Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2134
Revised: February 8, 2024
Accepted: March 29, 2024
Published online: April 26, 2024
Processing time: 117 Days and 12.7 Hours
The application of machine learning (ML) algorithms in various fields of hepa
Core Tip: Artificial intelligence is trending topic in healthcare research. Machine learning classifiers have been explored in the field of liver surgery and liver trans
- Citation: Calleja R, Durán M, Ayllón MD, Ciria R, Briceño J. Machine learning in liver surgery: Benefits and pitfalls. World J Clin Cases 2024; 12(12): 2134-2137
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2134.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2134
We read with interest the retrospective study by Dong et al[1] that developed a machine learning (ML) prediction model for acute kidney injury (AKI) following liver resection (LR). We thank the authors for their work and contribution in this field. LR is the first-line treatment of various liver lesions. However, the reported incidence of AKI after LR ranges from 10% to 15%[2], significantly impacting patient morbidity and mortality. Hence, identifying factors that may lead to the development of AKI is relevant. Dong et al[1] explored the potential contribution of ML classifiers to this issue.
The authors analyzed a retrospective cohort of 2450 patients and trained and validated four ML classifiers (logistic regression, random forest, support vector machine, extreme gradient boosting, and decision tree). The training methodology (10-fold cross-validation) and validation (a holdout technique with 30% patterns) were adequate. Random forest exhibited the highest performance [area under the curve (AUC) = 0.92] among the classifiers. Although the results were satisfactory, certain considerations must be addressed.
First, the rate of missing values should be reported because it can affect model training, subsequently affecting model performance and generalizability. Hence, random forest classifiers are the best algorithms for a significant rate of missing values[3]. Conversely, if this rate is low, artificial neural networks (ANNs) could offer promising dataset results. Second, several factors reported in the literature are associated with AKI after LR, such as major hepatectomy, surgery duration, hepatojejunostomy, increased Model for End-Stage Liver Disease score (MELD), and blood transfusion[2,4-8]. Among these factors, only surgery duration was included in the baseline characteristics. The inclusion of these variables may have increased the robustness of the model. Finally, performing external validation is challenging. Differences between the training and external validation cohorts may impact model accuracy. Therefore, a prospective validation may be an alternative.
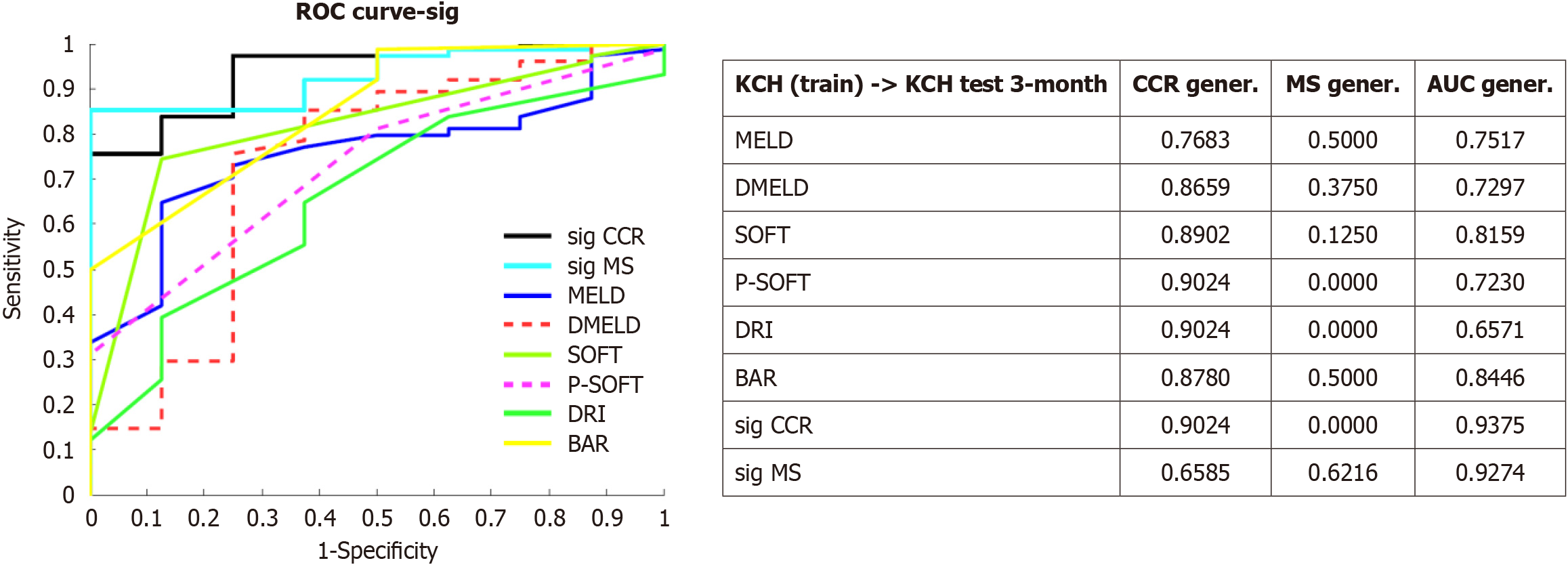
Some recent studies in ML applications ranges from protein structure prediction or COVID-19 diagnosis from X-ray images to optimizing donor-recipient matching to reduce waitlist mortality or improve post-transplant outcomes[9-11]. Our experience in the field of ML in liver surgery started from liver transplantation and efforts primarily focused on improving donor-recipient matching. Using graft survival as the endpoint, we developed an ANN model that achieved an AUC of approximately 0.8212[12]. This method was validated in an external cohort and improved AUC by 15%[13]. This ANN was integrated into a rule system with the MELD score to prioritize graft allocation. Although this method was explored in the United Network of Organ Sharing database, limited results were obtained because of a significant proportion of missing values were found[14]. Dong et al[1] found that the model performance was better than the current scores for AKI prediction. Similarly, we reported the difference of ML models that outperformed traditional scores, such as MELD, Survival Outcomes Following Liver Transplantation score, Donor Risk Index score, and Balance of Risk score (Figure 1). In medicine, certain variables do not necessarily have to assume a linear relationship. Hence, ML models are superior to statistical methods (linear regression), from which most of these scores are derived[15]. However, these findings may be attributed to model overtraining; therefore, validation is required.
The most significant lesson learned from using these models is their high dependency on the datasets on which they were trained. This issue affects the practical applicability. Retrospective data, external validation, the “black box issues” in ANN, and data-protection policies are considered significant contributing factors. To overcome these barriers, better data-handling policies are needed. Applicability relies on the clinicians’ confidence in using these models. Therefore, if external validation is impossible (region-specific rather than universal models), prospective validation should be considered. Moreover, the databases must be updated regularly to reinforce the learning of these models. Clinical scenarios are dynamic, and models must change accordingly.
Recently, interest in artificial intelligence and ML has increased. They can handle large amounts of data quickly and yield accurate results. However, we must note the limitations of these models and address them to achieve a real integration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu S, China S-Editor: Che XX L-Editor: A P-Editor: Zheng XM
| 1. | Dong JF, Xue Q, Chen T, Zhao YY, Fu H, Guo WY, Ji JS. Machine learning approach to predict acute kidney injury after liver surgery. World J Clin Cases. 2021;9:11255-11264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Lim C, Audureau E, Salloum C, Levesque E, Lahat E, Merle JC, Compagnon P, Dhonneur G, Feray C, Azoulay D. Acute kidney injury following hepatectomy for hepatocellular carcinoma: incidence, risk factors and prognostic value. HPB (Oxford). 2016;18:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Calleja Lozano R, Hervás Martínez C, Briceño Delgado FJ. Crossroads in Liver Transplantation: Is Artificial Intelligence the Key to Donor-Recipient Matching? Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Kambakamba P, Slankamenac K, Tschuor C, Kron P, Wirsching A, Maurer K, Petrowsky H, Clavien PA, Lesurtel M. Epidural analgesia and perioperative kidney function after major liver resection. Br J Surg. 2015;102:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Peres LA, Bredt LC, Cipriani RF. Acute renal injury after partial hepatectomy. World J Hepatol. 2016;8:891-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Reese T, Kröger F, Makridis G, Drexler R, Jusufi M, Schneider M, Brüning R, von Rittberg Y, Wagner KC, Oldhafer KJ. Impact of acute kidney injury after extended liver resections. HPB (Oxford). 2021;23:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Slankamenac K, Beck-Schimmer B, Breitenstein S, Puhan MA, Clavien PA. Novel prediction score including pre- and intraoperative parameters best predicts acute kidney injury after liver surgery. World J Surg. 2013;37:2618-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Tomozawa A, Ishikawa S, Shiota N, Cholvisudhi P, Makita K. Perioperative risk factors for acute kidney injury after liver resection surgery: an historical cohort study. Can J Anaesth. 2015;62:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 10. | Cai C, Gou B, Khishe M, Mohammadi M, Rashidi S, Moradpour R, Mirjalili S. Improved deep convolutional neural networks using chimp optimization algorithm for Covid19 diagnosis from the X-ray images. Expert Syst Appl. 2023;213:119206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24593] [Cited by in RCA: 22579] [Article Influence: 5644.8] [Reference Citation Analysis (0)] |
| 12. | Briceño J, Cruz-Ramírez M, Prieto M, Navasa M, Ortiz de Urbina J, Orti R, Gómez-Bravo MÁ, Otero A, Varo E, Tomé S, Clemente G, Bañares R, Bárcena R, Cuervas-Mons V, Solórzano G, Vinaixa C, Rubín A, Colmenero J, Valdivieso A, Ciria R, Hervás-Martínez C, de la Mata M. Use of artificial intelligence as an innovative donor-recipient matching model for liver transplantation: results from a multicenter Spanish study. J Hepatol. 2014;61:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Ayllón MD, Ciria R, Cruz-Ramírez M, Pérez-Ortiz M, Gómez I, Valente R, O'Grady J, de la Mata M, Hervás-Martínez C, Heaton ND, Briceño J. Validation of artificial neural networks as a methodology for donor-recipient matching for liver transplantation. Liver Transpl. 2018;24:192-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Guijo-Rubio D, Briceño J, Gutiérrez PA, Ayllón MD, Ciria R, Hervás-Martínez C. Statistical methods versus machine learning techniques for donor-recipient matching in liver transplantation. PLoS One. 2021;16:e0252068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Briceño J, Calleja R, Hervás C. Artificial intelligence and liver transplantation: Looking for the best donor-recipient pairing. Hepatobiliary Pancreat Dis Int. 2022;21:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |