Published online May 6, 2024. doi: 10.12998/wjcc.v12.i13.2218
Revised: March 10, 2024
Accepted: March 29, 2024
Published online: May 6, 2024
Processing time: 82 Days and 14.7 Hours
The specific benefits of Yangxinshi tablet (YXST) in the treating chronic heart failure (CHF) remain uncertain.
To systematically evaluate the efficacy and safety of YXST in the treatment of CHF.
Randomized controlled trials (RCTs) investigating YXST for CHF treatment were retrieved from eight public databases up to November 2023. Meta-analyses of the included clinical studies were conducted using Review Manager 5.3.
Twenty RCTs and 1845 patients were included. The meta-analysis results showed that the YXST combination group, compared to the conventional drug group, significantly increased the clinical efficacy rate by 23% [relative risk (RR) = 1.23, 95%CI: 1.17-1.29], P < 0.00001), left ventricular ejection fraction by 6.69% [mean difference (MD) = 6.69, 95%CI: 4.42-8.95, P < 0.00001] and 6-min walk test by 49.82 m (MD = 49.82, 95%C: 38.84-60.80, P < 0.00001), and reduced N-terminal pro-B-type natriuretic peptide by 1.03 ng/L [standardized MD (SMD) = -1.03, 95%CI:
YXST effectively improves clinical symptoms and cardiac function in patients with CHF while maintaining a favorable safety profile, suggesting its potential as a therapeutic strategy for CHF.
Core Tip: Chronic heart failure (CHF) represents a severe manifestation and late-stage complication of various heart diseases. This study aims to conduct a systematic evaluation of the efficacy and safety of Yangxinshi tablet (YXST) in the treating CHF through meta-analysis. The results indicate that YXST effectively improved clinical symptoms and cardiac function in patients with CHF while maintaining a favorable safety profile, suggesting its potential as a therapeutic strategy for CHF.
- Citation: Lu SH, Yu YF, Dai SS, Hu YQ, Liu JH. Efficacy and safety of Yangxinshi tablet for chronic heart failure: A systematic review and meta-analysis. World J Clin Cases 2024; 12(13): 2218-2230
- URL: https://www.wjgnet.com/2307-8960/full/v12/i13/2218.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i13.2218
Chronic heart failure (CHF) is a complex clinical syndrome characterized by ventricular systolic and/or diastolic dys
As research advances, an increasing number of researchers are recognizing the potential role of Chinese medicine in enhancing the prognosis of CHF[11,12]. The treatment of CHF with traditional Chinese medicine (TCM) involves multiple components, targets, and mechanisms[13]. The Yangxinshi tablet (YXST) is a kind of proprietary Chinese medicine composed of Panax ginseng C. A. Mey. (Renshen), Astragalus membranaceus (Fisch.) Bunge. (Huangqi), Salvia miltiorrhiza Bge. (Danshen), Corydalis yanhusuo W.T.Wang (Yanhusuo), Crataegus pinnatifida Bge (Shanzha), Codonopsis pilosula (Franch.) Nannf. (Dangshen), Ganoderma lucidum (Leyss. ex Fr.) Karst. (Lingzhi), Pueraria lobata (Willd.) Ohwi (Gegen), Angelica sinensis (Oliv) Diels (Danggui), Epimedium grandiflorum Morr (Yinyanghuo), Rehmannia glutinosa (Gaetn.) DC (Dihuang), Coptis chinensis Franch (Huanglian), and Glycyrrhizae radix et Rhizoma (Gancao)[14]. YXST benefits Qi, warms Yang, activates blood circulation and reduces blood stasis. Moreover, YXST has been widely used since its development to treat CHF, coronary heart disease, myocardial infarction, depression, and other diseases[14]. YXST was identified to inhibit myocardial fibrosis and resist ventricular remodeling by inhibiting cardiomyocyte apoptosis[15]. In patients with CHF, YXST improves cardiac function by modulating multiple metabolic pathways, including oxidative stress, energy metabolism, and fatty acid and amino acid metabolism[16]. In patients with CHF, YXST also relieves anxiety and depression and increases exercise tolerance, thereby improving quality of life[17]. This may serve as a potential treatment strategy for patients with CHF. However, owing to the lack of high-quality evidence, the specific benefits of YXST in patients with CHF remain unclear. This was a meta-analysis of randomized controlled trials (RCTs) that evaluated the efficacy of YXST for the treatment of CHF. This study aimed to provide evidence-based support for the clinical use of YXST.
This meta-analysis is registered with International Prospective Register of Systematic Reviews under registration number CRD42024507360.
A comprehensive search was conducted in English and Chinese databases to identify all relevant clinical studies from the time of database inception to November 2023. The search was conducted using English and Chinese databases, including PubMed, Cochrane Library, Web of Science, EMBASE, China National Knowledge Infrastructure, Wanfang, VIP, and China Biomedical Literature Database. The search strategy used a combination of subject terms and free words. The subject terms used were YXST and CHF, and the free terms were supplemented by MeSH and the Cochrane Library. The search was independently conducted by authors Lu and Yu and any differences were resolved by discussion.
The inclusion criteria were as follows: (1) The study was designed as a RCT; (2) the included participants were adults (≥ 18 years) who met the diagnostic criteria for CHF[18]; (3) the experimental group received YXST in combination with conventional treatment, whereas the control group received conventional treatment alone; and (4) the efficacy indicators included clinical efficacy rate, N-terminal pro-B-type natriuretic peptide (NT-proBNP), brain natriuretic peptide (BNP), left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), 6-min walk test (6-MWT), and readmission rate. The clinical efficacy rate represented the proportion of patients with signs and symptoms of CHF in remission. The safety indicator was adverse events.
The exclusion criteria were as follows: (1) The same research results were repeatedly reported; and (2) unavailable data.
Literature screening, data collection, and risk-of-bias evaluation were independently performed by Lu and Yu. First, two researchers independently screened the literature using NoteExpress 3.9.0 software. Second, the two researchers independently organized and filled in the basic characteristics and data statistics tables of the included studies. Furthermore, the two researchers independently assessed the risk of bias in each study with the help of the Cochrane tools. At each step, the two researchers ensured that the results were consistent. Any disagreements that arose during this period were discussed and resolved by both researchers involved.
RevMan 5.3 software was used to perform the meta-analysis. Dichotomous variables were expressed as relative risk (RR) and 95%CI, whereas continuous variables were expressed as mean difference (MD) or standardized MD (SMD) with a 95%CI. When I-squared statistic (I2) was < 50%, a fixed-effects model was used to analyze the data. When I2 was ≥ 50%, a sensitivity analysis was required if significant clinical or methodological heterogeneity existed. A random-effects model was used if no significant clinical or methodological heterogeneity was detected. Results were considered statistically significant at P < 0.05. Egger’s test was used to assess publication bias, with P > 0.1 indicating no publication bias in the results.
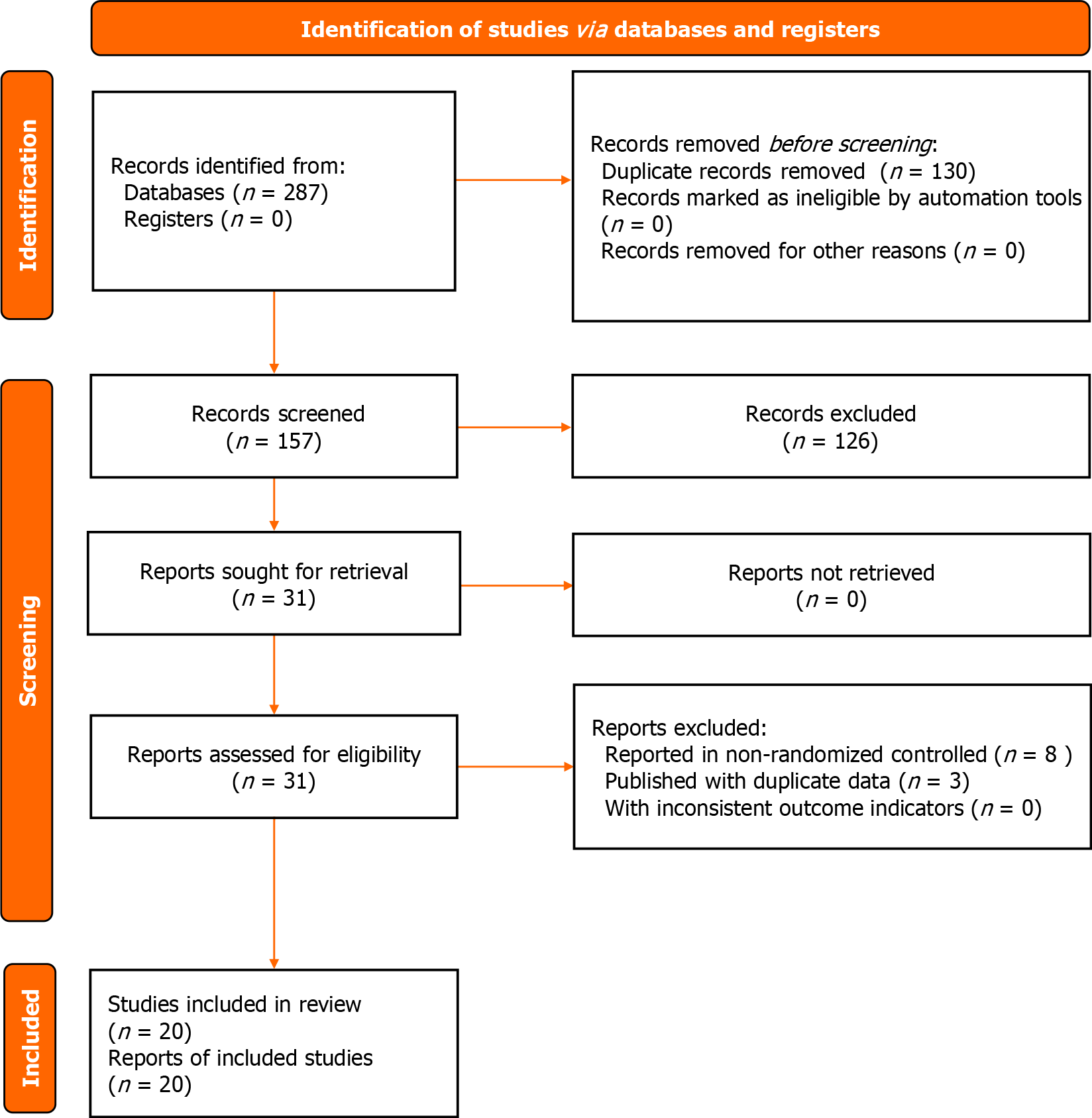
A total of 287 articles were retrieved from eight public databases. In the literature screening process, 130 duplicate articles were excluded along with 137 articles that did not conform to the research theme. Finally, 20 articles were included in this study[19-38]. The literature screening process is illustrated in Figure 1.
Twenty clinical trials and 1845 patients were included[19-38]. Of these, 935 patients were included in the YXST combi
| Ref. | Sample size | Male (%) | Age (yr) | Disease duration (yr) | Intervention | Treatment duration (wk) | Left ventricular function | Type of CHF | Baseline cardiac disease |
| Bai and Li[19], 2023 | 44 | 54.5 | 62.8 ± 5.3 | 10.6 ± 2.2 | Perindopril, metoprolol, trimetazidine, and YXST (0.18 g tid) | 4 | ICM | ||
| 44 | 52.3 | 62.0 ± 5.1 | 10.8 ± 2.4 | Perindopril, metoprolol, and trimetazidine | 4 | ||||
| Bai[20], 2019 | 50 | 50.0 | 66.6 ± 10.4 | Telmisartan, amlodipine, and YXST (0.18 g tid) | 4 | ICM | |||
| 50 | 52.0 | 66.5 ± 10.2 | Telmisartan and amlodipine | 4 | |||||
| Cheng et al[21], 2019 | 48 | 54.2 | 71.2 ± 3.6 | Levocarnitine and YXST (0.18 g tid) | 1 | Left ventricular diastolic dysfunction | HFpEF | ||
| 48 | 52.1 | 71.9 ± 3.8 | Levocarnitine | 1 | |||||
| Chen[22], 2019 | 20 | 70.0 | 64.0 ± 11.0 | ACEI/ARB, MRA, diuretic, cardiotonic, and YXST (0.18 g tid) | 4 | ||||
| 20 | 85.0 | 63.0 ± 10.0 | ACEI/ARB, MRA, diuretic, and cardiotonic | 4 | |||||
| Fan et al[23], 2020 | 63 | 52.4 | 66.0 ± 3.7 | Optimizing drug therapy and YXST (0.18 g tid) | 48 | ||||
| 63 | 57.1 | 67.0 ± 3.6 | Optimizing drug therapy | 48 | |||||
| Fu et al[26], 2014 | 64 | 65.6 | 65.0 ± 10.2 | 2.4 ± 1.2 | ACEI, diuretic, cardiotonic, and YXST (0.18 g tid) | 12 | NICM | ||
| 62 | 64.5 | 64.0 ± 10.8 | 2.3 ± 1.4 | ACEI, diuretic, and cardiotonic | 12 | ||||
| Gu et al[25], 2016 | 60 | 65.0 | 61.8 ± 11.8 | Perindopril, metoprolol, spironolactone, furosemide, isosorbide mononitrate, aspirin, clopidogrel, atorvastatin, and YXST (0.18 g tid) | 24 | ||||
| 60 | 70.0 | 62.5 ± 15.3 | Perindopril, metoprolol, spironolactone, furosemide, isosorbide mononitrate, aspirin, clopidogrel, and atorvastatin | 24 | |||||
| Gao and Zhang[24], 2021 | 39 | 51.3 | 62.54 ± 7.5 | Benazepril, metoprolol, furosemide, digoxin, and YXST (0.18 g tid) | 24 | ||||
| 39 | 53.8 | 63.0 ± 6.8 | Benazepril, metoprolol, furosemide, and digoxin | 24 | |||||
| Huang et al[27], 2009 | 63 | 58.7 | 59.8 ± 11.2 | 5.2 ± 4.3 | ACEI, β-blocker, diuretic, vasodilator, and YXST (0.18 g tid) | 4 | Left ventricular diastolic dysfunction | HFmrEF, HFpEF | |
| 62 | 58.1 | 61.2 ± 13.4 | 5.0 ± 4.9 | ACEI, β-blocker, diuretic, and vasodilator | 4 | ||||
| Li and Zhou[28], 2019 | 60 | 56.7 | 66.6 ± 12.5 | 5.7 ± 2.1 | Bisoprolol and YXST (0.12 g tid) | 24 | ICM | ||
| 60 | 43.3 | 64.9 ± 12.3 | 5.7 ± 2.0 | Bisoprolol | 24 | ||||
| Li[29], 2017 | 47 | 53.2 | 61.35 ± 8.7 | 9.3 ± 3.6 | Diuretic, vasodilator, trimetazidine, statin, and YXST (0.18 g tid) | 4 | ICM | ||
| 47 | 51.2 | 61.58 ± 7.6 | 9.52 ± 2.9 | Diuretic, vasodilator, trimetazidine, and statin | 4 | ||||
| Liu[30], 2022 | 33 | 48.5 | 58.4 ± 11.5 | 9.5 ± 3.1 | ACEI/ARB, β-blocker, MRA, diuretic, and YXST (0.18 g tid) | 12 | Left ventricular diastolic dysfunction | HFpEF | |
| 32 | 43.8 | 57.1 ± 12.8 | 10.2 ± 3.7 | ACEI/ARB, β-blocker, MRA, and diuretic | 12 | ||||
| Qian and Wei[31], 2012 | 56 | ACEI, β-blocker, diuretic, vasodilator, cardiotonic, and YXST (0.12g tid) | 12 | NICM | |||||
| 56 | ACEI, β-blocker, diuretic, vasodilator, and vardiotonic | 12 | |||||||
| Qu[32], 2008 | 89 | 61.8 | 52.0 ± 13.1 | ACEI, β-blocker, diuretic, vasodilator, cardiotonic, and YXST (0.18g tid) | 24 | ||||
| 82 | 62.2 | 53.3 ± 18.3 | ACEI, β-blocker, diuretic, vasodilator, and cardiotonic | 24 | |||||
| Sun et al[35], 2016 | 34 | 44.1 | 58.0 ± 13.1 | ACEI, β-blocker, diuretic, and YXST (0.18g tid) | 16 | ||||
| 34 | 52.9 | 54.3 ± 15.3 | ACEI, β-blocker, and diuretic | 16 | |||||
| Wang et al[33], 2011 | 34 | 64.7 | Spironolactone, hydrochlorothiazide, nitroglycerin, dobutamine, and YXST (0.24 g tid) | 2 | |||||
| 26 | 69.2 | Spironolactone, hydrochlorothiazide, nitroglycerin, and dobutamine | 2 | ||||||
| Yuan[34], 2012 | 40 | 52.5 | 68.7 ± 10.2 | 3.2 ± 0.7 | ACEI, β-blocker, diuretic, vasodilator, and YXST (0.24 g tid) | 4 | Left ventricular diastolic dysfunction | HFpEF | |
| 35 | 51.4 | 71.3 ± 13.1 | 2.9 ± 0.9 | ACEI, β-blocker, diuretic, and vasodilator | 4 | ||||
| Zhang and Niu[36], 2017 | 34 | 52.9 | 55.7 ± 9.6 | 6.0 ± 3.3 | Benazepril, metoprolol, losartan potassium, hydrochlorothiazide, and YXST (0.18 g tid) | 8 | Left ventricular diastolic dysfunction | HFmrEF, HFpEF | |
| 33 | 63.6 | 54.1 ± 9.6 | 6.1 ± 3.2 | Benazepril, metoprolol, losartan potassium, and hydrochlorothiazide | 8 | ||||
| Zhang[37], 2018 | 30 | 60.0 | 63.8 ± 4.8 | ACEI, β-blocker, MRA, and YXST (0.18 g tid) | 12 | ||||
| 30 | 56.7 | 62.6 ± 5.2 | ACEI, β-blocker and MRA | 12 | |||||
| Zhang[38], 2022 | 27 | 48.1 | 65.2 ± 5.3 | 5.2 ± 1.0 | ARNI/ARB, β-blocker, MRA, diuretic, vasodilator, cardiotonic, and YXST (0.18 g tid) | 8 | HFrEF | ||
| 27 | 58.6 | 64.1 ± 6.0 | 5.1 ± 1.2 | ARNI/ARB, β-blocker, MRA, diuretic, vasodilator, and cardiotonic | 8 |
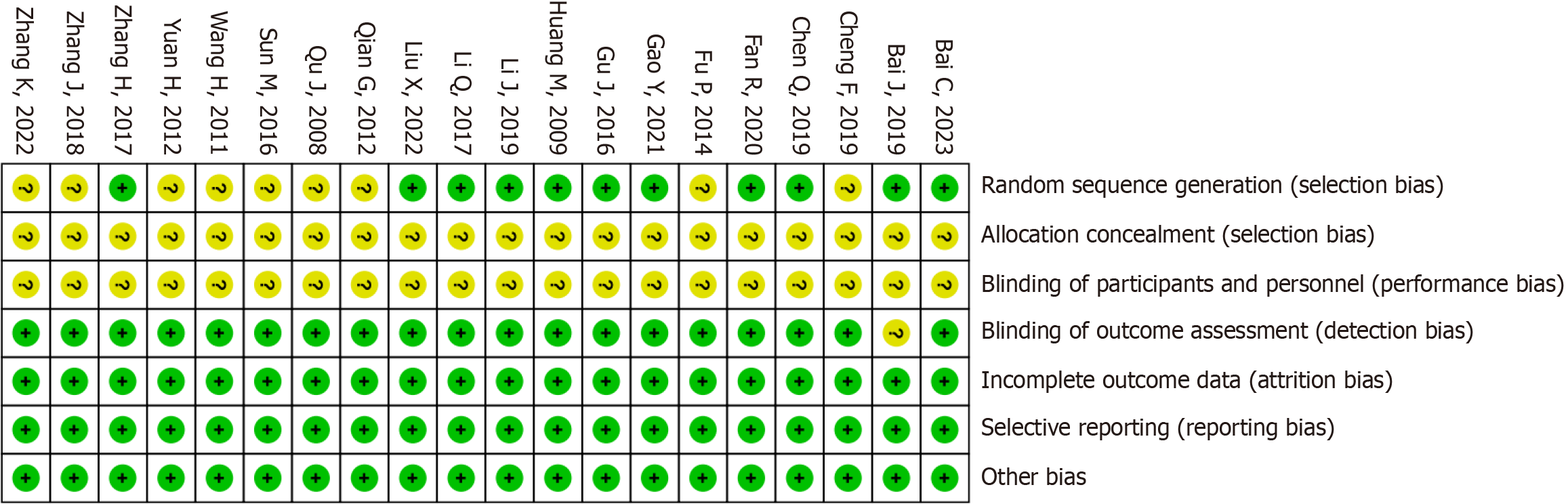
The risk of bias associated with the randomized approach was unclear in nine studies. Additionally, the risk of bias due to allocation concealment and intervention blinding was unclear in 20 studies. The risk of bias for the remaining areas was low. The risk of bias assessment is displayed in Figure 2.
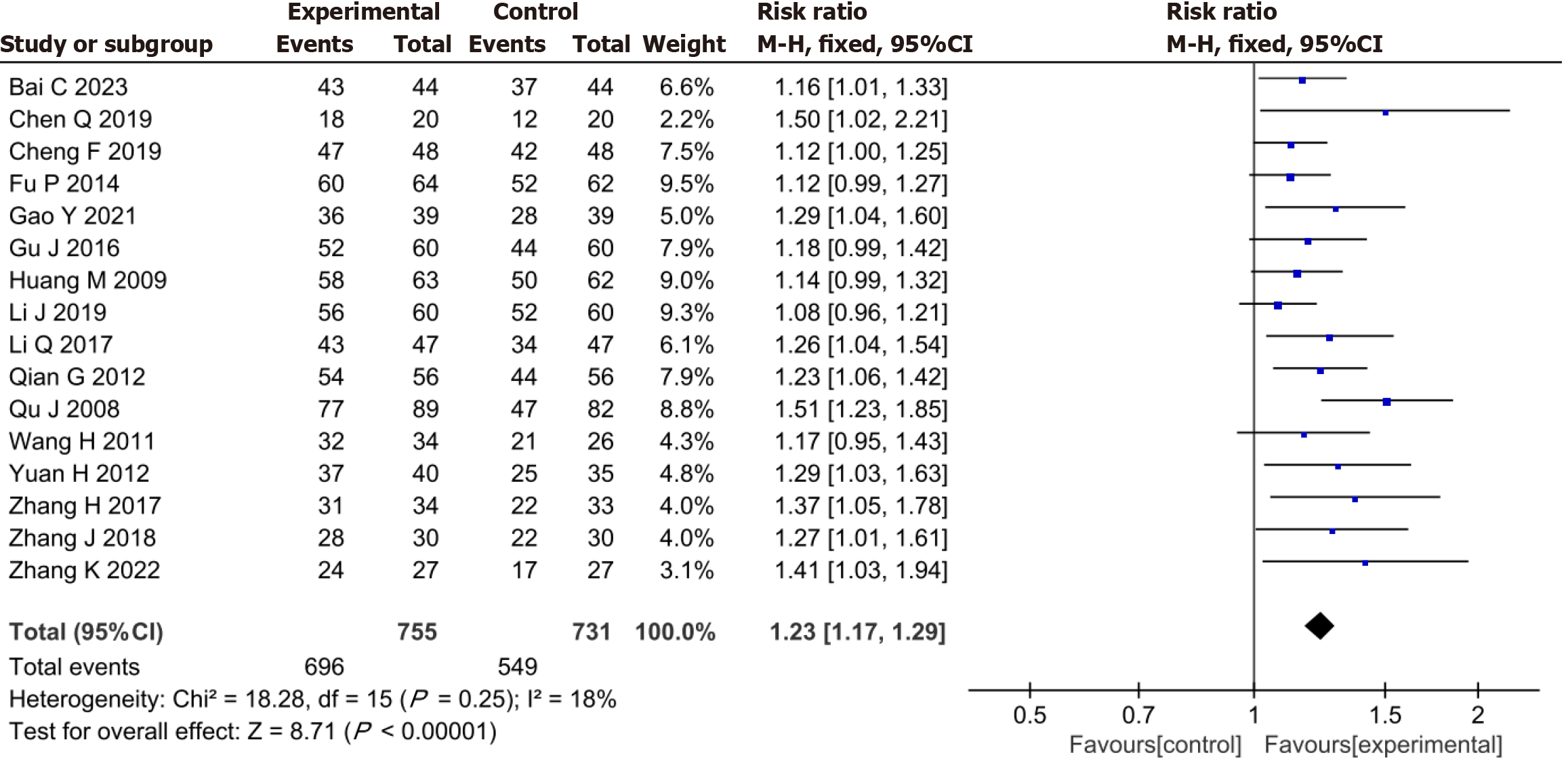
The meta-analysis demonstrated that the YXST combination group had a significantly increased clinical efficacy rate by 23% compared to that of the conventional drug group (RR = 1.23, 95%CI: 1.17-1.29, P < 0.00001) (Figure 3).
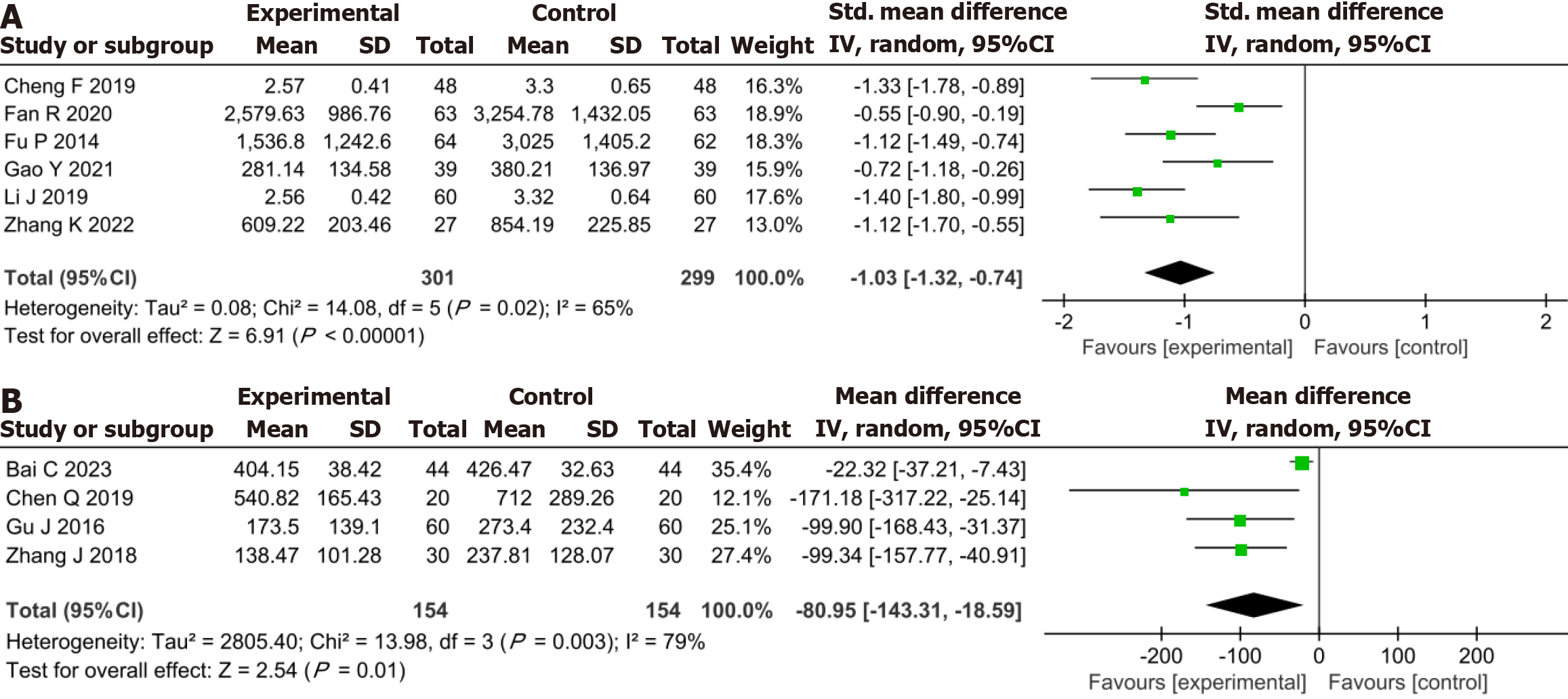
Meta-analysis demonstrated that in comparison to the conventional drug group, the YXST combination group reduced NT-proBNP by 1.03 ng/L (SMD = -1.03, 95%CI: -1.32 to -0.74, P < 0.00001) and BNP by 80.95 ng/L (MD = -80.95, 95%CI:
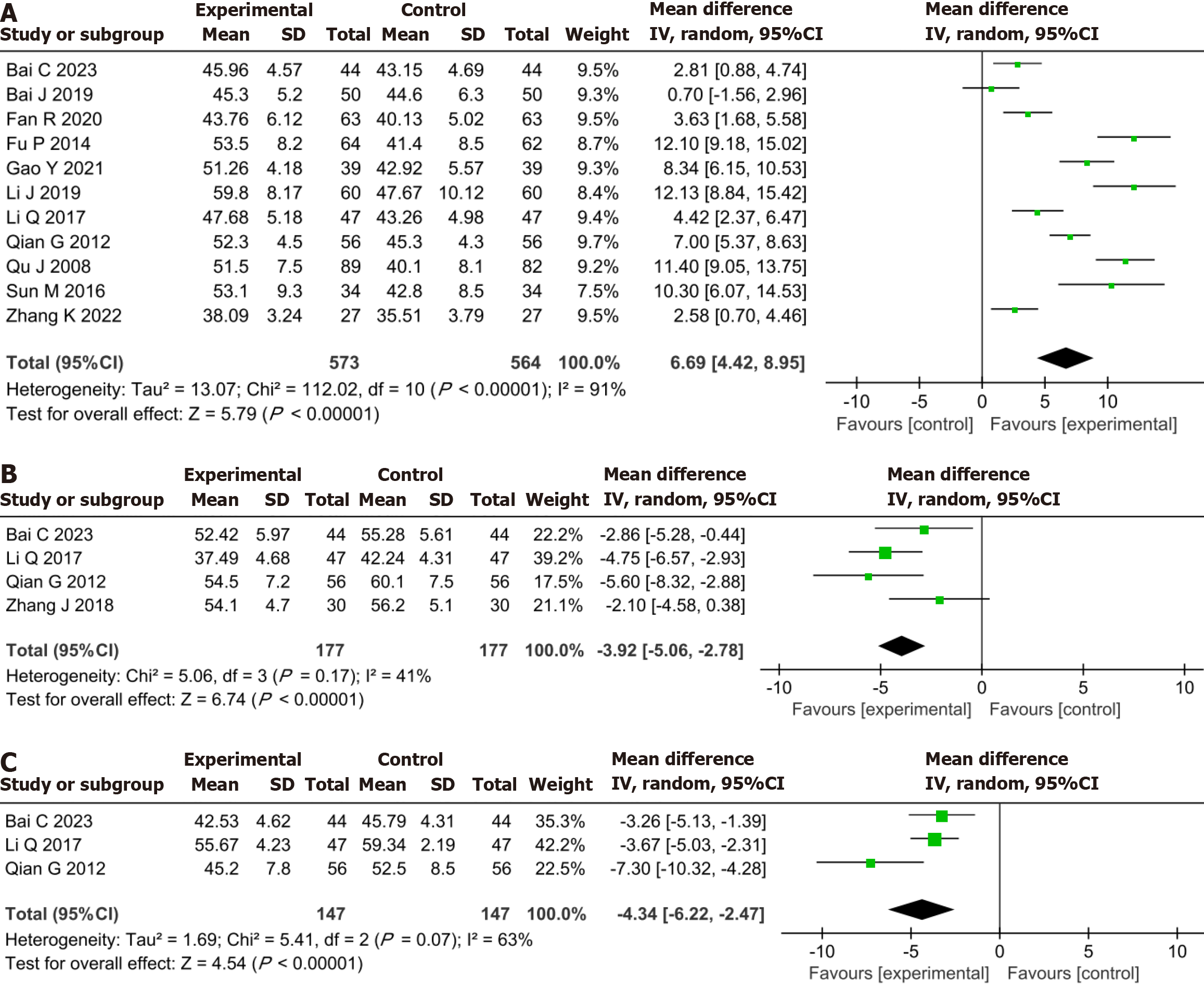
Meta-analysis demonstrated that the YXST combination group significantly increased LVEF by 6.69% (MD = 6.69, 95%CI: 4.42-8.95, P < 0.00001), reduced LVEDD by 3.92 mm (MD = -3.92, 95%CI: -5.06 to -2.78, P < 0.00001) and LVESD by 4.34 mm (MD = -4.34, 95%CI: -6.22 to -2.47, P < 0.00001) compared to the conventional drug group (Figure 5).
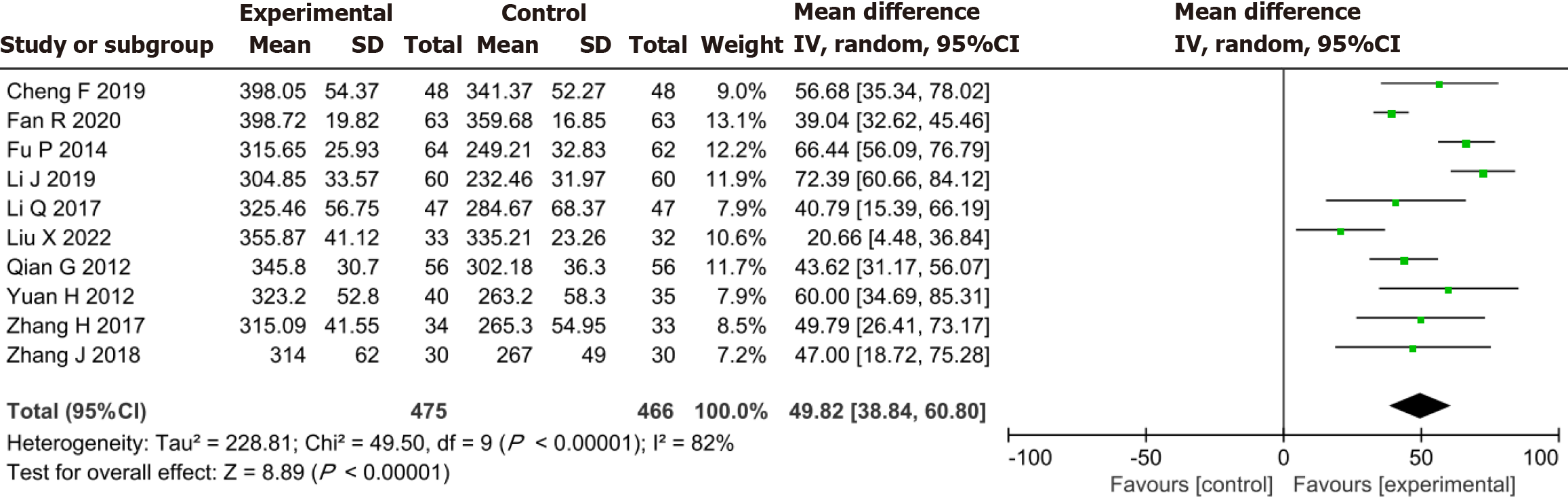
Meta-analysis established that the YXST combination group significantly increased 6-MWT by 49.82 m compared to the conventional drug group (MD = 49.82, 95%CI: 38.84-60.80, P < 0.00001) (Figure 6).
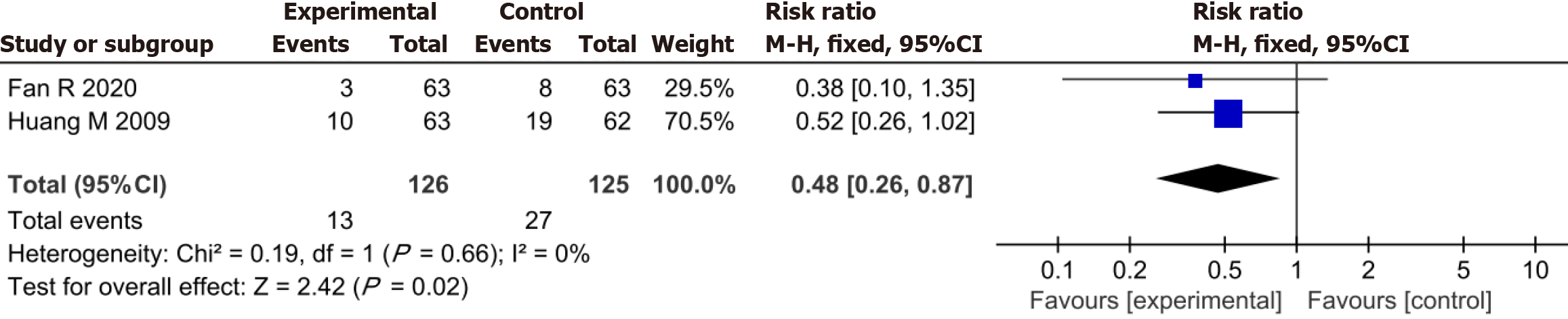
Thirteen patients in the YXST combination group and 27 in the conventional drug group were readmitted due to relapse during treatment. The readmission rate in the combined YXST group was significantly lower than that in the conventional drug group (RR = 0.48, 95%CI: 0.26-0.87, P = 0.02) (Figure 7).
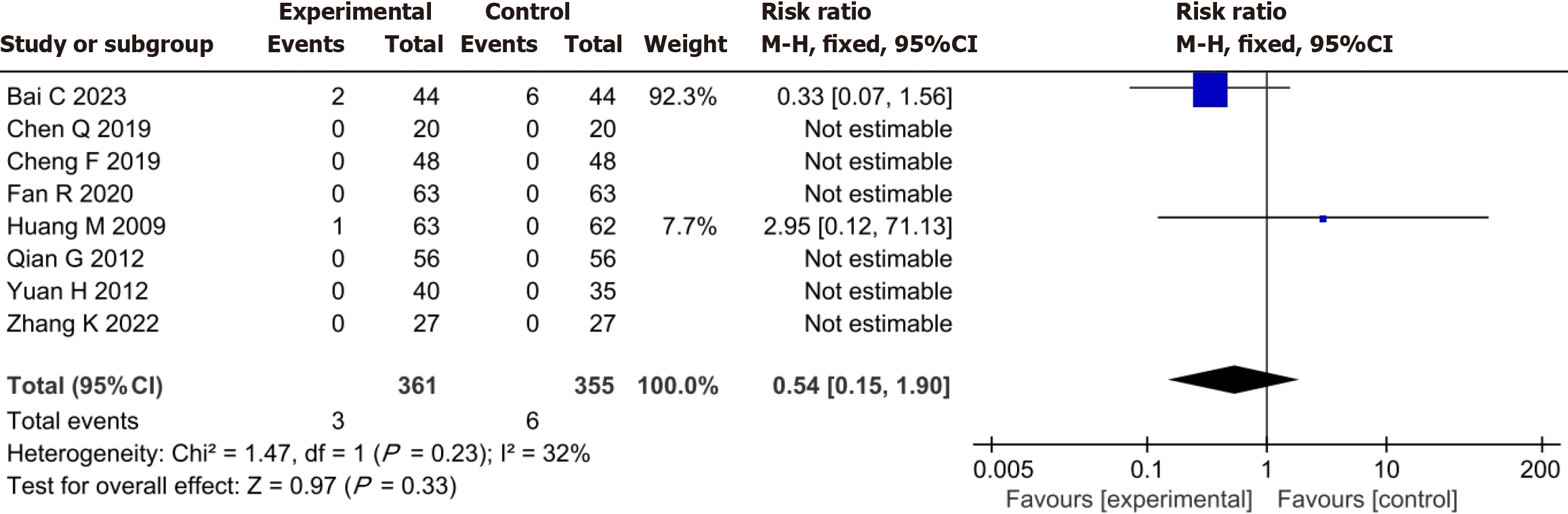
Three patients in the YXST combination group experienced adverse events, including one case of nausea, one case of slightly dry mouth, and one case of itchy skin. Six adverse events occurred in the conventional drug group, including three cases of nausea, two cases of abdominal distension, and one case of slightly dry mouth. No significant difference was observed in adverse events between the YXST combination group and the conventional drug group (RR = 0.54, 95%CI: 0.15-1.90, P = 0.33) (Figure 8).
HF with preserved ejection fraction (HFpEF) subgroup analysis was employed to explore the clinical efficacy of YXST in the treatment of HFpEF. The results confirmed that, compared to the conventional drug group, the YXST combination group significantly improved the clinical effective rate by 19% (RR = 1.19, 95%CI: 1.06-1.33, P = 0.003), and increased the 6-MWT by 44.61 m (MD = 44.61, 95%CI: 17.58-71.65, P = 0.001). Additionally, the YXST combination group decreased the NT-proBNP by 0.73 ng/L (MD = -0.73, 95%CI: -0.95 to -0.51, P < 0.00001). As shown in Table 2.
| Outcome | Sample size (E/C) | I2/% | MD/RR (95%CI) | P value |
| Clinical efficacy rate | 88/83 | 37 | 1.19 (1.06-1.33) | 0.003 |
| NT-proBNP | 48/48 | 0 | -0.73 (-0.95 to -0.51) | < 0.00001 |
| 6-MWT | 121/115 | 80 | 44.61 (17.58-71.65) | 0.001 |
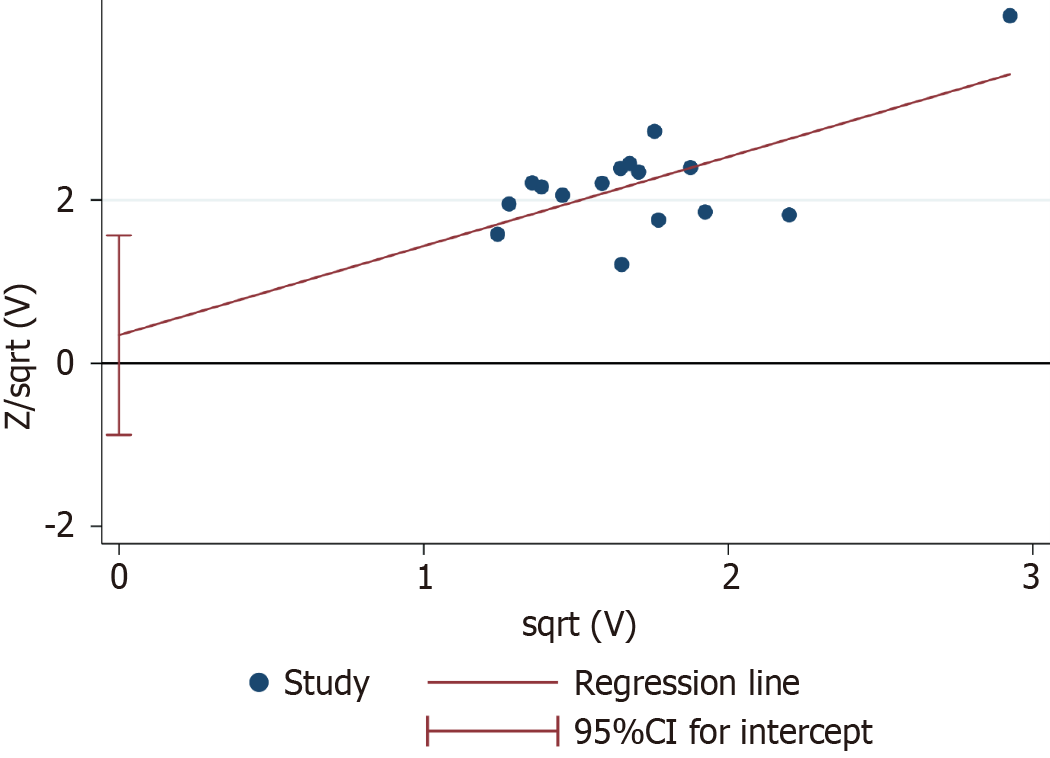
The clinical efficacy rate was defined as the primary efficacy endpoint. Egger’s test of the clinical efficacy rate demon
CHF is a severe manifestation or late stage of various heart diseases with high mortality and readmission rates[39]. The prevention and treatment of CHF have become a global public health concern. The pathogenesis of CHF is mainly related to ventricular remodeling. The overactivation of neuroendocrine and cytokine factors is closely related to the occurrence of ventricular remodeling. As the understanding of the pathogenesis of CHF has deepened, the treatment concept for CHF has produced a major shift from traditional cardiotonic, diuretic, and vasodilator approaches to the inhibition of excessive activation of the neuroendocrine system and ventricular remodeling[40,41]. An increasing number of studies have demonstrated that YXST can improve coronary blood flow, alleviate symptoms, such as shortness of breath caused by myocardial ischemia, and inhibit myocardial fibrosis and ventricular remodeling through its anti-inflammatory and antioxidant properties. This suggests that YXST may serve as a complementary treatment strategy for CHF[16]. This study included 20 RCTs involving 1845 patients. This is the first systematic evaluation and meta-analysis of YXST for the treatment of CHF intending to provide evidence-based support for the clinical use of YXST.
Our findings revealed that the YXST combination group significantly improved the clinical effective rate by 23% and 6-MWT by 49.82 m compared to the conventional treatment group. This suggests that YXST effectively reduces the signs and symptoms of HF and enhances exercise tolerance in patients with CHF. Furthermore, the combination group of YXST reduced NT-proBNP by 1.03 ng/L and BNP by 80.95 ng/L, indicating its role in slowing down the progression of CHF, as BNP and NT-proBNP are important reference indexes for measuring the overall prognostic efficacy of CHF. In terms of cardiac function, the combination group of YXST significantly increased LVEF by 6.69%, reduced LVEDD by 3.92 mm, and LVESD by 4.34 mm. LVEF represents the ratio of stroke volume to the left ventricular end-diastolic volume. The parameter serves as an objective indicator of the severity of HF. Mortality in patients with HF is closely correlated with the LVEF. Additionally, LVESD and LVEDD are indicative of the volume load on the left ventricle. Increases in LVEDD and LVESD signify cardiac dilation and compromised ventricular compliance. Both LVEF and LVEDD reflect the extent of left ventricular remodeling. These three outcome indicators suggest that YXST improves cardiac function and reverses ventricular remodeling to a certain extent. This confirms that YXST improves the patients' clinical symptoms and cardiac function, which may be the reason for the reduction in the readmission rate.
Regarding safety endpoints, the YXST combination group exhibited an adverse event rate of 0.83% (3/361), whereas the conventional drug group had an adverse event rate of 1.69% (6/355). Adverse event rates were comparable between the two groups. This suggests that YXST has a favorable safety profile. The adverse events that occurred in both groups mainly involved gastrointestinal events. As the researchers did not identify a correlation between these adverse events and YXST, we hypothesized that they may have been caused by conventional medications such as aspirin. However, owing to the narrow study base and sample size, more studies are required to further explore the safety of YXST.
HFpEF is the most common type of CHF, accounting for more than 50% of all cases[42]. An observational study in a western country demonstrated that the 1-year mortality rate of patients with HFpEF was 20%–29%, whereas the 5-year mortality rate was as high as 53%–74%[43]. SGLT2i and angiotensin receptor/neprilysin inhibitors (ARNI) are commonly used for HFpEF and they effectively improve its prognosis[18,44]. However, apart from SGLT2i and ARNI, few beneficial drugs are available for HFpEF. The current treatment regimens are still inadequate for the management of all patients with HFpEF[45]. In this study, we evaluated the clinical efficacy of YXST in treating HFpEF. The results of the HFpEF subgroup analysis demonstrated that YXST significantly increased the clinical effective rate by 19%, 6-MWT by 44.61 m, and decreased NT-proBNP by 0.73 ng/L in patients with HFpEF. This suggests that YXST can reduce clinical symptoms, enhance exercise tolerance, and improve the overall prognosis of patients with HFpEF. Therefore, we hypothesized that YXST has the potential to complement SGLT2i and ARNI in the treatment of HFpEF.
According to the TCM theory, CHF is attributed to prolonged involvement of the heart, leading to a deficiency of Yangqi and blood stasis. The key to the treatment of CHF is to benefit Qi, warm Yang, and invigorate blood circulation to eliminate blood stasis[22]. The compositional characteristics of YXST, with multiple drugs and components, determine its pharmacological mechanism of action through multitarget synergistic effects. Moreover, YXST regulates neuroendocrine and cytokine levels through various pathological and physiological pathways, thereby enhancing its effectiveness in preventing and treating CHF. A previous study has reported that Panax ginseng C. A. Mey. (Renshen), Astragalus membranaceus (Fisch.) Bunge. (Huangqi), and Salvia miltiorrhiza Bge. (Danshen) are the main contributors to the blood-entry components of YXST[46]. Ginsenoside Rb1 inhibits calcium ion channel activity in the cell membrane and enhances myocardial contractility. Astragalus membranaceus (Fisch.) Bunge. (Huangqi) is mainly composed of saponins and flavonoids. Total Astragalus saponin can increase coronary blood flow and relieve myocardial ischemia. Salvia officinalis is mainly composed of Salvia quinone/ketones and salvianolic acid components, which can reduce blood viscosity and enhance blood fluidity[46]. Gao[47] discovered that YXST could protect the myocardium at the metabolic level, mainly by regulating energy metabolism and the inflammatory immune response, thus exerting an anti-HF effect using ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry coupled with principal component analysis. Owing to the limited number of mechanistic studies related to YXST, further research is required to elucidate the specific mechanisms of action of the drug.
The study has some limitations: (1) The study only included a sample size of 1845, which may result in a lack of precision in the study's findings due to insufficient statistical validity; (2) the included studies may have potential selectivity and implementation biases, which may have reduced the confidence in the meta-analysis; (3) the duration of each included study ranged from 1 to 48 wk, and the lack of long-term follow-up results did not confirm the long-term effects of YXST on CHF; and (4) YXST is a common proprietary Chinese medicine that is currently being used mainly in China, leading to the fact that the experimental centers of the published clinical trials were all in China. This meta-analysis predominantly explains the role of YXST in people of Chinese ethnicity, and how the drug works in other ethnicities is not clear. In the future, more multicenter, double-blind, stratified RCTs are needed to further investigate the effects of factors such as ethnicity and treatment duration on the clinical efficacy of YXST and to provide high-quality evidence-based confirmation of the clinical significance of the drug.
YXST effectively improves clinical symptoms and cardiac function in patients with CHF while maintaining a favorable safety profile, suggesting its potential as a therapeutic strategy for CHF.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ito S, Japan S-Editor: Liu H L-Editor: A P-Editor: Zhao S
| 1. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4368] [Cited by in RCA: 4920] [Article Influence: 546.7] [Reference Citation Analysis (4)] |
| 2. | Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J Card Fail. 2022;28:e1-e167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 227] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 3. | Zhang Y, Gao C, Greene SJ, Greenberg BH, Butler J, Yu J, Zheng Z, Ma G, Wang L, Yang P, Ji X, Xu D, Wang J, Zhang Y, Liu Y, Zhao Y, Qi H, Zhai M, Feng J, Huang Y, Zhou Q, Zhang J. Clinical performance and quality measures for heart failure management in China: the China-Heart Failure registry study. ESC Heart Fail. 2023;10:342-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7267] [Article Influence: 1816.8] [Reference Citation Analysis (0)] |
| 5. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 1278] [Article Influence: 426.0] [Reference Citation Analysis (0)] |
| 6. | Sandri M, Viehmann M, Adams V, Rabald K, Mangner N, Höllriegel R, Lurz P, Erbs S, Linke A, Kirsch K, Möbius-Winkler S, Thiery J, Teupser D, Hambrecht R, Schuler G, Gielen S. Chronic heart failure and aging - effects of exercise training on endothelial function and mechanisms of endothelial regeneration: Results from the Leipzig Exercise Intervention in Chronic heart failure and Aging (LEICA) study. Eur J Prev Cardiol. 2016;23:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Wang H, Liu YJ, Yang JF. [Epidemiology of heart failure]. Linchuang Xinxueguanbing Zazhi.. 2023;39:243-247. [DOI] [Full Text] |
| 8. | Emmons-Bell S, Johnson C, Roth G. Prevalence, incidence and survival of heart failure: a systematic review. Heart. 2022;108:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 171] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 9. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 5484] [Article Influence: 1096.8] [Reference Citation Analysis (1)] |
| 10. | Shu H, Hang W, Peng Y, Nie J, Wu L, Zhang W, Wang DW, Zhou N. Trimetazidine Attenuates Heart Failure by Improving Myocardial Metabolism via AMPK. Front Pharmacol. 2021;12:707399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Zhu MJ. [Evaluation and consideration of the curative effects of traditional Chinese medicine in the treatment of chronic heart failure]. Beijing Zhongyiyao Daxue Xuebao. 2023;46:902-907. |
| 12. | Gao F, Fu Q, Bao SY, Ao WL. [Research progress of TCM in treatment of CHF]. Zhongyiyao Xinxi. 2022;39:85-89. [DOI] [Full Text] |
| 13. | Zang YB, Wan JJ, Zhang Z, Huang S, Liu X, Zhang W. An updated role of astragaloside IV in heart failure. Biomed Pharmacother. 2020;126:110012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Liu HX. [Interpretation of Consensus of Experts on Clinical Application of Yangxinshi Tablets in Treatment of Coronary Heart Disease]. Shijie Zhongyiyao. 2020;15:637-642. [DOI] [Full Text] |
| 15. | Guo ZG, Yu CX, Song XQ. [Effect of Yangxinshi Tablets on myocardial apoptosis in rats with heart failure]. Zhongguo Laonianxue Zazhi. 2018;38:3032-3035. [DOI] [Full Text] |
| 16. | Wang H, Cao YJ, Zhao ZY. [Progress of Yangxin tablet in the treatment of cardiovascular diseases and its mechanism of action]. Zhongxiyi Jiehe Xinnaoxueguanbing Zazhi. 2023;21:3188-3192. [DOI] [Full Text] |
| 17. | Chen YR, Lu SH, Wang Z, Cheng Y. [Based on network pharmacology and molecular docking to investigate the mechanism of Yangxinshi Tablet against myocardial fibrosis]. Zhongxiyi Jiehe Xinnaoxueguanbing Zazhi. 2023;21:1195-1201. [DOI] [Full Text] |
| 18. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Skibelund AK; ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 919] [Article Influence: 459.5] [Reference Citation Analysis (0)] |
| 19. | Bai CY, Li R. [Effect of Yangxin Tablet as an adjuvant therapy on patients with coronary heart disease and heart failure]. Sichuan Shenglikexue Zazhi. 2023;45:423-425. |
| 20. | Bai JB. [Evaluation of the clinical effect of Yangxinshi tablet in the intervention of heart failure caused by coronary heart disease]. Yixue Shiliao Yu Jiankang. 2019;14:128. |
| 21. | Cheng F, Wei YJ, Xu HJ, Gao Z, Chen JG, Zhou M. [Clinical study on Yangxinshi Tablets combined with levocarnitine in treatment of diastolic heart failure]. Xiandai Yaowu Yu Linchuang. 2019;34:1007-1011. [DOI] [Full Text] |
| 22. | Chen QY. [Clinical study of Yangxinshi tablet treating chronic heart failure with Qi deficiency and blood stasis syndrome]. M.Sc. Thesis, Anhui University of Chinese Medicine, 2019; 1-47. |
| 23. | Fan RY, Li WG, Liu YM, Liu P, Wang H. [Effect of Yangxinshi Tablet on cardiac function, neutrophil lymphocyte ratio and bilirubin in patients with chronic heart dysfunction]. Jiefangjun Yixue Zazhi. 2020;38:42-45. [DOI] [Full Text] |
| 24. | Gao YN, Zhang J. [Clinical observation of Yangxinshi Tablet in the treatment of type 2 diabetes mellitus complicated with chronic heart failure]. Shijie Zhongyiyao. 2021;16:961-964. [DOI] [Full Text] |
| 25. | Gu JL, Ye K, Wang XL, Zhou H, Xue JG. [Clinical observation on Yangxinshi Tablets on heart failure of coronary heart disease]. Zhongguo zhongyi jizhneg. 2016;25:868-871. [DOI] [Full Text] |
| 26. | Fu P, Huang ZD, Xie D. [Observation on curative effect of Yangxinshi Tablet treating heart failure caused by dilated cardiomyopathy]. Shijie Zhongyiyao. 2014;9:577-578. [DOI] [Full Text] |
| 27. | Huang M, Li HH, Zhang XN. [Clinical observation of Yangxinshi Tablet for treatment of diastolic heart failure]. Zhongxiyi Jiehe Xinnaoxueguanbing Zazhi. 2009;7:135-136. [DOI] [Full Text] |
| 28. | Li J, Zhou XL. [Clinical study on Yangxinshi Tablets combined with bisoprolol in the treatment of coronary heart disease and heart failure]. Shijie Zhongyiyao. 2019;14:3278-3281. [DOI] [Full Text] |
| 29. | Li Q. [Effect of Yangxinshi Tablets combined with Trimetazidine Hydrochloride Tablets on vascular endothelial function in coronary heart disease complicated with chronic heart failure]. Hebei Yiexue Zazhi. 2017;39:1222-1224. [DOI] [Full Text] |
| 30. | Liu XM. [Intervention studies of Yangxinshi on exercise intensity and quality of life on heart failure with preserved ejection fraction]. M.Sc. Thesis, Shandong University of Chinese Medicine, 2022; 1-46. |
| 31. | Qian GQ, Wei FP. [Observation of Yangxinshi Tablet treating 56 cases of heart failure with dilated cardiomyopathy]. Zhejiang Zhongyi Zazhi. 2012;47:850-851. [DOI] [Full Text] |
| 32. | Qu JW. [The effect of Yangxinshi on patients with chronic heart failure]. Xibu Yixue. 2008;20:989-990. [DOI] [Full Text] |
| 33. | Wang HL, Cheng XM, Guo JX, Guo YM. [Yangxinshi Tablet combined with western medicine to treat 60 cases of refractory heart failure]. Zhongguo Meirong Yixue Zazhi. 2011;20:502. [DOI] [Full Text] |
| 34. | Yuan H. [Clinical observation of Yangxinshi Tablet in adjuvant treatment of 75 cases of diastolic heart failure]. Haixia Yaoxue Zazhi. 2012;24:145-146. [DOI] [Full Text] |
| 35. | Sun ML, Wang P, Qiu TG, Liu WT. [Clinical observation of Yangxinshi Tablets in treatment of heart function in chronic heart failure with Qi deficiency and blood stasis syndrome]. Shijie Zhongyiyao. 2016;11:1507-1509. [DOI] [Full Text] |
| 36. | Zhang H, Niu TF. [Clinical observation of Yangxinshi Tablet treating 34 cases of diastolic heart failure of Qi deficiency and blood stasis syndrome]. Zhongxiyi Jiehe Xinnaoxueguanbing Zazhi. 2017;24:3148-3150. [DOI] [Full Text] |
| 37. | Zhang JL. [Effects of Yangxinshi Tablet on cardiac function and plasma levels of brain natriuretic peptide of patients with chronic heart disease]. Shijie Zhongyiyao. 2018;13:2148-2150. [DOI] [Full Text] |
| 38. | Zhang K. Effect of loading treatment with Yangxin's tablets on cardiac function and quality of life in patients with HFrEF (qi deficiency and blood stasis type). M.Sc. Thesis, Nanjing University of Chinese Medicine, 2022; 1-35. |
| 39. | Tomasoni D, Adamo M, Lombardi CM, Metra M. Highlights in heart failure. ESC Heart Fail. 2019;6:1105-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 40. | Chen J, Chen S, Zhang B, Liu J. SIRT3 as a potential therapeutic target for heart failure. Pharmacol Res. 2021;165:105432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43:2549-2561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 285] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 42. | Lloji A, Sreenivasan J, Novograd J, Aronow WS, Pan S, Lanier GM. Heart failure with preserved ejection fraction: key stumbling blocks for experimental drugs in clinical trials. Expert Opin Investig Drugs. 2022;31:463-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 999] [Article Influence: 124.9] [Reference Citation Analysis (0)] |
| 44. | Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1601] [Article Influence: 266.8] [Reference Citation Analysis (0)] |
| 45. | Meier ML, Pierce KN. New therapies for the treatment of heart failure with preserved ejection fraction. Am J Health Syst Pharm. 2022;79:1424-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 46. | Liu H, Li J, Guo CJ, Meng PP, Li Y, Yu HC. [Study and clinical efficacy of Yangxinshi Tablets in treatment of cardiovascular diseases]. Zhongguo Xunzheng Xinxueguan Yixue Zazhi. 2022;5:638-640. [DOI] [Full Text] |
| 47. | Gao Y. [Study on the anti-heart failure mechanism of Yangxinshi Tablet based on omics and network pharmacology]. MD Thesis, Second Military Medical University, 2017; 1-137. |