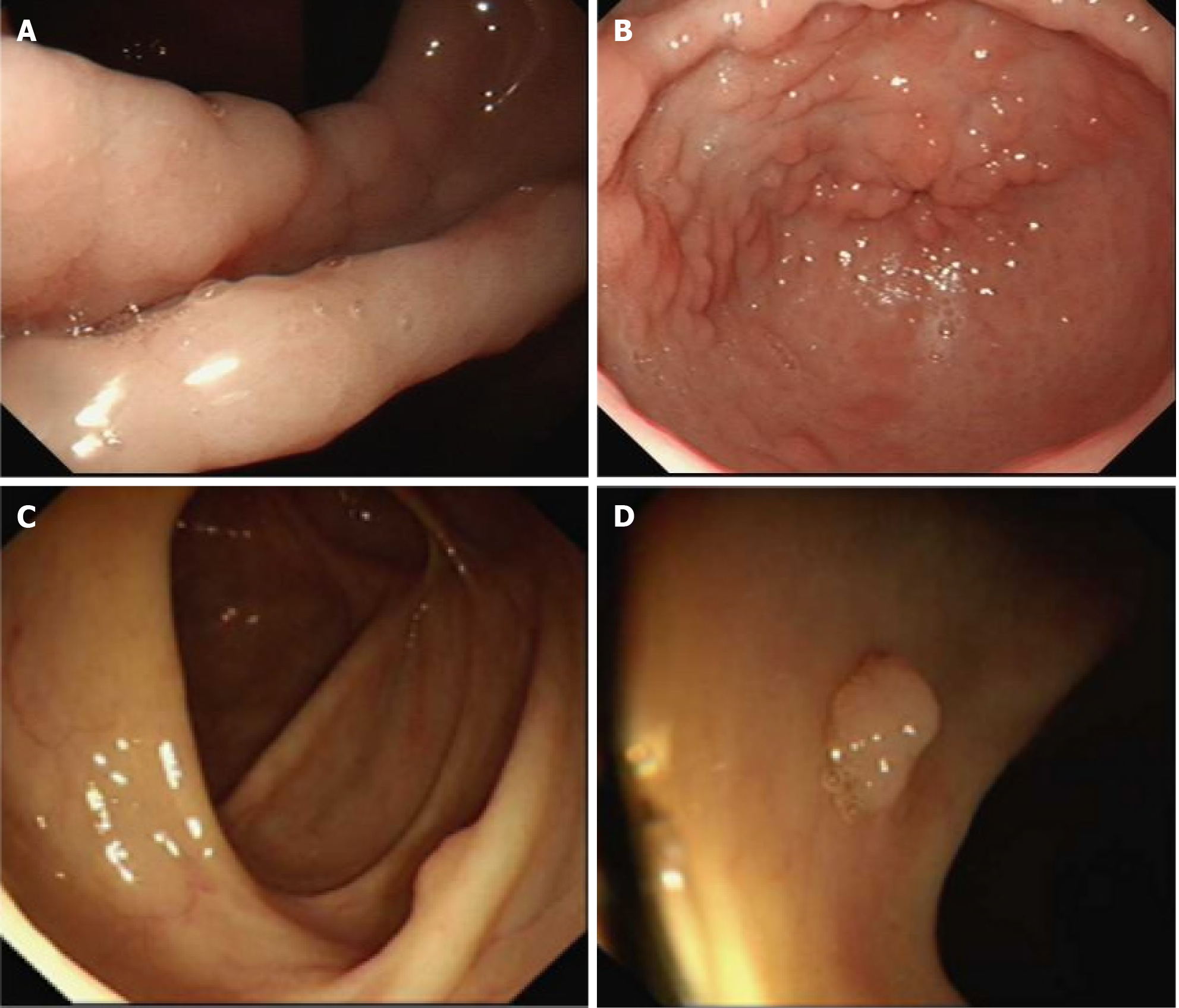
Endoscopic findings revealed numerous polyps in the stomach (Figure 2A and B) and colon (Figure 2C and D). Polyps were mostly pedunculated or fatty, around 2-5 cm in diameter, with different morphologies: Nodular, grape-like, or coral-like. Histologic findings of the polyps revealed prominent cystic dilation of the crypts and expanded inflamed lamina propria, consistent with inflammatory polyps (Figure 3).
The final diagnosis was CCS.
The patient's symptoms were significant, thus methylprednisolone (40 mg/d) was administered. Her symptoms (i.e., nausea, vomiting, and diarrhea) improved within 1 wk and disappeared after 1 mo. The drug was then reduced by 4 mg/d every 2 wk. After review, hormone reduction gradually began, and the hormone level was maintained at 8 mg/d after 6 mo. Despite the age of the patient and the side effects of hormones, the patient had no obvious discomfort. However, hormone drugs were discontinued, and mesalazine was administered orally at 3 g/d.
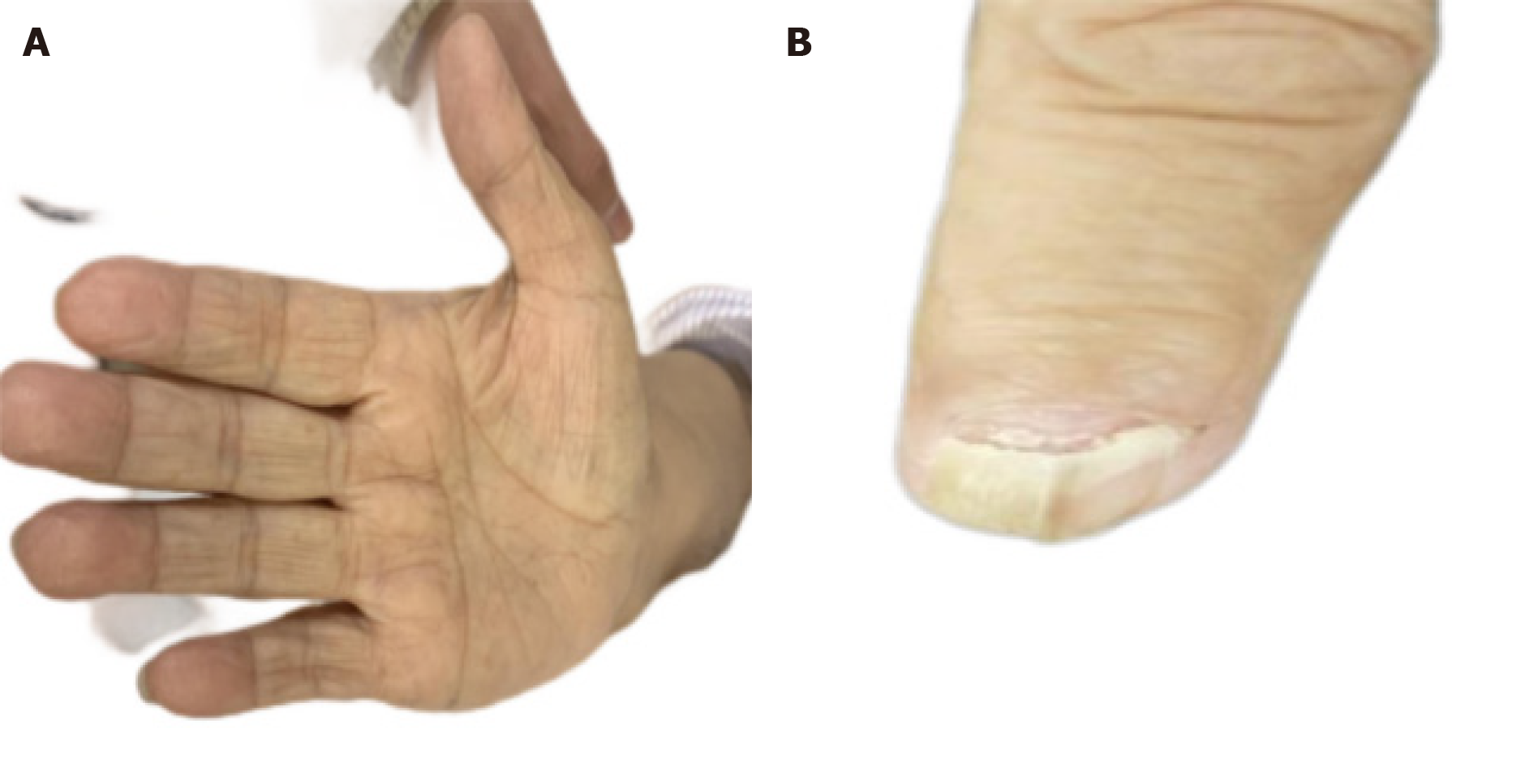
After 3 mo of hormone therapy, the patient had reduced lower limb edema, normal electrolytes and albumin, and skin color (Figure 4A). Gastrointestinal symptoms were also resolved. However, her nails did not improve (Figure 4B). Polyps were significantly reduced on gastroscopy and colonoscopy (Figure 5), especially since the colon was scattered with polyps.
The patient's symptoms continued to improve after a follow-up of 5 years. No obvious nausea, vomiting, or diarrhea occurred after mesalazine administration. However, the patient refused to undergo gastroenteroscopy, thus the effect of mesalazine could not be evaluated more accurately.
CCS is an exceedingly rare clinical syndrome first reported by the American physician Cronkhite and the radiologist Canada in the New England Journal of Medicine in 1955[2]. The term “Cronkhite-Canada syndrome” was first identified by Jarnum and Jensen[7] in 1966. The main clinical manifestations of CCS are alopecia, skin pigmentation, finger (toe) nail malnutrition, and gastrointestinal polyps on endoscopy. CCS is also called (gastrointestinal) polyposis-pigmentation-alopecia-finger (toe) nail malnutrition (atrophy) syndrome[8,9].
Over 60 years have passed since CCS was first reported, but its pathophysiology remains unclear. Given its rarity, a standard treatment for CCS has not yet been established. Currently, CSS treatment strategies include corticosteroids, anabolic steroids, proton pump inhibitors, H2 receptor antagonists, nutritional support, sodium glyonate, immunosuppressive agents, antibiotics, surgery, 5-aminosalicylic acid, rituximab, eradication of H. pylori, and a combination of these therapies[6]. Corticosteroids are considered the mainstay treatment in CCS because of their high efficacy. To date, no guidelines[6] on the recommended dose and duration of corticosteroids in CCS exist, and the prognosis is unclear[10]. However, not all patients respond sufficiently; some may relapse when steroids are tapered[5], and serious adverse reactions may occur during hormone therapy[6].
In the current case report, the patient showed a favorable initial response to corticosteroid therapy paired with nutritional supplements. However, the risk of severe infection and femoral head necrosis due to long-term, heavy use of hormones was high, given the patient’s older age. Therefore, an alternative treatment was required when her symptoms had improved. Mesalazine has been successfully administered for the treatment of inflammatory bowel disease. It can reduce intestinal inflammation and promote mucosal repair. Independent of its anti-inflammatory features, it appears to have an antiproliferative effect on colorectal adenomas[11]. Prior studies[12,13] demonstrated sustained remission after the use of oral mesalazine and corticosteroids. The patient's symptoms were notable at presentation, thus prednisone therapy (40 mg/d) was initiated. After 3 mo, the patient had no obvious symptoms. Polyps were significantly reduced on gastroscopy and colonoscopy, and hormone reduction gradually began. The dose was maintained at 10 mg/d after 6 mo. Thereafter, hormone drugs were discontinued, and mesalazine was administered orally at 3 g/d instead. The patient's symptoms continued to improve after a follow-up of 5 years.
In conclusion, the current study suggests that the 5-year mortality rate of CCS is as high as 55%. It is a rare, non-hereditary disease of unknown origin. The etiology and optimal treatment for this syndrome remain unknown. To date, no standard therapeutic strategies for CCS have been established. Although improvement in gastrointestinal symptoms may result from high-dose corticosteroid treatment, mesalazine has also been shown to maintain remission of intestinal tract lesions. Therefore, mesalazine could be an additional or alternative modality for the treatment of CCS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kroupa R, Czech Republic S-Editor: Zheng XM L-Editor: Wang TQ P-Editor: Guo X
| 1. | Zhao R, Huang M, Banafea O, Zhao J, Cheng L, Zou K, Zhu L. Cronkhite-Canada syndrome: a rare case report and literature review. BMC Gastroenterol. 2016;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Cronkhite LW Jr, Canada WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med. 1955;252:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 247] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Patil V, Patil LS, Jakareddy R, Verma A, Gupta AB. Cronkhite-Canada syndrome: a report of two familial cases. Indian J Gastroenterol. 2013;32:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Chaisidhivej N, Maneerattanaporn M, Pongpaibul A, Trongtorsak A, Kinnucan J. Cronkhite-Canada Syndrome: A Rare Case of Chronic Diarrhea With Ectodermal Changes. Cureus. 2022;14:e29298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Jha AK, Kumar A, Singh SK, Madhawi R. Panendoscopic characterization of Cronkhite-Canada syndrome. Med J Armed Forces India. 2018;74:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Watanabe C, Komoto S, Tomita K, Hokari R, Tanaka M, Hirata I, Hibi T, Kaunitz JD, Miura S. Endoscopic and clinical evaluation of treatment and prognosis of Cronkhite-Canada syndrome: a Japanese nationwide survey. J Gastroenterol. 2016;51:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Jarnum S, Jensen H. Diffuse gastrointestinal polyposis with ectodermal changes. A case with severe malabsorption and enteric loss of plasma proteins and electrolytes. Gastroenterology. 1966;50:107-118. [PubMed] [DOI] [Full Text] |
| 8. | Wang JY, Lu WQ, Ning HB. [Clinical analysis of Cronkhite-Canada syndrome]. Henan Yixue Yanjiu. 2019;28:1931-1935. |
| 9. | Cao XC, Wang BM, Zhang J, Tan J, Li W, Ding JJ, Lian J, Lu N, Jiang K, Huang NX. [Cronkhite-Canada syndrome 32 clinical analysis]. Zhongguo Shiyong Neike Zazhi. 2006;26:9-11. |
| 10. | Yamakawa K, Yoshino T, Watanabe K, Kawano K, Kurita A, Matsuzaki N, Yuba Y, Yazumi S. Effectiveness of cyclosporine as a treatment for steroid-resistant Cronkhite-Canada syndrome; two case reports. BMC Gastroenterol. 2016;16:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Munding J, Ziebarth W, Pox CP, Ladigan S, Reiser M, Hüppe D, Brand L, Schmiegel W, Tannapfel A, Reinacher-Schick AC. The influence of 5-aminosalicylic acid on the progression of colorectal adenomas via the β-catenin signaling pathway. Carcinogenesis. 2012;33:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 418] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | Schulte S, Kütting F, Mertens J, Kaufmann T, Drebber U, Nierhoff D, Töx U, Steffen HM. Case report of patient with a Cronkhite-Canada syndrome: sustained remission after treatment with corticosteroids and mesalazine. BMC Gastroenterol. 2019;19:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |