Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2308
Revised: February 14, 2024
Accepted: April 2, 2024
Published online: May 16, 2024
Processing time: 128 Days and 19.9 Hours
Type 2 diabetes mellitus (T2DM) is a leading risk factor for the development and progression of chronic kidney disease (CKD). However, an accurate and con
To investigate the relationship between NLR and renal function in T2DM patients.
This study included 1040 adults aged 65 or older with T2DM from Shanghai's Community Health Service Center. The total number of neutrophils and lym
Significant differences were found in terms of sex, serum creatinine, blood urea nitrogen, total cholesterol, and low-density lipoprotein cholesterol among patients with T2DM in different NLR groups (P < 0.0007). T2DM patients in the highest NLR quartile had a higher prevalence of CKD (P for trend = 0.0011). Multivariate logistic regression analysis indicated that a high NLR was an independent risk factor for CKD in T2DM patients even after adjustment for important clinical and pathological parameters (P = 0.0001, odds ratio = 1.41, 95% confidence intervals: 1.18-1.68).
An elevated NLR in patients with T2DM is associated with higher prevalence of CKD, suggesting that it could be a marker for the detection and evaluation of diabetic kidney disease.
Core Tip: In elderly type 2 diabetes mellitus (T2DM) patients, elevated neutrophil-to-lymphocyte ratio (NLR) is strongly linked to an increased risk of chronic kidney disease (CKD), uncovering NLR as a potential independent biomarker for early detection of renal damage. This finding holds significant promise for addressing the current challenge of delayed CKD diagnosis in T2DM, signifying the potential utility of NLR as a convenient and sensitive detection method for identifying CKD in diabetic patients.
- Citation: Gao JL, Shen J, Yang LP, Liu L, Zhao K, Pan XR, Li L, Xu JJ. Neutrophil-to-lymphocyte ratio associated with renal function in type 2 diabetic patients. World J Clin Cases 2024; 12(14): 2308-2315
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2308.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2308
Diabetes is a prevalent metabolic disease with significant implications for global health, and type 2 diabetes mellitus (T2DM) represents the predominant form of diabetes in China, accounting for over 90% of cases[1]. T2DM is one of the leading risk factors for the development and progression of chronic kidney disease (CKD)[2]. In Asia, it is estimated that > 60% of patients with diabetes will develop kidney complications, compared with 30%-40% in Europeans despite having a similar duration of diabetes[3,4]. Furthermore, CKD in diabetic individuals is associated with increased morbidity and premature mortality, which impose a substantial economic burden on healthcare systems[5,6]. Given the continuous in
Recent studies have shown that the neutrophil-to-lymphocyte ratio (NLR), an inflammatory marker, strongly correlates with acute ischemic stroke, tumors, sepsis, and CKD[7-10]. However, prior research has mostly examined hospitalized patients with more severe conditions, overlooking diabetic patients in the general population. This article mainly ex
This study involved 1040 adults aged 65 years old or above diagnosed with T2DM from the health examination platform of the Community Health Service Center in Songnan Town, Baoshan District, Shanghai, China from June to August 2021. The investigation focused on three medical service stations located in the community health service center and affiliated institutes of Songnan Town. The diagnosis of T2DM was in accordance with Chinese guidelines for the prevention and treatment of T2DM (2020 edition)[11], namely, a fasting plasma glucose (FPG) level ≥ 7.0 mmol/L or a previous diagnosis of T2DM and currently receiving oral medication. Participants were excluded if they had incomplete or uncertain basic information, type 1 DM, a history of hormone therapy within a year, any type of malignancy, a current acute infection, or an established hematologic disease. Oral consent was obtained from all participants, and the study was approved by the central ethics committee.
Demographics (sex and age) and disease history, such as hypertension, were self-reported by all participants through comprehensive questionnaires completed by trained general practitioners and volunteers. Height and weight were mea
For laboratory testing, the participants’ whole blood was drawn into an EDTA vacuum anticoagulant tube and mixed by inversion several times. Additionally, 6 mL of fasting venous blood was obtained in the early morning, and the su
The estimated glomerular filtration rate (eGFR), expressed in ml/min/1.73 m2, was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation on the basis of Scr[12]. The formula was as follows: (1) if female: Scr ≤ 0.7 mg/dL, eGFR = 144 × (Scr/0.7)-0.329 × (0.993)age; Scr > 0.7 mg/dL, eGFR = 144 × (Scr/0.7)-1.209 × (0.993)age; and (2) if male: Scr ≤ 0.9 mg/dL, eGFR = 141 × (Scr/0.9)-0.411 × (0.993)age; Scr > 0.9 mg/dL, eGFR = 141 × (Scr/0.9)-1.209 × (0.993)age. CKD was defined as an eGFR of 60 mL/min/1.73 m2 or less[13].
(1) Hypertension was defined according to the Chinese guidelines for the prevention and treatment of hypertension (2018 Revision)[14], with systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, or with a previous diagnosis of hypertension and currently being treated with oral antihypertensive drugs; (2) Smoking referred to those who smoked ≥ 1 cigarette per day on average, continuously or cumulatively for the past 6 months; (3) Drinking was defined as consuming alcohol at least once a week at a dose of ≥ 50 g of ethanol per occasion, continuously or cu
Continuous variables were expressed as the mean ± SD or median with interquartile range and were analyzed by an in
All statistical analyses were performed using SAS software (version 9.4). A two-tailed P value < 0.05 was considered statistically significant.
Table 1 shows the general characteristics of the study population. A total of 1040 participants diagnosed with T2DM were included in the analysis and divided into four groups according to their NLR: quartile 1 (NLR < 1.38, 261 patients), quartile 2 (1.38 ≤ NLR < 1.76, 258 patients), quartile 3 (1.76 ≤ NLR < 2.30, 262 patients), and quartile 4 (NLR ≥ 2.30, 259 patients). No significant difference was observed among the groups concerning age, BMI, SBP, DBP, FPG, TG, platelet counts, smoking, drinking, regular exercise, and hypertension (Table 1). However, significant differences were found in terms of sex, BUN, Scr, eGFR, TC, LDL-C, HDL-C, neutrophil, lymphocyte and white blood cell (WBC) counts among the four groups (P < 0.05).
| Variables | Total | Quartile 1 (NLR < 1.38) | Quartile 2 (1.38 ≤ NLR < 1.76) | Quartile 3 (1.76 ≤ NLR < 2.30) | Quartile 4 (NLR ≥ 2.30) |
| N | 1040 | 261 | 258 | 262 | 259 |
| Male1, n (%) | 490 (47.1) | 99 (37.9) | 119 (46.1) | 130 (49.6) | 142 (54.8) |
| Age (yr) | 71.9 ± 5.5 | 71.5 ± 5.5 | 72.0 ± 5.7 | 71.9 ± 5.4 | 72.3 ± 5.5 |
| BMI (kg/m2) | 25.0 ± 4.0 | 25.1 ± 3.6 | 25.3 ± 4.6 | 25.1 ± 3.7 | 24.7 ± 4.2 |
| SBP (mmHg) | 145.3 ± 20.3 | 144.8 ± 19.7 | 144.4 ± 19.4 | 146.5 ± 20.2 | 145.3 ± 22.0 |
| DBP (mmHg) | 78.7 ± 11.7 | 77.8 ± 11.5 | 79.0 ± 10.8 | 78.9 ± 11.0 | 79.2 ± 13.5 |
| FPG (mmol/L) | 7.8 ± 2.4 | 7.6 ± 1.8 | 8.0 ± 2.8 | 7.8 ± 2.4 | 8.0 ± 2.3 |
| BUN1 (mmol/L) | 6.8 ± 2.1 | 6.4 ± 1.6 | 6.9 ± 2.2 | 6.6 ± 2.0 | 7.1 ± 2.3 |
| Scr1 (mmol/L) | 74.1 ± 21.8 | 68.2 ± 16.1 | 73.1 ± 19.4 | 75.3 ± 22.1 | 79.8 ± 26.8 |
| eGFR1 (mL/min/1.73 m2) | 80.2 ± 15.7 | 83.6 ± 12.9 | 80.7 ± 15.0 | 79.3 ± 16.0 | 77.7 ± 17.0 |
| TC1 (mmol/L) | 4.7 ± 1.2 | 5.0 ± 1.2 | 4.8 ± 1.3 | 4.7 ± 1.1 | 4.6 ± 1.1 |
| TG (mmol/L) | 1.7 ± 1.5 | 1.6 ± 1.2 | 1.8 ± 2.0 | 1.7 ± 1.5 | 1.5 ± 1.1 |
| LDL-C1 (mmol/L) | 3.0 ± 0.9 | 3.2 ± 1.0 | 3.0 ± 1.0 | 2.9 ± 0.9 | 2.9 ± 0.9 |
| HDL-C1 (mmol/L) | 1.5 ± 0.4 | 1.5 ± 0.3 | 1.4 ± 0.3 | 1.5 ± 0.4 | 1.5 ± 0.4 |
| Neutrophil1 | 3.7 ± 1.0 | 2.9 ± 0.7 | 3.5 ± 0.8 | 3.9 ± 0.9 | 4.5 ± 1.0 |
| Lymphocyte1 | 2.1 ± 0.6 | 2.6 ± 0.7 | 2.2 ± 0.5 | 1.9 ± 0.4 | 1.5 ± 0.4 |
| WBC1 | 6.3 ± 1.4 | 6.1 ± 1.3 | 6.3 ± 1.4 | 6.3 ± 1.4 | 6.5 ± 1.4 |
| Platelet | 189.1 ± 48.5 | 187.6 ± 46.3 | 191.7 ± 46.2 | 191.2 ± 52.0 | 185.8 ± 49.4 |
| Smoking, n (%) | 174 (16.7) | 36 (13.8) | 48 (18.6) | 47 (17.9) | 43 (16.6) |
| Drinking, n (%) | 179 (17.2) | 36 (13.8) | 54 (20.9) | 48 (18.3) | 41 (15.8) |
| Regular exercise, n (%) | 197 (18.9) | 53 (20.3) | 52 (20.2) | 51 (19.5) | 41 (15.8) |
| Hypertension, n (%) | 714 (68.7) | 171 (65.5) | 182 (70.5) | 187 (71.4) | 174 (67.2) |
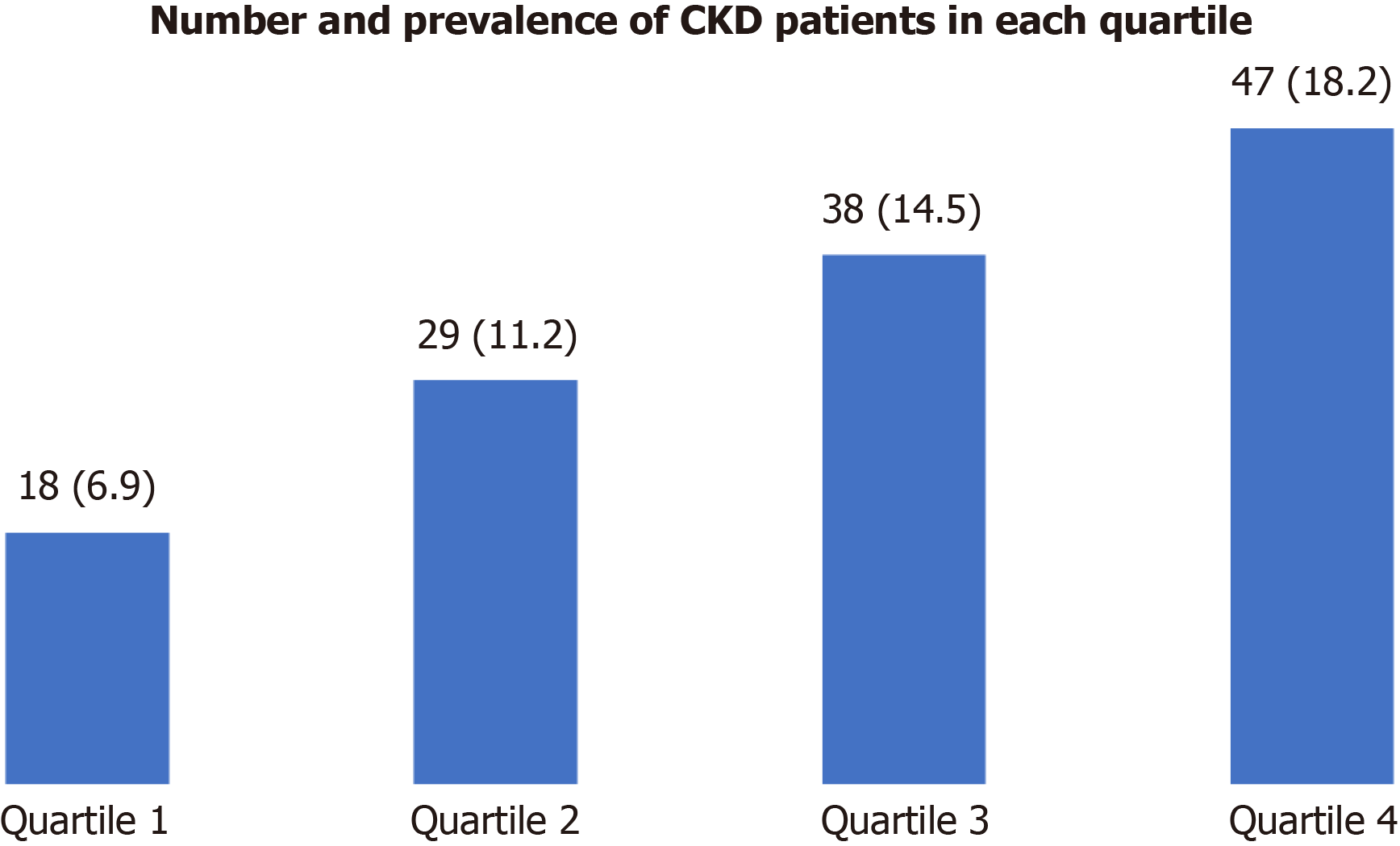
Using the definition of CKD, there were 132 cases in total. As shown in Figure 1, patients in the higher NLR quartile group had a greater prevalence of CKD (P for trend = 0.0011).
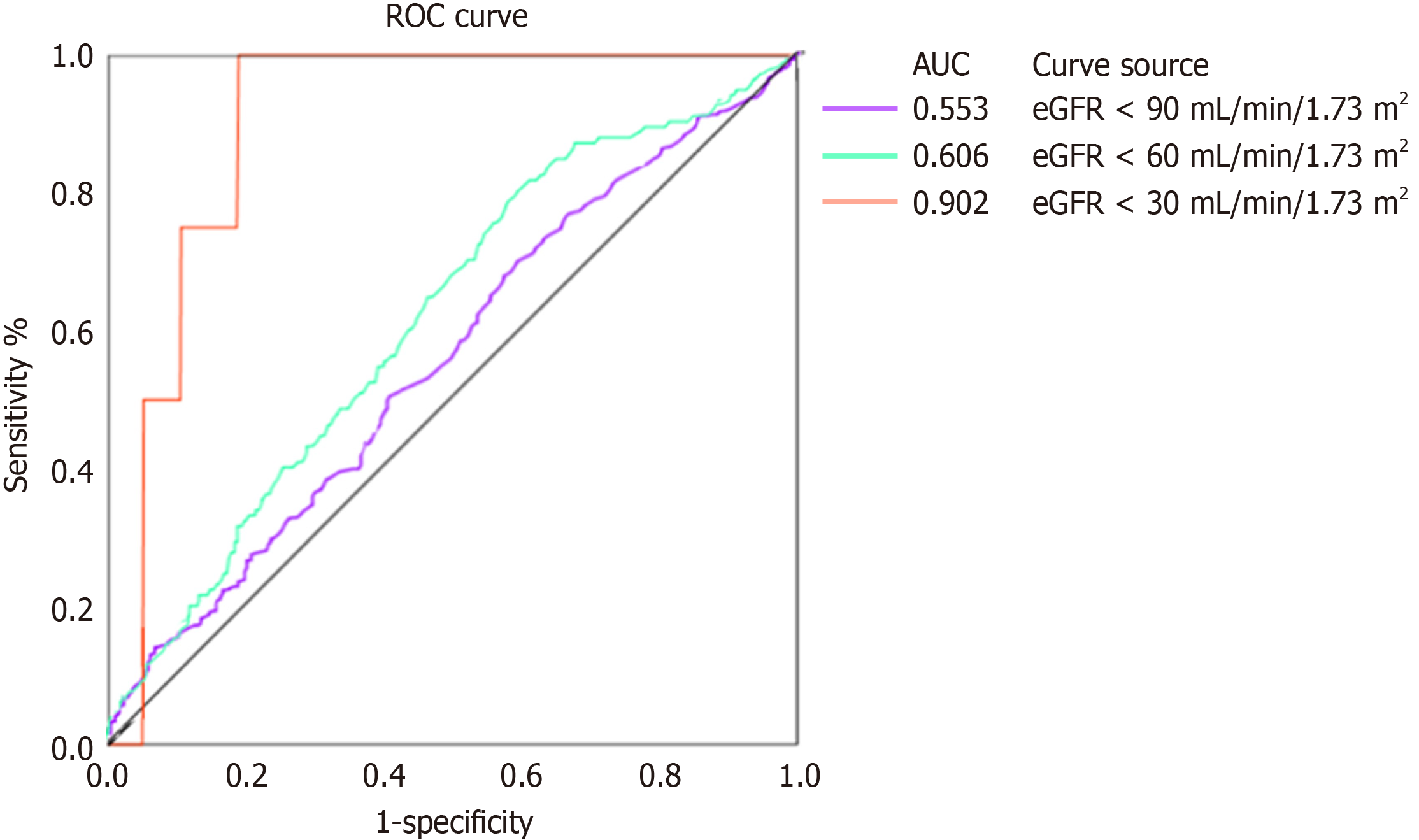
As shown in Table 2, we found that high NLR was associated with a higher prevalence of CKD in T2DM patients in multivariate regression models even after adjustment for important clinical parameters, including age, sex, smoking, drinking, regular exercise, BMI, SBP, FPG, TG and LDL-C (P value < 0.0001). With an SD increase in the NLR, the prevalence of CKD increased by 44%. Furthermore, when NLR was categorized into quartiles, the association between NLR and CKD remained, and there was a 3.3-fold increased prevalence of CKD in T2DM patients in the highest quartile of NLR (OR 3.30, 95%CI: 1.78-6.12, P = 0.0001) compared to those in the lowest quartile. AUROC was analyzed to de
| Exposure | Age-and-sex adjusted model | Multivariate model | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| NLR (per SD) | 1.39 (1.17-1.65) | 0.0002 | 1.44 (1.21-1.72) | < 0.0001 |
| Quartile 1 (< 1.38) | Reference | Reference | ||
| Quartile 2 (1.38–1.76) | 1.69 (0.89-3.22) | 0.1100 | 1.57 (0.81-3.04) | 0.1785 |
| Quartile 3 (1.76–2.30) | 2.42 (1.30-4.50) | 0.0052 | 2.52 (1.35-4.73) | 0.0039 |
| Quartile 4 (≥ 2.30) | 3.07 (1.68-5.62) | 0.0003 | 3.30 (1.78-6.12) | 0.0001 |
| P for trend | 0.0001 | < 0.0001 | ||
In this cross-sectional study comprising 1040 patients diagnosed with T2DM, we observed that higher NLR was sig
CKD is a common and severe complication in individuals with diabetes, although its precise pathogenesis remains poorly understood. It is recognized that a sequence of pathological events, including parenchymal cell loss, chronic in
NLR has recently emerged as a discerning inflammatory indicator that provides insights into the equilibrium between neutrophils and lymphocytes, two critical constituents of the immune system. While neutrophils function as nonspecific instigators of inflammation, lymphocytes play regulatory and protective roles in the context of inflammatory responses[18]. NLR has garnered recognition as a reliable metric for gauging the extent of systemic inflammation[19,20]. Previous studies have shown a positive association between NLR and well-established inflammation markers, such as interleukin-6 and C-reactive protein[21,22]. Notably, in comparison to other inflammatory markers, the NLR exhibits advantages in terms of stability, cost-effectiveness, and accessibility[23].
Growing evidence substantiates the connection between elevated NLR and progression and prognosis of CKD[24-28]. Although DKD is the most common cause of CKD, the relationship between NLR and CKD in T2DM patients is still relatively understudied[29]. Notably, a previous study showed a positive correlation between neutrophil levels and uri
Our study adds to the literature by providing compelling evidence of a significant association between NLR and CKD prevalence in patients with T2DM. However, it should be noted that this study had some limitations. First, as a cross-sectional study, a causal relationship between NLR and CKD could not be established. Second, the small sample might have introduced selection bias. Third, due to data constraints, our study population was not assessed for urine albumin and CRP, consequently relying on eGFR to evaluate kidney function and WBC counts to assess inflammatory markers as a control for NLR. Last, as this study was conducted at a single center and only included patients aged 65 years and older, the generalizability of our findings to other settings may be limited. Therefore, future investigations should consider lar
Our findings revealed a significant association between elevated NLR levels and reduced eGFR, as well as a higher prevalence of CKD in Chinese adults with T2DM. These results suggest that NLR has potential as a valuable biomarker for early detection of kidney damage in diabetes patients. However, it is crucial to emphasize the need for further re
The authors thank all team members and participants in the Community Health Service Center of Songnan Town, Baoshan District, Shanghai, China.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kurasawa S, Japan; Ohashi N, Japan S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition). Zhonghua Neike Zazhi. 2022;61:12-50. [RCA] [DOI] [Full Text] [Cited by in RCA: 83] [Reference Citation Analysis (0)] |
| 2. | Martínez-Castelao A, Navarro-González JF, Górriz JL, de Alvaro F. The Concept and the Epidemiology of Diabetic Nephropathy Have Changed in Recent Years. J Clin Med. 2015;4:1207-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 3. | Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao MH, Wang H. Trends in Chronic Kidney Disease in China. N Engl J Med. 2016;375:905-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S135-S151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 5. | Wu AY, Kong NC, de Leon FA, Pan CY, Tai TY, Yeung VT, Yoo SJ, Rouillon A, Weir MR. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the MicroAlbuminuria Prevalence (MAP) Study. Diabetologia. 2005;48:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Jha V. Current status of chronic kidney disease care in southeast Asia. Semin Nephrol. 2009;29:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1645] [Cited by in RCA: 2212] [Article Influence: 201.1] [Reference Citation Analysis (0)] |
| 8. | Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 9. | Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, Zhou F, Duan R, Chen W, Huang T, Wang M, Deng Q, Shi H, Zhou J, Jiang T, Zhang Y. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 10. | Ao G, Wang Y, Qi X, Wang F, Wen H. Association of neutrophil-to-lymphocyte ratio and risk of cardiovascular or all-cause mortality in chronic kidney disease: a meta-analysis. Clin Exp Nephrol. 2021;25:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Diabetics Branch of Chinese Medical Association. Guidelines for Prevention and Treatment of Type 2 Diabetes in China (2020 edition). Zhongguo Tangniaobing Zazhi. 2021;13:315-409. [DOI] [Full Text] |
| 12. | Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 566] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 13. | Revision Committee of Guidelines on Hypertension Prevention and Treatment. Guidelines for Hypertension Prevention and Treatment in China (2018 Revision). Zhongguo Xinxueguan Zazhi. 2019;24:24-56. [DOI] [Full Text] |
| 14. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 19983] [Article Influence: 1248.9] [Reference Citation Analysis (0)] |
| 15. | Ruiz-Ortega M, Rayego-Mateos S, Lamas S, Ortiz A, Rodrigues-Diez RR. Targeting the progression of chronic kidney disease. Nat Rev Nephrol. 2020;16:269-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 559] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 16. | Matoba K, Takeda Y, Nagai Y, Kawanami D, Utsunomiya K, Nishimura R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 17. | Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013;124:139-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 582] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 18. | Khandare SA, Chittawar S, Nahar N, Dubey TN, Qureshi Z. Study of Neutrophil-lymphocyte Ratio as Novel Marker for Diabetic Nephropathy in Type 2 Diabetes. Indian J Endocrinol Metab. 2017;21:387-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Winter L, Wong LA, Jerums G, Seah JM, Clarke M, Tan SM, Coughlan MT, MacIsaac RJ, Ekinci EI. Use of Readily Accessible Inflammatory Markers to Predict Diabetic Kidney Disease. Front Endocrinol (Lausanne). 2018;9:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Ko HL, Jung J, Lee J, Lim JH, Im DW, Kim YC, Paek JH, Park WY, Kim KM, Lee S, Lee SW, Shin SJ, Kim DK, Han SS, Baek CH, Kim H, Park JY, Ban TH, Kim K. Dynamic nature and prognostic value of the neutrophil-to-lymphocyte ratio in critically ill patients with acute kidney injury on continuous renal replacement therapy: A multicenter cohort study. Front Med (Lausanne). 2023;10:1162381. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Okyay GU, Inal S, Oneç K, Er RE, Paşaoğlu O, Paşaoğlu H, Derici U, Erten Y. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Karava V, Kondou A, Dotis J, Taparkou A, Farmaki E, Kollios K, Printza N. Exploring systemic inflammation in children with chronic kidney disease: correlates of interleukin 6. Pediatr Nephrol. 2024;39:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Yan YT, Liu HM, Kong YF, Liu JM, Li C, Zhao BC, Liu KX. Association of preoperative neutrophil-lymphocyte ratio with acute kidney injury in patients with non-cardiac surgery: difference among surgical types. Int Urol Nephrol. 2023;55:2647-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Yoshitomi R, Nakayama M, Sakoh T, Fukui A, Katafuchi E, Seki M, Tsuda S, Nakano T, Tsuruya K, Kitazono T. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren Fail. 2019;41:238-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Yuan Q, Wang J, Peng Z, Zhou Q, Xiao X, Xie Y, Wang W, Huang L, Tang W, Sun D, Zhang L, Wang F, Zhao MH, Tao L, He K, Xu H; C-STRIDE study group. Neutrophil-to-lymphocyte ratio and incident end-stage renal disease in Chinese patients with chronic kidney disease: results from the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J Transl Med. 2019;17:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Roumeliotis S, Neofytou IE, Maassen C, Lux P, Kantartzi K, Papachristou E, Schurgers LJ, Liakopoulos V. Association of Red Blood Cell Distribution Width and Neutrophil-to-Lymphocyte Ratio with Calcification and Cardiovascular Markers in Chronic Kidney Disease. Metabolites. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Lin CH, Li YH, Wang YY, Chang WD. Higher Neutrophil-To-Lymphocyte Ratio Was Associated with Increased Risk of Chronic Kidney Disease in Overweight/Obese but Not Normal-Weight Individuals. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Kuo YT, Wang YY, Lin SY, Chang WD. Age and sex differences in the relationship between neutrophil-to-lymphocyte ratio and chronic kidney disease among an adult population in Taiwan. Clin Chim Acta. 2018;486:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1762] [Article Influence: 220.3] [Reference Citation Analysis (0)] |
| 30. | Chung FM, Tsai JC, Chang DM, Shin SJ, Lee YJ. Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes Care. 2005;28:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Li L, Shen Q, Rao S. Association of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio with Diabetic Kidney Disease in Chinese Patients with Type 2 Diabetes: A Cross-Sectional Study. Ther Clin Risk Manag. 2022;18:1157-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 32. | Wan H, Wang Y, Fang S, Chen Y, Zhang W, Xia F, Wang N, Lu Y. Associations between the Neutrophil-to-Lymphocyte Ratio and Diabetic Complications in Adults with Diabetes: A Cross-Sectional Study. J Diabetes Res. 2020;2020:6219545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Huang W, Huang J, Liu Q, Lin F, He Z, Zeng Z, He L. Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol (Oxf). 2015;82:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Jaaban M, Zetoune AB, Hesenow S, Hessenow R. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as novel risk markers for diabetic nephropathy in patients with type 2 diabetes. Heliyon. 2021;7:e07564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Liu J, Liu X, Li Y, Quan J, Wei S, An S, Yang R, Liu J. The association of neutrophil to lymphocyte ratio, mean platelet volume, and platelet distribution width with diabetic retinopathy and nephropathy: a meta-analysis. Biosci Rep. 2018;38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Azab B, Daoud J, Naeem FB, Nasr R, Ross J, Ghimire P, Siddiqui A, Azzi N, Rihana N, Abdallah M, Patel P, Kleiner M, El-Sayegh S. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study). Ren Fail. 2012;34:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |