Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2128
Peer-review started: January 16, 2024
First decision: February 6, 2024
Revised: February 9, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: April 26, 2024
Processing time: 91 Days and 5.2 Hours
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease primarily affecting young females. SLE can invade any organ, and various forms of splenic invasion have been reported. Manifestations include splenomegaly and splenic infarction, rupture, and calcification. The study encountered a rare case of splenic involvement, with nodules of various sizes without calcifications or ruptures.
A 15-year-old girl presented with arthralgia, weight loss, fever, increased levels of inflammatory markers, and positive antinuclear antibody test results. The patient was diagnosed with SLE. She was asymptomatic while taking steroids and hydro
As disease activity increased, multiple nodules in the spleen that were previously unseen were observed using AP-CT and histologically confirmed. Spleen invasion by SLE can appear in multiple nodular forms and patterns. Therefore, physicians should consider these findings when differentiating these nodules from infections and malignancies.
Core Tip: Systemic lupus erythematosus (SLE) is a chronic inflammatory disease primarily affecting young females. Here, the study reports a rare case of a 15-year-old female with SLE. During follow-up, the patient developed splenic involvement with nodules of various sizes, which are not typical manifestations. The patient was diagnosed with an SLE flare-up and was maintained on high-dose steroids. The study emphasizes the importance of an accurate diagnosis and careful observation of rare splenic manifestations of SLE.
- Citation: Kang MI, Kwon HC. Systemic lupus erythematosus in a 15-year-old female with multiple splenic nodules: A case report. World J Clin Cases 2024; 12(12): 2128-2133
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2128.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2128
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease mainly affecting young females, including those of childbearing age[1]. SLE is commonly observed in children, and less than 20% of all cases occur at a young age[2]. SLE can invade organs of the body, resulting in various symptoms. Spleen invasion often manifests as splenomegaly, splenic infarction, and rupture[3]. Similar to other rheumatic diseases, such as rheumatoid arthritis and systemic sclerosis, splenic calcification may occur in SLE[4]. It is often necessary to differentiate SLE from other diseases, such as infections or malignancies; thus, understanding the various clinical findings in the affected organs is crucial. Herein, the study reports a rare case of splenic involvement with nodules of various sizes in a 15-year-old girl with SLE.
A 15-year-old female patient presented to the Department of Rheumatology with a 3-month history of multiple articular pain and weight loss.
The patient was admitted to our hospital. Approximately 3 months before admission, she experienced weight loss of 6 kg and pain in both shoulders and knees. She had left pleural pain and a fever of up to 38.3 °C that began 2 wk before admission. She also had bilateral pelvic pain that began 1 wk before admission.
She had no relevant medical history.
Personal or family history was unremarkable.
Physical examination revealed a blood pressure of 100/66 mmHg, pulse rate of 115 beats/min, and respiratory rate of 20 breaths/min. Her body temperature was 36.5 °C at the time of admission.
Laboratory results showed normal levels of white blood cells (3.89 × 103/μL); lymphocytes (0.65 × 103/μL); hemoglobin (8.6 g/dL); platelet (331 × 103/μL); C-reactive protein (1.25 mg/dL); erythrocyte sedimentation rate (63 mm/h); serum creatinine (0.43 mg/dL); antinuclear antibodies (1:1280 with a homogeneous pattern); anti-double-stranded DNA antibodies (2470.4 IU/mL) (normal range < 7); complement component 3 (70.6 mg/dL) (normal range: 70-206); and complement component 4 (6.3 mg/dL) (normal range: 11-61). The patient tested positive for anti-cardiolipin IgG (46.0 GPL) (normal range < 23), anti-Ro (2.43 AI) (normal range ≤ 0.90), anti-La (6.60 AI) (normal range ≤ 0.90), anti-Smith (1.93 AI) (normal range ≤ 0.90), and anti-RNP antibodies (6.69 AI) (normal range ≤ 0.90). Proteinuria was not observed on urinalysis. All bacterial cultures and tuberculosis yielded negative results.
Left costophrenic angle blunting was observed during chest examination, and electrocardiography showed sinus tachycardia.
SLE was diagnosed based on clinical symptoms, antibody test results, and blood test results, according to the 2012 Systemic Lupus International Collaborating Clinics criteria[5].
High-dose steroids, hydroxychloroquine, and nonsteroidal anti-inflammatory drugs were administered, and almost all symptoms improved. After the prednisolone dose reduction to 20 mg, outpatient follow-up was performed every 2 or 4 wk. Afterward, the prednisolone dose was gradually reduced to 5 mg. Additionally, the interval between outpatient visits was increased to 2 months. Topical tacrolimus was administered because of facial malar rash and photosensitivity.
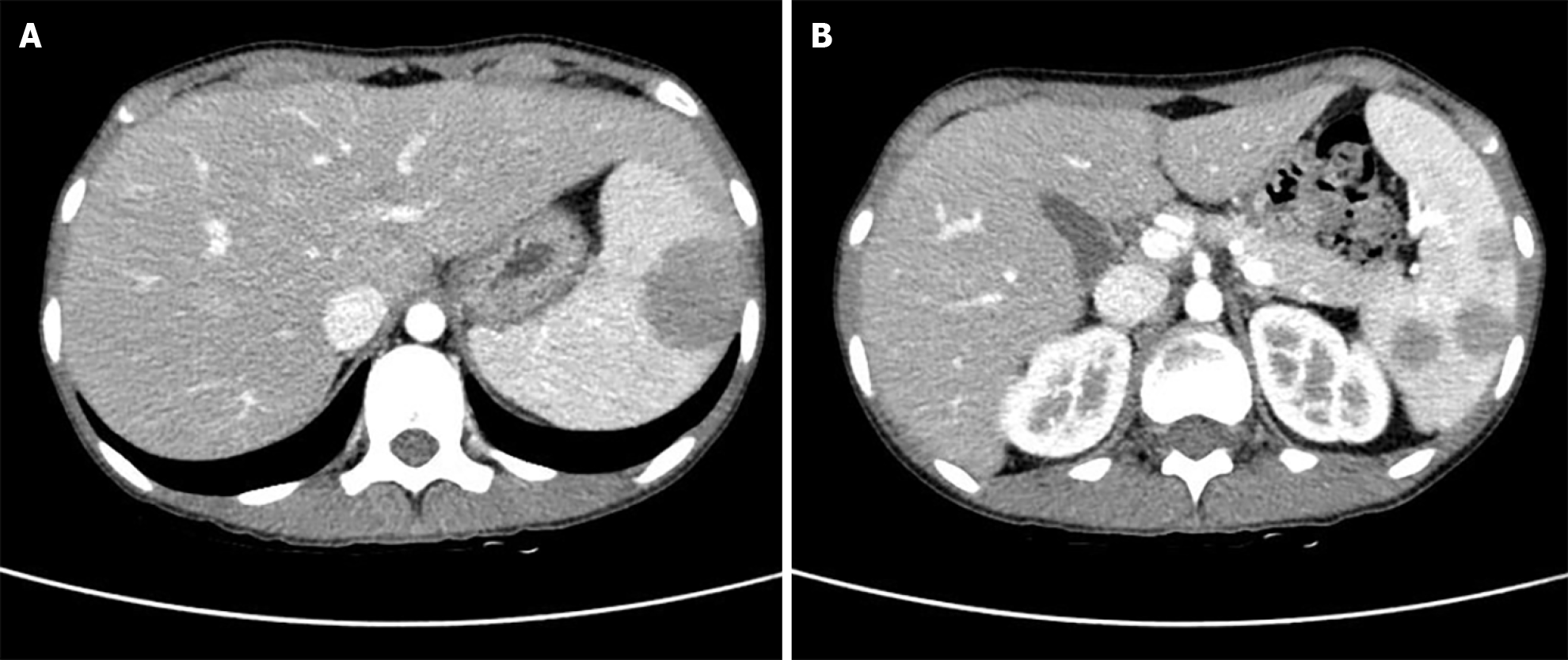
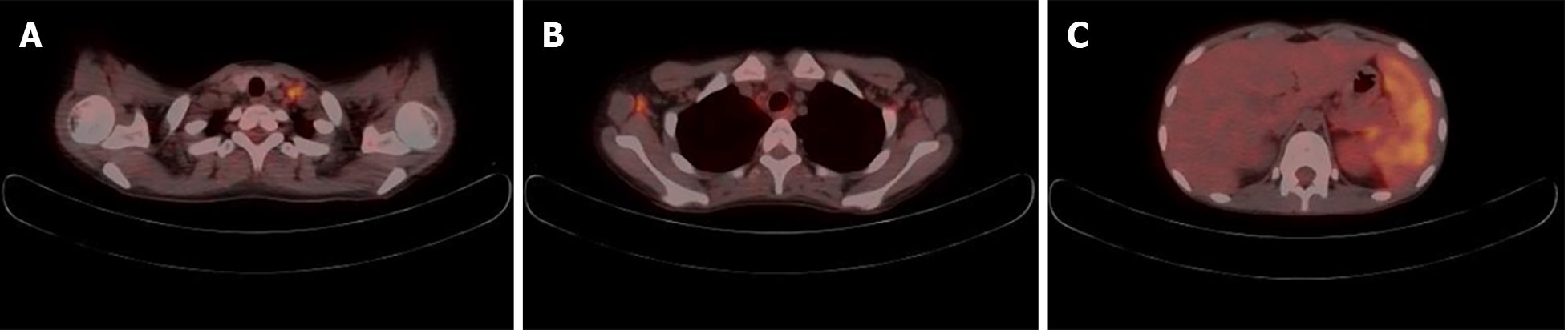
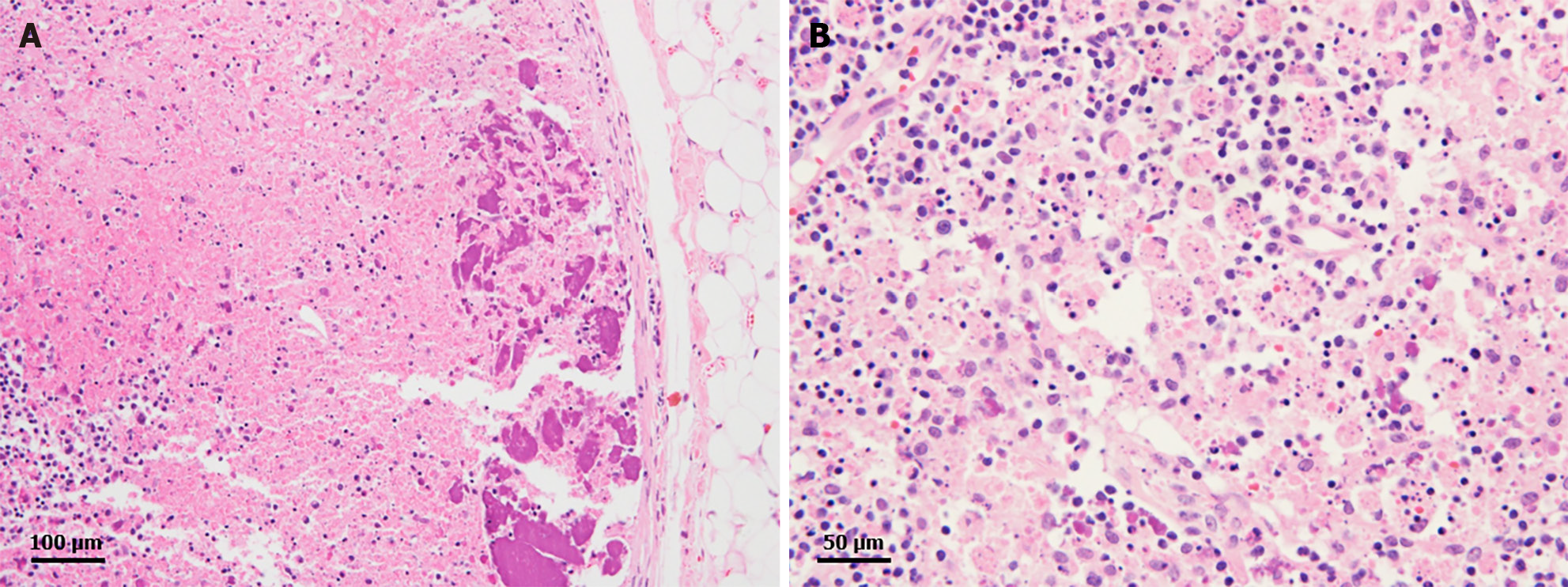
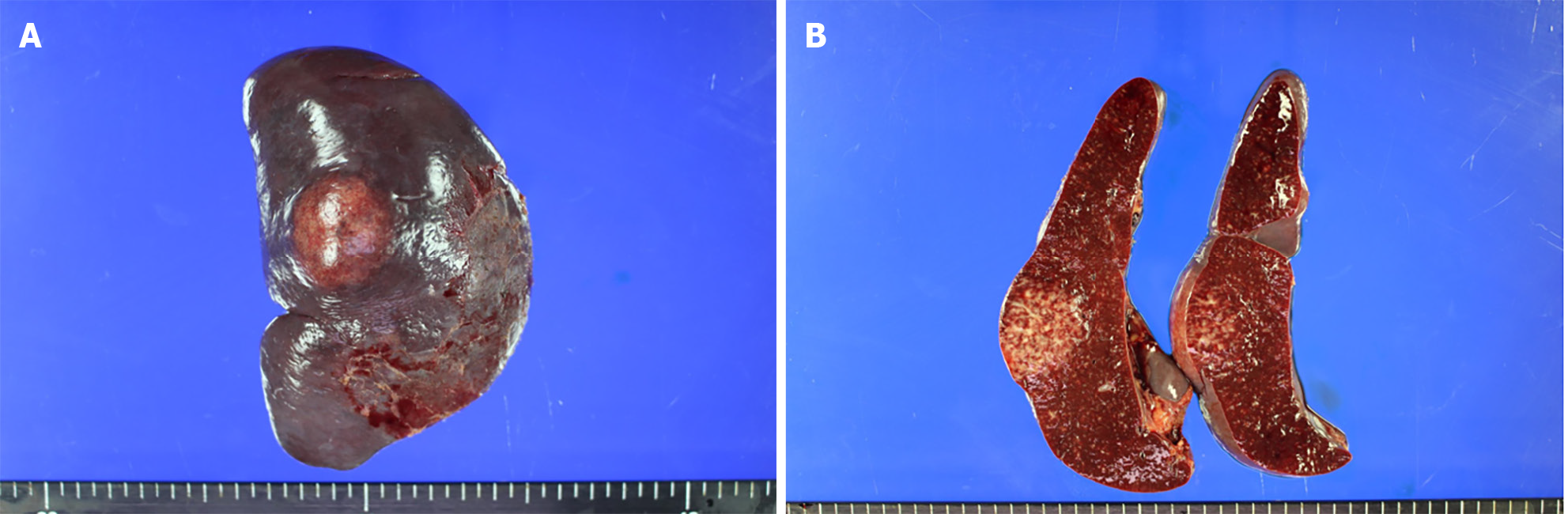
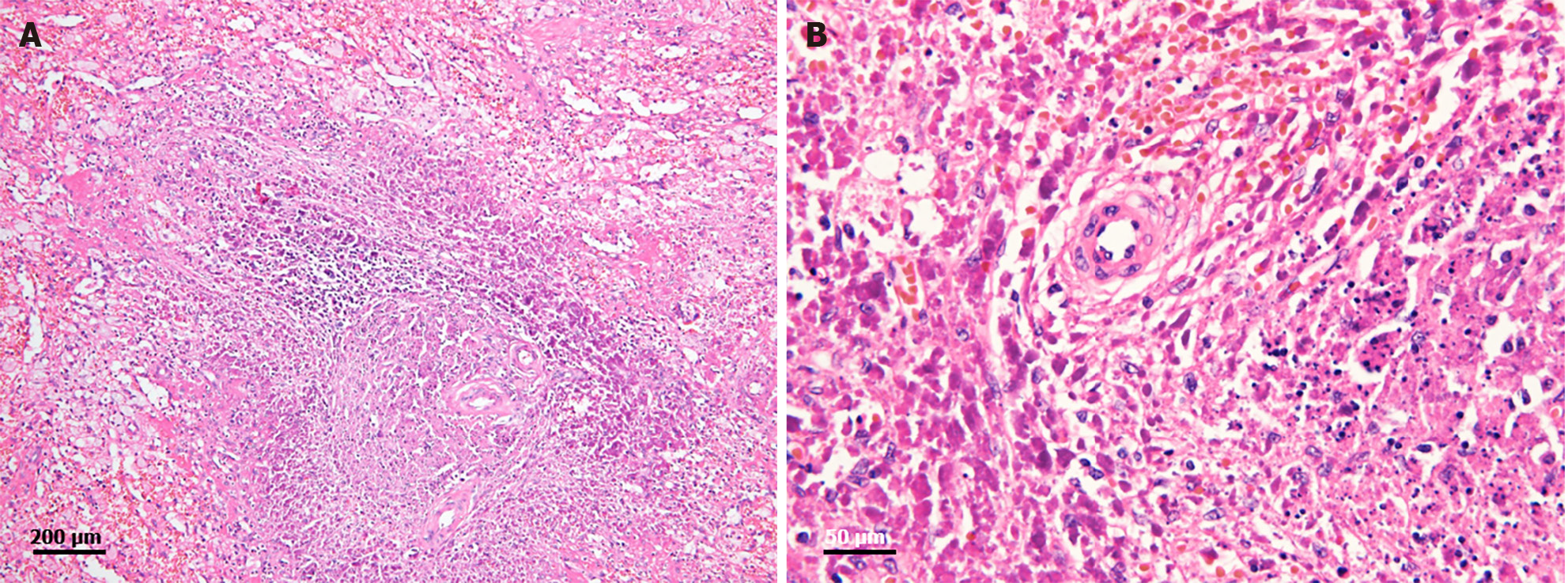
Two months after the last visit, she presented to the hospital with nausea, abdominal pain, weight loss of 3 kg, and a fever of up to 38.2 °C. Abdominopelvic computed tomography (AP-CT) was performed to rule out lupus gastroenteritis or other infections. Imaging revealed no specific findings in the gastrointestinal tract. However, splenomegaly with a maximum diameter of 11.8 cm was observed. Additionally, several round, low-density nodules with a maximum diameter of 4 cm were observed in the spleen (Figure 1). The results of the interferon-gamma release assay, cytomegalovirus polymerase chain reaction, and Epstein-Barr virus polymerase chain reaction were all negative, and reticulocytes were normal. According to the radiologist, it was necessary to differentiate between hemangiomas and hematologic malignancies in the splenic nodules. First, a red blood cell single-photon emission computed tomography (CT) was performed to discriminate between hemangiomas. Cold lesions were found, and hemangiomas were excluded. Hematologic malignancy could not be ruled out; therefore, positron emission tomography-CT (PET-CT) was performed, and the left sternoclavicular lymph node [maximum standardized uptake value corrected for lean body mass (SULmax), 3.6], right axillary lymph node (SULmax, 2.7), and splenic fluorodeoxyglucose (FDG) uptake (SULmax, 3.1) increased (Figure 2). A sternoclavicular lymph node biopsy was performed because the possibility of lymphoma could not be ruled out. Histological findings revealed extensive subcapsular necrosis with hematoxylin bodies, suggesting a high possibility of SLE (Figure 3), and a scheduled splenectomy was performed after the pneumococcal vaccination was administered. Macroscopic examination of the spleen revealed round nodules without calcification or rupture of approximately 13 cm × 8 cm in diameter (Figure 4). The histological findings of the spleen included extensive periarteriolar necrosis with hematoxylin bodies and numerous karyorrhectic debris. These findings were diagnostic of SLE activation; however, there were no findings suggestive of infection or malignancy (Figure 5). Subsequently, the patient was diagnosed with an SLE flare-up, and high-dose steroids and immunosuppressants were administered. Since then, the overall symptoms have improved, and she has been undergoing follow-up without any specific symptoms.
SLE can invade multiple organs and presents with various clinical symptoms and imaging findings. Previous case reports have described calcified fibrotic nodules of various sizes in the spleen of patients with SLE[4,6]. Splenic calcifications form granulomas caused by histoplasma infection; other causes include tuberculosis, Brucella infection, and auto-infarction[6]. However, there were no suggestive findings or history of infection in this case. Splenomegaly is common in patients with SLE. It is associated with lymphoid hyperplasia, enlarged lymphoid follicles, and SLE activation. The activation of macrophages and plasma cells around the arterioles also contributes to splenomegaly[3]. In this case, the previously reported splenic calcifications, ruptures, and atrophy were not observed. As far as we know, there have been no splenic multiple nodules that were not accompanied by these changes. Instead, AP-CT revealed round, low-density nodules of various sizes and splenomegaly. Lymphoma could not be ruled out because of the increased FDG uptake in the spleen and various lymph nodes observed on PET-CT. PET-CT has also shown lymphadenopathy and increased FDG uptake in patients with SLE[7]. Furthermore, the risk of non-Hodgkin lymphoma has been reported to increase in patients with SLE[8,9]. Additionally, because changes in the shape of nodules of various sizes in the spleen were not previously known to be associated with SLE flare-ups, a biopsy was performed to confirm the diagnosis. One study reported a low-density splenic nodule in a patient with lupus. However, aseptic necrosis and infarction were also observed[10]. Many SLE patients show positive antiphospholipid antibodies, and these antibodies are involved in splenic infarction and necrosis through the upregulation of surface adhesion molecules and the release of proinflammatory cytokines and procoagulants[11-13]. In this case, periarteriolar necrosis was observed during histological examination, but infarction, necrosis, and calcification were not clearly observed during imaging. The histological examination revealed hematoxylin bodies, crescentic histiocytes, and abundant karyorrhectic debris.
In this case, as disease activity increased, multiple nodules in the spleen that were previously unseen were observed on AP-CT and histologically confirmed. Thus, the invasion of the spleen by SLE can appear in multiple nodular forms and patterns without calcifications or ruptures. Physicians should consider these findings in order to differentiate them from other infections or malignancies.
We would like to thank the patient in this case and all those who collected and organized the medical records and data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu HB, China S-Editor: Che XX L-Editor: A P-Editor: Yu HG
| 1. | Barber MRW, Drenkard C, Falasinnu T, Hoi A, Mak A, Kow NY, Svenungsson E, Peterson J, Clarke AE, Ramsey-Goldman R. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17:515-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 334] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 2. | Charras A, Smith E, Hedrich CM. Systemic Lupus Erythematosus in Children and Young People. Curr Rheumatol Rep. 2021;23:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Fishman D, Isenberg DA. Splenic involvement in rheumatic diseases. Semin Arthritis Rheum. 1997;27:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Tieng AT, Sadow CA, Hochsztein JG, Putterman C. Diffuse calcifications of the spleen: a novel association with systemic lupus erythematosus. Semin Arthritis Rheum. 2011;41:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG Jr, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G Jr, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2816] [Cited by in RCA: 3539] [Article Influence: 272.2] [Reference Citation Analysis (0)] |
| 6. | Danieli MG, Marchetti A, Monteforte F, Calabrese V, Pettinari L, Gabrielli A. Splenic microcalcifications in systemic lupus erythematosus. Lupus. 2009;18:570-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Curiel R, Akin EA, Beaulieu G, DePalma L, Hashefi M. PET/CT imaging in systemic lupus erythematosus. Ann N Y Acad Sci. 2011;1228:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Blanco R, McLaren B, Davis B, Steele P, Smith R. Systemic lupus erythematosus-associated lymphoproliferative disorder: report of a case and discussion in light of the literature. Hum Pathol. 1997;28:980-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Buskila D, Gladman DD, Hannah W, Kahn HJ. Primary malignant lymphoma of the spleen in systemic lupus erythematosus. J Rheumatol. 1989;16:993-996. [PubMed] |
| 10. | Bounaim A, Serhane K, Mejdane A, Janati IM. [Necrosis: aseptic necrosis of the enlarged spleen as the presenting symptom of systemic lupus erythematosus]. J Chir (Paris). 2005;142:269-270. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Wand O, Tayer-Shifman OE, Khoury S, Hershko AY. A practical approach to infarction of the spleen as a rare manifestation of multiple common diseases. Ann Med. 2018;50:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Garcia D, Erkan D. Diagnosis and Management of the Antiphospholipid Syndrome. N Engl J Med. 2018;378:2010-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 489] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 13. | Arnold MH, Schrieber L. Splenic and renal infarction in systemic lupus erythematosus: association with anti-cardiolipin antibodies. Clin Rheumatol. 1988;7:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |