Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2342
Revised: February 16, 2024
Accepted: April 2, 2024
Published online: May 16, 2024
Processing time: 105 Days and 2.8 Hours
Helicobacter pylori (H. pylori) infection is closely related to the development of gastric cancer (GC). However, GC can develop even after H. pylori eradication. Therefore, it would be extremely useful if GC could be predicted after eradication. The Kyoto classification score for gastritis (GA) is closely related to cancer risk. However, how the score for GC changes after eradication before onset is not well understood.
To investigate the characteristics of the progression of Kyoto classification scores for GC after H. pylori eradication.
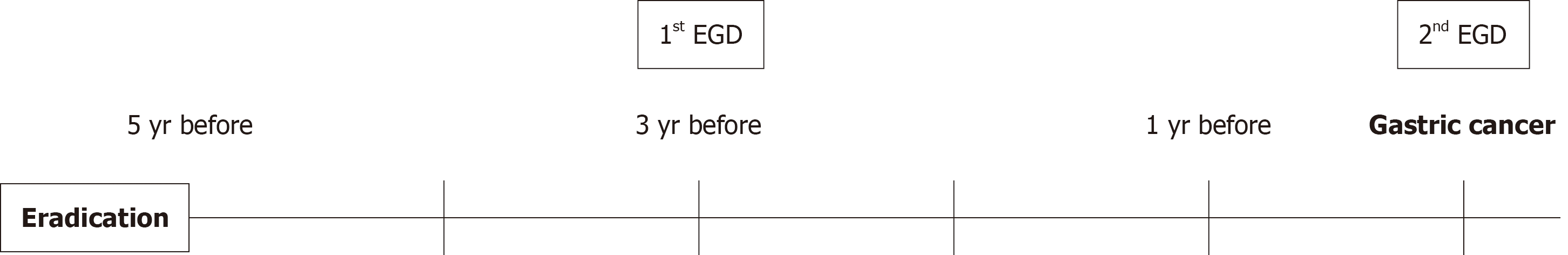
Eradication of H. pylori was confirmed in all patients using either the urea breath test or the stool antigen test. The Kyoto classification score of GC patients was evaluated by endoscopy at the time of event onset and three years earlier. In ad
In total, 30 cases of early GC and 30 cases of chronic GA were evaluated. The pathology of the cancer cases was differentiated adenocarcinoma, except for one case of undifferentiated adenocarcinoma. The total score of the Kyoto classifi
The course of the modified atrophy score is useful for predicting the onset of GC after eradication. Patients with severe atrophy after H. pylori eradication require careful monitoring.
Core Tip:Helicobacter pylori (H. pylori) infection is closely related to the development of gastric cancer (GC). Therefore, H. pylori eradication therapy is very important. However, GC can develop even after H. pylori eradication. Thus, it would be very useful if the onset of GC could be predicted. The Kyoto classification of gastritis is useful for endoscopic diagnosis. In this study, we showed that a modified atrophy score may be useful for predicting GC after eradication. In cases of GC after eradication, the modified atrophy score did not decrease during endoscopic follow-up. Gastric mucosal atrophic findings should be noted during post-eradication surveillance.
- Citation: Suzuki Y, Katayama Y, Fujimoto Y, Kobori I, Tamano M. Non-improvement of atrophic gastritis in cases of gastric cancer after successful Helicobacter pylori eradication therapy. World J Clin Cases 2024; 12(14): 2342-2349
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2342.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2342
In 1983, Helicobacter pylori (H. pylori) was successfully isolated and cultured, and this bacterium was found to be the cause of histological gastritis (GA)[1]. Accumulation of genetic abnormalities due to persistent inflammation leads to gastric carcinogenesis[2]. Many studies, basic, clinical, and epidemiological, have shown the relationship between H. pylori and gastric cancer (GC)[3-5]. Though eradication of H. pylori prevents GC development[6]. The incidence of GC does not completely disappear after eradication[7]. Therefore, it is very important to identify risk factors for carcinogenesis during post-eradication surveillance. The most reliable method may be to screen for genetic mutations and methylation levels in gastric mucosa[6,8]. Methylation levels of certain microRNAs after eradication are associated with an increased risk of developing metachronous GC[9]. However, this method cannot yet be easily used in general practice. Therefore, regular endoscopy after eradication is important.
The Kyoto classification of GA aims to predict the onset of GC by scoring and evaluating the mucosal condition of GA[10]. It can be easily scored using an endoscope, and there have been several reports of the usefulness of the score at the onset of GC [11]. We have also reported that scores obtained several years before the onset of GC are useful for predicting the subsequent onset of GC[12]. However, its usefulness for predicting GC after eradication is unknown. In this study, whether the time course of Kyoto classification scores for GC after eradication shows any specific characteristic was investigated.
Patients were selected from among cases who underwent endoscopic submucosal dissection (ESD) for GC after eradi
The urea breath test (UBT tablets 100 mg; Otsuka Pharmaceutical, Tokyo, Japan) or a stool antigen test (Meridian HpSA ELISA2, Fujirebio, Tokyo, Japan) was also used to confirm eradication of H. pylori. If a patient showed negative results for all tests and had a history of eradication, the patient was diagnosed as an H. pylori-eradication patient.
Endoscopic examinations were performed using Olympus electroscopes (GIF-260H, GIF-290H, GIF-290Z; Olympus, Tokyo, Japan). The Kyoto classification score of GA was evaluated: Atrophy (Kimura-Takemoto classification: C0-CI = A0, CII-CIII = A1, and OI-OIII = A2), intestinal metaplasia (none, IM0; intra-antral, IM1; up to the body, IM2), hypertro
| Parameter | Score | |
| Atrophy | 0 | C0–CI according to Kimura-Takemoto classification |
| 1 | CII–CIII | |
| 2 | OI–OIII | |
| Intestinal metaplasia | 0 | None |
| 1 | Within the antrum | |
| 2 | Up to corpus | |
| Hypertrophy of gastrid folds | 0 | None |
| 1 | Positive | |
| Nodularity | 0 | None |
| 1 | Positive | |
| Diffuse redness | 0 | None |
| 1 | Mild (with RAC) | |
| 2 | Severe |
| Modified atrophy score | Kimura-Takemoto classification |
| 0 | C0 |
| 1 | CI |
| 2 | CII |
| 3 | CIII |
| 4 | OI |
| 5 | OII |
| 6 | OIII |
The outcome was the risk score obtained using the Kyoto classification of changes in gastric mucosa at the time of GC onset and three years earlier.
Fisher’s exact tests were used for sex comparisons. The Wilcoxon rank-sum test was performed to compare age and risk scores obtained using the Kyoto classification of GA between the GC and GA groups. The modified atrophy score was compared by the Wilcoxon rank-sum test. Changes over time in each of the two groups were examined using the Wilco
Thirty cases of early GC and thirty cases of chronic GA were evaluated. Although no difference in mean age was ob
| Characteristics | Gastric cancer | Gastritis | P value |
| Number | 30 | 30 | |
| Age (yr ± SD) | 75.3 | 73.9 | 0.695 |
| Sex (M:F) | 21:9 | 17:13 | 0.422 |
| Differentiation (tub1-2: Sig-por) | 29:1 | ||
| Depth (m: Sm) | 30:0 |
In the case of GC, the examination findings taken three years before the onset of cancer were evaluated to see if any cancer had been missed, focusing on the area where ESD was performed. No oversights could be confirmed.
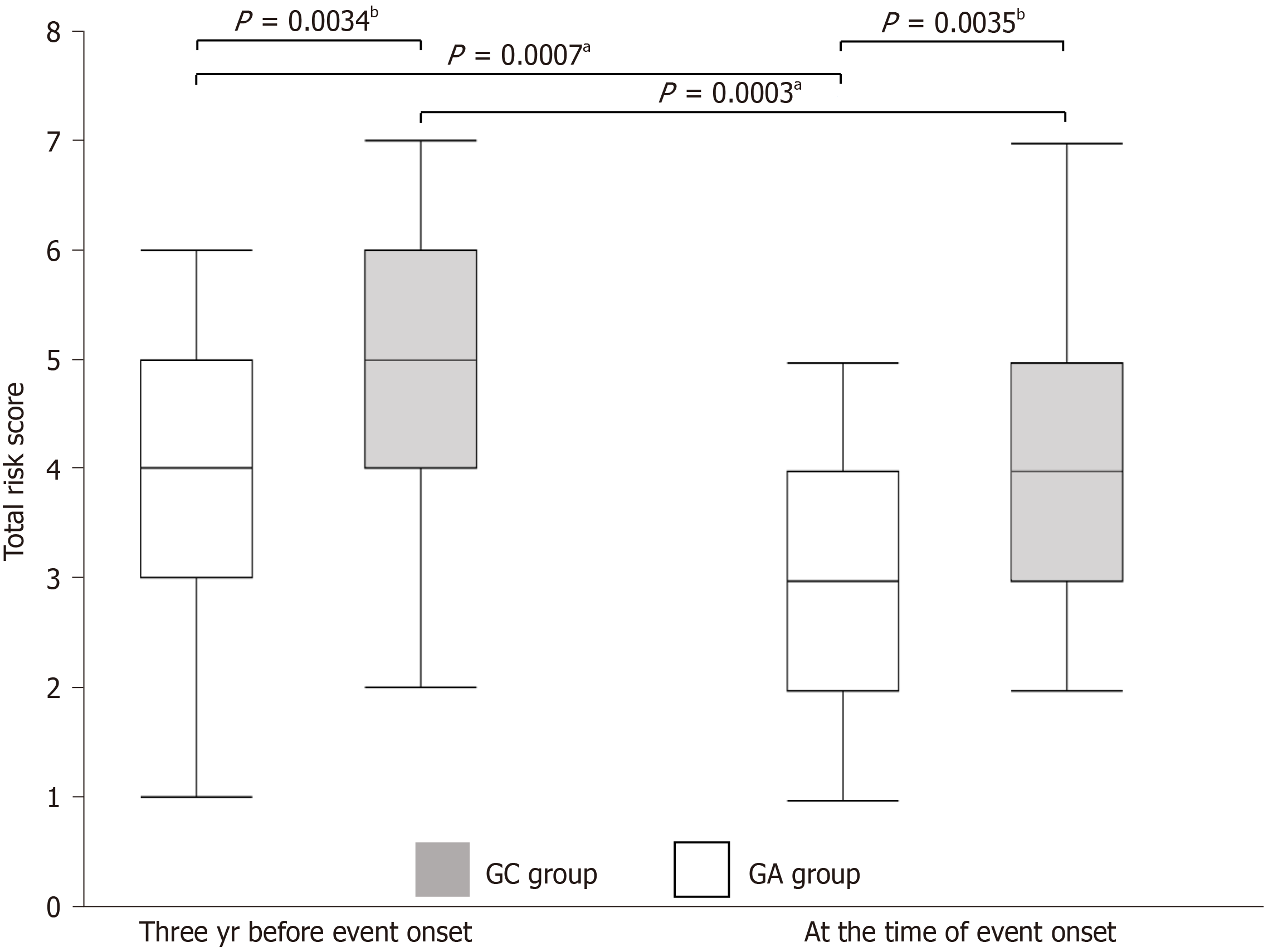
The total score was significantly higher in the GC group both at the time of cancer onset and three years earlier (4.97 vs 3.73, P = 0.0034; 4.2 vs 3.1, P = 0.0035, respectively). In terms of changes over time, total scores decreased significantly in both groups (4.97 vs 4.2, P = 0.0003; 3.73 vs 3.1, P = 0.0007, respectively) (Figure 2). The atrophy score was higher in the GC group at all time points, and no significant changes over time were observed in either group. The intestinal meta
| Parameters | 1st EGD | 2nd EGD | P value | 1st EGD | 2nd EGD | P value | 1st EGD | 2nd EGD | P value |
| A | IM | H | |||||||
| GC | 2 | 2 | 1 | 1.27 ± 0.74 | 1.27 ± 0.74 | 1 | 0.43 ± 0.09 | 0.50 ± 0.504 | 0.543 |
| GA | 1.9±0.305 | 1.9±0.305 | 1 | 0.8 ± 0.76 | 0.87 ± 0.78 | 0.326 | 0.37 ± 0.09 | 0.367 ± 0.490 | 0.184 |
| P value | 0.0415 | 0.0415 | 0.0192 | 0.046 | 0.605 | 0.281 | |||
| N | DR | ||||||||
| GC | 0.03 ± 0.183 | 0.03 ± 0.183 | 1 | 1.27 ± 0.83 | 0.5 ± 0.82 | 0.0004 | |||
| GA | 0.03 ± 0.183 | 0 | 0.326 | 0.95 ± 0.17 | 0.07 ± 0.37 | 0.0008 | |||
| P value | 1 | 0.326 | 0.017 | 0.012 | |||||
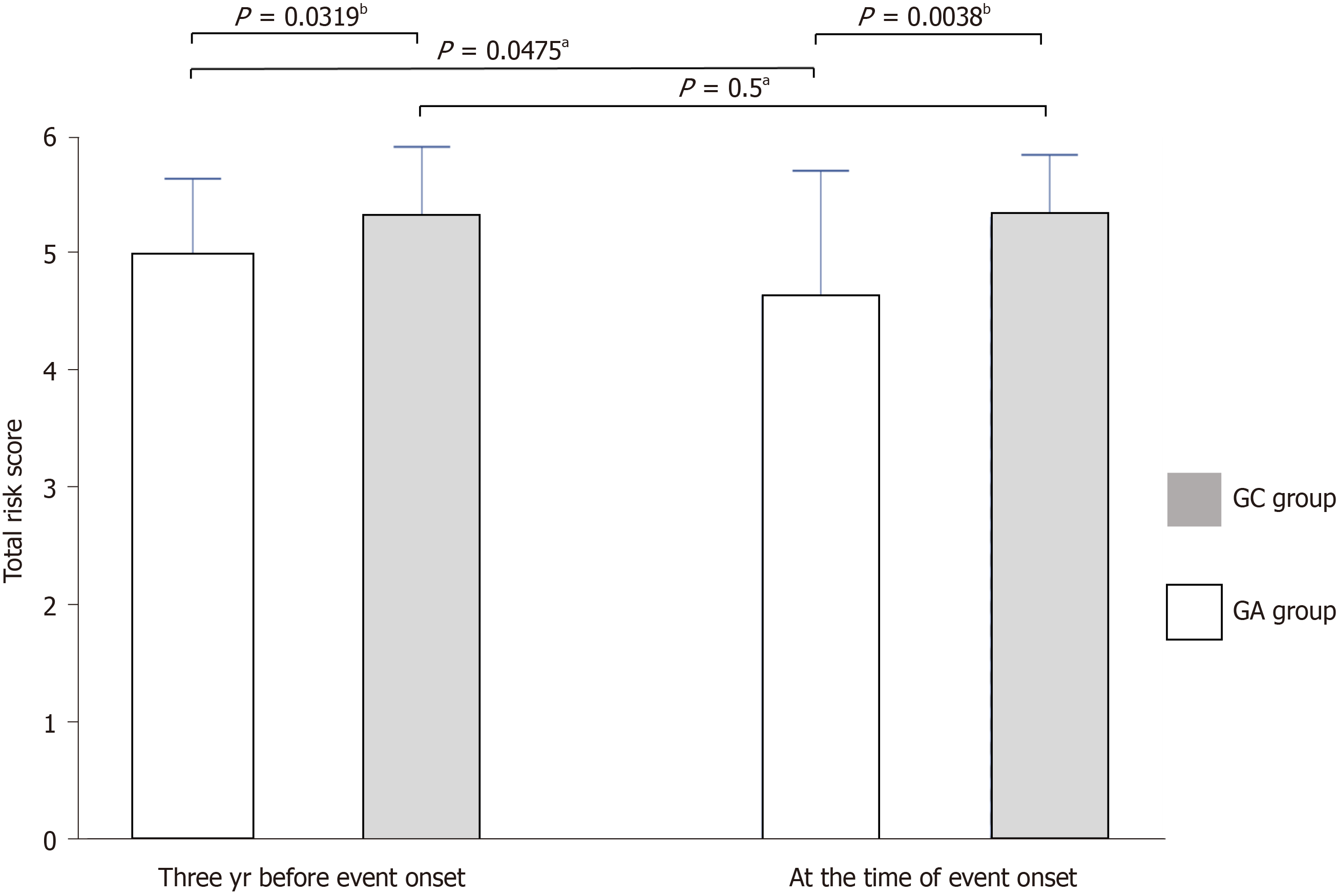
The modified atrophy score was significantly higher in the GC group both at the time of cancer onset and three years earlier (5.3 vs 4.93, P = 0.0319; 4.93 vs 4.63, P = 0.0038, respectively), and it was significantly improved only in the GA group (5.3 vs 5.3, P = 0.5; 3.73 vs 3.1, P = 0.0475, respectively) (Figure 3).
Although the number of deaths from GC is decreasing, its incidence remains high[13]. H. pylori infection is the most important cause of GC, and eradication therapy reduces the risk of GC[14,15]. However, the risk of cancer is not elimi
There have been many reports of the relationship between endoscopically diagnosed GA and GC. Therefore, endo
The Kyoto classification of GA is based on the previous diagnosis and classification of GA, and it distinguishes among those uninfected, currently infected, and previously infected (including after eradication) with H. pylori[19]. The Kyoto classification of GA aims to reflect GC risk. Five elements are included in the endoscopic finding score for GC risk: Atrophy, intestinal metaplasia, diffuse redness, hypertrophy of gastric folds, and nodularity[10].
Sugimoto et al[20] reported a comparison of risk scores obtained using the Kyoto classification of GA at the time of GC detection[20]. Scores of GC were significantly higher than GA scores in both non-eradicated and eradicated cases. They identified atrophic GA and intestinal metaplasia as related factors. Shichijo et al[21] also reported that atrophy was more involved in the risk score for GC[21]. However, those reports evaluated the risk score obtained using the Kyoto classification of GA at the time of diagnosis of GC. It would be clinically very useful if the Kyoto classification score could be used to show changes in the gastric mucosa after eradication until the onset of gastric cancer. Because it is difficult to conduct a prospective study at a single institution, the present study was a retrospective review.
In the present study, the atrophy score, intestinal metaplasia, diffuse redness, and total score were significantly higher both at initial endoscopy and at the onset of GC after eradication (Table 4). There were no differences in fold enlargement and nodularity between GC cases and GA cases at the time of initial endoscopy or at the time of GC onset. The modified atrophy score was significantly higher both at initial endoscopy and at the onset of GC after eradication. The modified atrophy score did not improve during the course of GC cases after eradication. It is a very important finding that atrophy does not improve in GC cases compared to GA even after eradication. The atrophy score of the Kyoto classification is only 0, 1, or 2, making it difficult to distinguish between the two groups and difficult to interpret the results. In the pre
We previously reported that post-eradication atrophy scores are useful for predicting subsequent cancer[12]. In addi
All GC in the present study were differentiated adenocarcinomas except for one case, and all were intramucosal car
This was a single-center, retrospective study with a limited number of cases. In this study, image-enhanced endoscopy was used only for the diagnosis of some cases of intestinal metaplasia. Since endoscopic data at the time of eradication were not available for all cases, endoscopic data from three years before the onset of GC after eradication were used.
In conclusion, the results of this retrospective study suggested that the course of the modified atrophy score is useful for predicting the onset of GC after eradication. In particular, patients with severe atrophy even after H. pylori eradication should be carefully monitored to detect GC early.
The authors would like to thank the staff of their endoscopy center, whose important contributions to this study were indispensable to its success.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leowattana W, Thailand; Liu TF, China S-Editor: Liu H L-Editor: A P-Editor: Xu ZH
| 1. | Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] |
| 2. | Hatakeyama M, Brzozowski T. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2006;11 Suppl 1:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 3. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 783] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 5. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1234] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 6. | Irie T, Yamada H, Takeuchi C, Liu YY, Charvat H, Shimazu T, Ando T, Maekita T, Abe S, Takamaru H, Kodama M, Murakami K, Sugimoto K, Sakamoto K, Ushijima T. The methylation level of a single cancer risk marker gene reflects methylation burden in gastric mucosa. Gastric Cancer. 2023;26:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 7. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 936] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 8. | Usui Y, Taniyama Y, Endo M, Koyanagi YN, Kasugai Y, Oze I, Ito H, Imoto I, Tanaka T, Tajika M, Niwa Y, Iwasaki Y, Aoi T, Hakozaki N, Takata S, Suzuki K, Terao C, Hatakeyama M, Hirata M, Sugano K, Yoshida T, Kamatani Y, Nakagawa H, Matsuda K, Murakami Y, Spurdle AB, Matsuo K, Momozawa Y. Helicobacter pylori, Homologous-Recombination Genes, and Gastric Cancer. N Engl J Med. 2023;388:1181-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 9. | Asada K, Nakajima T, Shimazu T, Yamamichi N, Maekita T, Yokoi C, Oda I, Ando T, Yoshida T, Nanjo S, Fujishiro M, Gotoda T, Ichinose M, Ushijima T. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut. 2015;64:388-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Kamada T, Haruma K, Inoue K, Shiotani A. [Helicobacter pylori infection and endoscopic gastritis -Kyoto classification of gastritis]. Nihon Shokakibyo Gakkai Zasshi. 2015;112:982-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 11. | Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (2)] |
| 12. | Fujimoto Y, Katayama Y, Gyotoku Y, Oura R, Kobori I, Kitagawa T, Tamano M. Predictive value of risk score using Kyoto classification of gastritis a few years prior to diagnosis of early gastric cancer. JGH Open. 2021;5:280-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 983] [Article Influence: 196.6] [Reference Citation Analysis (1)] |
| 14. | Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 644] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 15. | Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, You J, Wong BCY, Chen J, Ye W. Effect of Helicobacter pylori Eradication on Gastric Cancer Prevention: Updated Report From a Randomized Controlled Trial With 26.5 Years of Follow-up. Gastroenterology. 2022;163:154-162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 16. | Ito M, Tanaka S, Chayama K. Characteristics and Early Diagnosis of Gastric Cancer Discovered after Helicobacter pylori Eradication. Gut Liver. 2021;15:338-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Schindler R. Chronic gastritis. Bull NY Acad Med. 1939;15:322-327. [PubMed] |
| 18. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;3:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (3)] |
| 19. | Sakae H, Iwamuro M, Okamoto Y, Obayashi Y, Baba Y, Hamada K, Gotoda T, Abe M, Kono Y, Kanzaki H, Kawano S, Kawahara Y, Tanaka T, Yanai H, Okada H. Evaluation of the Usefulness and Convenience of the Kyoto Classification of Gastritis in the Endoscopic Diagnosis of the Helicobacter pylori Infection Status. Digestion. 2020;101:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Koike K. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol. 2017;32:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Kawamura M, Uedo N, Koike T, Kanesaka T, Hatta W, Ogata Y, Oikawa T, Iwai W, Yokosawa S, Honda J, Asonuma S, Okata H, Ohyauchi M, Ito H, Abe Y, Ara N, Kayaba S, Shinkai H, Shimokawa T. Kyoto classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan. Dig Endosc. 2022;34:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | Han F, Sun LP, Liu S, Xu Q, Liang QY, Zhang Z, Cao HC, Yu J, Fan DM, Nie YZ, Wu KC, Yuan Y. Promoter methylation of RNF180 is associated with H.pylori infection and serves as a marker for gastric cancer and atrophic gastritis. Oncotarget. 2016;7:24800-24809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, Tamura G, Saito D, Sugimura T, Ichinose M, Ushijima T. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 25. | Chan AO, Peng JZ, Lam SK, Lai KC, Yuen MF, Cheung HK, Kwong YL, Rashid A, Chan CK, Wong BC. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut. 2006;55:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 26. | Chen CL, Wang SH, Chan PC, Shen MR, Chen HC. Phosphorylation of E-cadherin at threonine 790 by protein kinase Cδ reduces β-catenin binding and suppresses the function of E-cadherin. Oncotarget. 2016;7:37260-37276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |