Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2092
Revised: February 22, 2024
Accepted: March 21, 2024
Published online: April 26, 2024
Processing time: 133 Days and 20.1 Hours
This study aimed to explore the relationship between gene mutations and early embryonic development arrest and to provide more possibilities for the diagnosis and treatment of repeated implantation failure.
Here, we collected and described the clinical data of a patient with early em
A novel mutant of the TUBB8 gene [c.602G>T(p.C201F)] was identified, and this mutant provided new data on the genotype-phenotype relationships of related diseases.
Core Tip: A clinical case (28 years old) in which a new mutation in the TUBB8 gene caused repeated arrest of early embryonic development to expand our understanding of the genetic basis of female infertility and lay the groundwork for future genetic counseling.
- Citation: Zhang XY, Zhang XX, Wang L. Early embryonic failure caused by a novel mutation in the TUBB8 gene: A case report. World J Clin Cases 2024; 12(12): 2092-2098
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2092.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2092
With the development of assisted reproductive technology, an increasing number of couples are conceiving through in vitro fertilization (IVF) embryo transfer. However, there are still some couples who are unable to conceive successfully[1]. The normal progression of meiosis and mitosis is one of the basic conditions for oocyte maturation and early embryo development. In the process of cell division, homologous chromosomes are symmetrically arranged through the microtubule organizing center, and the chromosomes divide to form the next stage of cells under the traction of bipolar spindles. Spindle assembly and chromosome separation are inseparable from the basic role of tubulin, and TUBB8 is a special subtype of tubulin that plays an important role in human oocytes and early embryonic cells[2,3]. Previous studies have shown that mutation of the TUBB8 gene leads to disorders of oocyte maturation, fertilization and early embryo development stagnation[4,5]. This paper reports a clinical case in which a novel mutation in the TUBB8 gene led to repeated early embryonic development arrest. This finding expands our understanding of the genetic basis of female infertility and lays the foundation for future genetic counseling.
A 2-year history of primary infertility after marriage.
The patient was a 28-year-old female with a 2-year history of primary infertility after marriage. Her menstrual history was as follows: menarche at age 14, a cycle of 7/-30 days, normal volume and color of menstrual blood, and no dysmenorrhea. The male partner was 26 years old. Results of the routine semen analysis were normal (sperm concentration 34.7 × 106; percentage of progressive motility 64.6%; sperm DNA fragmentation index: 14.38%) according to World Health Organization 5th Edition criteria.
In 2019, the patient underwent hysteroscopy and hysteroscopic endometrial polypectomy at another hospital due to abnormal echoes in the uterine cavity, and pathological examination of the uterine specimen indicated the presence of endometrial polyps.
The patient had no pertinent personal or family history. Both partners had no bad living habits or hobbies, and were not engaged in work related to reproductive toxicity.
The patient had a negative vulva, a normal uterus, and a negative bilateral adnexal area. Her body mass index was 21.23 kg/m2.
The concentration of anti-Mullerian hormone (AMH) was 2.634 ng/mL (1 ng/mL = 7.14 pmol/L). There were no obvious abnormalities in basic hormone levels or thyroid function. The patient’s karyotype was 46, XX, and the male partner’s karyotype was 46, XY.
Hysterosalpingography revealed that the uterine cavity was normal, bilateral fallopian tubes were developed, and the spread of the pelvic contrast agent was diffuse and limited.
Primary infertility.
In March 2021 and May 2021, the patient underwent two cycles of artificial insemination in the Department Reproductive Center. The first cycle was a natural cycle, and the second cycle was an ovulation induction cycle. Both cycles exhibited the development of dominant follicles and did not result in pregnancy.
From August 2021 to November 2022, 5 IVF cycles of assisted pregnancy treatment were performed in the Department Reproductive Center (Table 1).
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | |
| COH protocol | GnRH-A Long | GnRH-Ant | Natural cycle | Luteal phase | Natural cycle |
| Gonadotropin | r-FSH | r-FSH | - | HMG | - |
| Initiation dose (IU) | 200 | 225 | - | 225 | - |
| Total dose of gonadotropin (IU) | 1975 | 2025 | - | 2025 | - |
| Duration of gonadotropin (d) | 9 | 9 | - | 9 | - |
| E2 level on the day of HCG injection | 6605.24 | 5822.55 | 189.60 | 2239.37 | 172.30 |
| LH level on the day of HCG injection | 1.54 | 0.57 | 4.87 | 1.86 | 4.04 |
| P level on the day of HCG injection | 2.05 | 2.56 | 0.28 | 7.38 | 0.21 |
| No. of follicles (≥ 18 mm) | 3 | 6 | 0 | 4 | 0 |
| No. of follicles (≥ 14 mm) | 9 | 8 | 1 | 8 | 1 |
| Ovulation trigger (dose) | 6000 IU (HCG) | 2000 IU (HCG) + 0.2 mg (GnRH agonist) | 0.1 mg (GnRH agonist) | 6000 IU (HCG) | 0.1 mg (GnRH agonist) |
| Interval between HCG administration and oocyte retrieval (h) | 35.5 h | 35 h | 35 h | 35 h | 34 h |
| Number of retrieved oocytes | 12 | 15 | 1 | 12 | 1 |
| Fertilization mode | R-ICSI | ICSI | ICSI | ICSI | - |
| No. of MII | 8 | 13 | 0 | 11 | 0 |
| No. of GV | 2 | 1 | 0 | 0 | - |
| No. of 2PN | 5 | 10 | - | 8 | - |
| No. of D2 zygote | 5 | 10 | - | 8 | - |
| No. of D3 embryos | 5 | 7 | - | 6 | - |
| No. of blastocysts | 0 | 0 | - | 0 | - |
| No. of transferred embryos | 1 (FET) | - | - | - | - |
| Pregnancy outcome | Not pregnant | No transferable embryos | No transferable embryos | No transferable embryos | No transferable embryos |
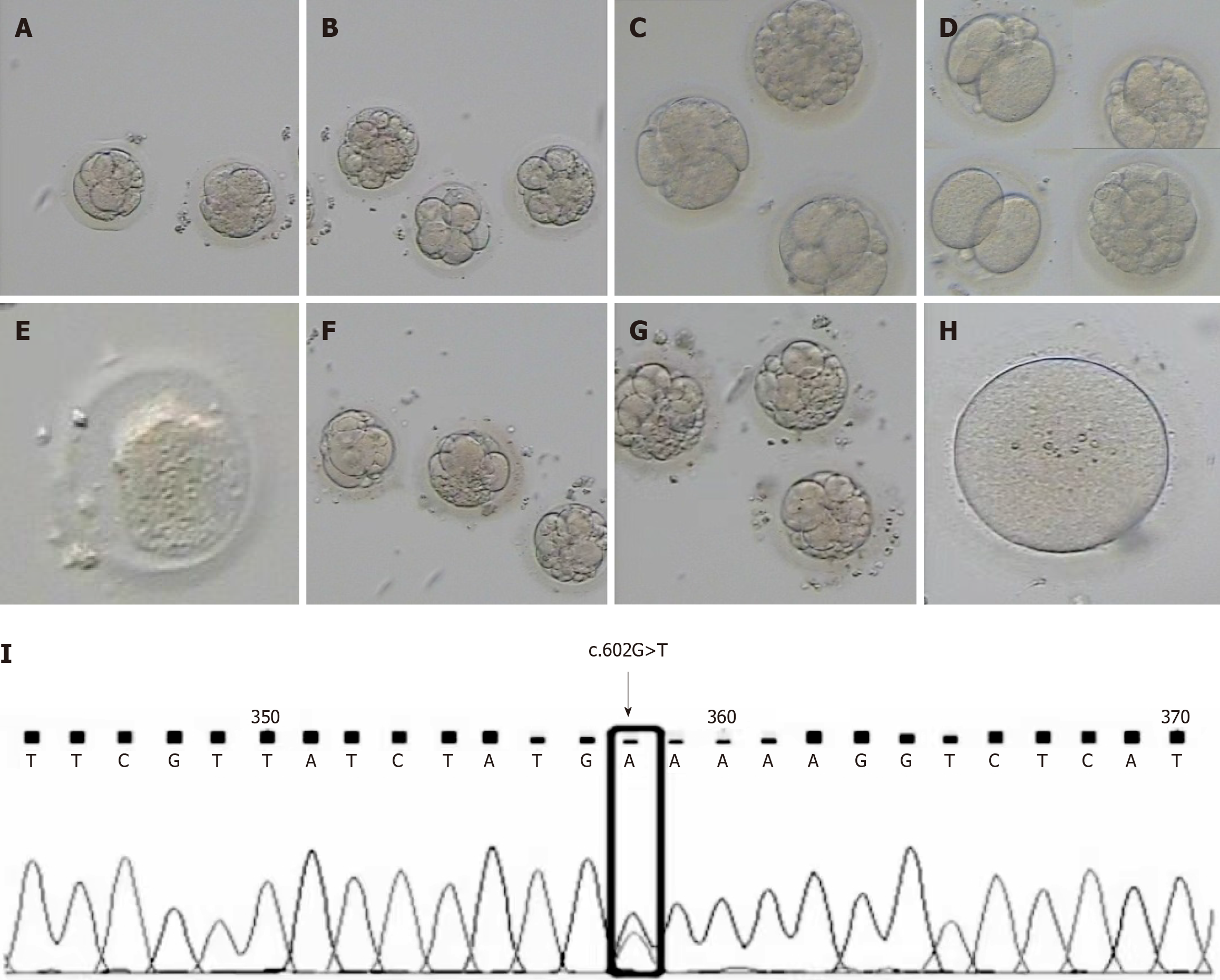
Cycle 1: In August 2021, the short-acting long protocol in the luteal phase (GnRh-a long protocol) was used to promote ovulation. After ovulation during the mid-menstrual period, 0.1 mg of triptorelin acetate (Triptorelin®, 0.1 mg/piece, Ferring GmbH, Germany) was injected subcutaneously for 7 d, followed by 0.05 mg qd for 7 d. After 14 d, ultrasound and sex hormone examinations revealed that the ovarian follicles were in the basic state, gonadotropin (Gn) was activated, and recombinant follicle stimulating hormone was injected with recombinant follitropin β (Pouliquen®, 600 IU/piece, Merck & Co. Inc, United States) at 200 IU qd and Gn for 9 d. Three follicles were ≥ 18 mm, and nine follicles were ≥ 14 mm on the day of hCG trigger (trigger day). An intramuscular injection of 6000 IU of human chorionic gonadotropin (HCG) (2000 IU/tube, Lizhu Group, China) was administered, and 35.5 h later, oocyte retrieval surgery was performed. On the day of oocyte retrieval (D0), 12 oocytes were obtained. The male partner's semen volume on the trigger day and the sperm concentration after treatment showed no significant abnormalities, and routine IVF was performed. After 4-6 h of fertilization, the maturation of oocytes was observed after removing the extracellular granular cells: two oocytes were in the GV stage, two in the MI stage, and eight in the MII stage. However, due to the absence of second polar bodies in the perioocyte space of all MII oocytes, rescue intracytoplasmic spermatozoid injection (R-ICSI) fertilization was performed due to fertilization disorder. Five zygotes with two pronuclei (2PN) were observed on the first day (D1) after oocyte collection, and five zygotes were cleaved on the second day (D2). On the 3rd day (D3), one 8CII embryo was observed and frozen. Three 6CIII embryos and one 5CII embryo continued to incubate without forming a blastocyst. In December 2021, thawing cycle transplantation was performed, and artificial cycle preparation of the endometrium was carried out. On the second day of menstruation, 2 mg/d oral estradiol valerate (Bujiale® 1 mg/tablet, Bayer Vital GmbH, Germany) was given. On the 13th day of menstruation, ultrasound revealed 0.8 cm A-B of the endometrium, and an intramuscular injection of 40 mg qd progesterone (Xianju-®, 20 mg/piece, Zhejiang Xianju Pharmaceutical, China) was administered. Three days later, one 10CIII embryo was transferred, but no pregnancy occurred (Figure 1A and B, the embryos).
Cycle 2: In the second cycle in January 2022, the antagonist protocol (GnRH-Ant protocol) was used to promote ovulation. Beginning on the third day of menstruation, recombinant follicle stimulating hormone (Jin Saiheng®, 75 IU/piece, Jin Sai Group, China) was administered subcutaneously for nine days. On the 6th day of Gn treatment, 0.25 mg of GnRH-Ant (Orgalutran®, Merck & Co. Inc, United States) was administered by intramuscular injection until the trigger day. Six follicles were ≥ 18 mm, and eight follicles were ≥ 14 mm on the trigger day. An intramuscular injection of HCG 2000 IU and a subcutaneous injection of triptorelin acetate 0.1 mg as a double trigger were administered, and the oocytes were harvested 35 h later. Fifteen oocytes were obtained by D0. Considering the fertilization disorder that occurred in the first cycle, ICSI fertilization was performed directly. The maturation of oocytes was observed after removing the granulosa cells outside the oocytes: one in the GV stage, one in the MI stage, and thirteen in the MII stage. Ten fertilized oocytes with 2PN were observed on D1, and nine fertilized oocytes were cleaved on D2. On D3, one 8CIII embryo, two 6CIII embryos, two 4CIII embryos, one 3CIIImbryo, one 2CIII embryo, two IV embryos were observed, and no blastocysts formed after continuous feeding (Figure 1C and D, the embryos).
Cycle 3: Growth hormone (GH, Sai Zeng, Jin Lei®, 30 IU/piece, Jin Sai Group, China) at a dose of 2 IU was ad
Cycle 4: In June 2022, the luteal phase of the fourth cycle was designed to promote ovulation. Menotrophin (Li Zhu®, 75 IU/tube, Lizhu Group, China) was injected intramuscularly for 10 d, with four follicles ≥ 18 mm and eight follicles ≥ 14 mm on the trigger day. HCG at a dose of 6000 IU was injected intramuscularly for the trigger, and the oocytes were retrieved 35 h later. Twelve oocytes were obtained at D0, and ICSI was performed. Oocyte maturation was observed after removing the granulosa cells outside the oocytes: one in the MI stage, and eleven in the MII stage. Eight fertilized oocytes with 2PN were observed on D1, and eight fertilized oocytes with cleavage were observed on D2. On D3, there were two 6CIII embryos, four 5CIII embryos, and two IV embryos, and no blastocysts formed after continuous feeding (Figure 1F and G, the embryos)
Cycle 5: In November 2022, in the fifth natural cycle, one oocyte was obtained (MI). After continuous culture, the oocyte did not fertilized (Figure 1H, the embryo).
In August 2022, the whole exomes of the couple and the woman's parents were sequenced (Yikang Gene Testing Company). The results of high-throughput sequencing confirmed the presence of a TUBB8 gene mutation in the woman [c.602G>T(p.C201F)], but no mutation was found in the man. Sanger sequencing of the woman's parents was used to verify the c.602G>T locus of the TUBB8 gene. The results showed that the genotype of the patient's father was the same as that of the patient, and the patient's mother had no variation at this locus (Tables 2 and 3, and Figure 1I, the locus of the TUBB8 gene).
| Gene | Chromosomal location | Variant naming | Frequency | Zygotic type | Source of variation |
| TUBB8 | Chr 93730 | NM_177987.3: c.602G>T(p.C201F) | Not included | Heterozygous | Unknown |
| Relationship | Gene | Transcript | Verification site | Verification results |
| Father | TUBB8 | NM_177987.3 | c.602G>T | Heterozygous variation |
| Mother | TUBB8 | NM_177987.3 | c.602G>T | Without-variation |
The patient utilized donated oocytes at another hospital and is currently pregnant.
The patient suffered from primary infertility and had a history of infertility for 2 years. After excluding other infertility-related factors, she first underwent 2 cycles of artificial insemination that failed and then underwent IVF treatment. Both the number of follicles in the bilateral ovarian sinuses and the AMH levels suggested normal ovarian function. In the first cycle, the conventional short-term and long-term follicular phase scheme was used, and the fertilization disorder was remedied quickly. Despite the development of an embryo, the patient failed to conceive after transplantation. In subsequent treatments, whether an antagonist scheme, a luteal phase scheme, or the addition of growth hormone to improve the quality of oocytes was used, although the patient had mature oocytes, the subsequent cleavage of fertilized -eggs and early division of embryos were not satisfactory, and the development of all embryos was still stagnant in the cleavage stage, accompanied by embryo fragmentation. After five cycles of assisted reproductive technology, we found that although the patient could produce mature oocytes, there were obstacles to fertilization of the oocytes and stagnation of early embryo development. By consulting the literature and tracking the results of previous similar research[6,7], we determined that maternal RNA and protein present in oocytes will still have an impact on embryo development after fertilized oocytes are formed, and maternal gene mutation may be one of the reasons for early embryo development stagnation[8]. We sequenced the whole exomes of the patient and her spouses and detected a mutation in the TUBB8 gene [c.602G>T(p.C201F)] in the patient. The mutant gene was subsequently verified in the parents of the patient, and it was found that the TUBB8 mutation was inherited from the father of the female patient.
The human β -tubulin family consists of nine β -tubulin isoforms[9], but TUBB8 is the only gene specifically expressed in human oocytes and early embryos[4], and spindle assembly and chromosome separation are inseparable from their basic functions during meiosis and mitosis of early embryos[2]. The TUBB8 gene is a highly conserved genotype that exists only in primates. Missense mutations of TUBB8 may interfere with the maturation of human oocytes, which is a key prerequisite for fertilization and subsequent embryo development. To date, 109 unique TUBB8 mutations have been reported, including 87 heterozygous mutations, 13 homozygous mutations and 9 compound heterozygous mutations, which exist in 8 families. According to these reports, TUBB8 mutations account for approximately 31.96% of all cases of primary oocyte maturation stagnation[10], and different mutant genotypes are associated with different clinical phenotypes, including the following[11]: (1) Formation of fully developed immature oocytes; (2) formation of unfertilized MII oocytes; (3) formation of a fertilized oocyte that cannot be cleaved; and (4) stagnation of early embryo development, including: (a) Oocytes that completely stagnate at the MI stage, (b) MII oocytes that cannot be fertilized, (c) fertilized oocytes that can be fertilized but the embryo does not cleave, and (d) embryos that can be fertilized and the embryo can cleave but then stagnate at the early stage to form embryos with a normal appearance but repeated implantation failures. Therefore, our results extend the mutation and phenotype spectrum of TUBB8 in patients with oocyte maturation, fertilization and early embryonic development arrest. The heterozygous mutation of TUBB8 described in this study c.602G>T(p.C201F) is a newly discovered variant, that has not been reported in previous literature. The clinical phenotype of this genotype is early embryonic development stagnation; that is, this mutation does not hinder the maturation and fertilization of oocytes, so we can see that this patient can form MII oocytes that can be fertilized and cleaved normally. However, the development of all the embryos stopped at the 2-8-cell stage, and they did not further develop into normal blastocysts, which was similar to the phenotypes of the mutations found by Yuan et al[6] and Jia et al[12]. Because the TUBB8 gene plays an important role in the formation and assembly of spindles during oocyte meiosis, we speculate that the stagnation of early embryonic development in patients may be partially caused by defects in nuclear maturity[13].
There are two genetic modes of disease inheritance related to TUBB8 gene mutations: autosomal dominant inheritance and autosomal recessive inheritance. To further explore the source of TUBB8 mutations, we collected as much family gene information as possible. We tested the blood samples of the woman's parents (the patient was the only daughter), and it was verified that the patient's mutation was inherited from her father. Although dozens of pathogenic TUBB8 gene mutations have been reported in the literature, there is still a lack of effective treatment methods. It has been reported that the duration of oocyte activation, the mode of calcium oscillation and calcium channel blockers affect the early embryonic development of mice[14-16]. Some studies have suggested that calcium supplementation may have a positive therapeutic effect on the embryonic development arrest caused by TUBB8 gene mutation and ultimately overcome early embryonic development arrest[17]. Jia et al[12] proposed that intracellular injection of cDNA of the normal TUBB8 gene can improve the spindle assembly of mouse cells, allowing the embryos to develop normally and enabling the birth of living offspring after injection; however the early embryo division mode of mice is different from that of humans, so there is still no experimental evidence to support the safety and effectiveness of intracellular injection of cDNA of the normal TUBB8 gene into the human body. Therefore, for the treatment of patients, in view of the current medical means, the best choice is to use donated oocytes.
In summary, this study demonstrated that the TUBB8 gene plays an important role in early human embryo development, and that mutation of this gene leads to early embryo development stagnation. In this patient, a novel TUBB8 gene mutation, c.602G>T(p.C201F) was found, expanding the range of TUBB8 gene mutations and providing new clues for genetic counseling, assisted reproductive risk prediction and optimization of the clinical treatment of infertility. This case also illustrates the importance of screening for TUBB8 gene mutations in patients who have multicycle oocyte maturation disorder, fertilization failure or early embryo development stagnation.
We would like to thank all the patients and medical staff included in our study for their contributions to this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shah RA, India S-Editor: Liu JH L-Editor: A P-Editor: Zheng XM
| 1. | Yatsenko SA, Rajkovic A. Genetics of human female infertility†. Biol Reprod. 2019;101:549-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 2. | Yang P, Yin C, Li M, Ma S, Cao Y, Zhang C, Chen T, Zhao H. Mutation analysis of tubulin beta 8 class VIII in infertile females with oocyte or embryonic defects. Clin Genet. 2021;99:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril. 2010;94:2507-2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y, Li B, Yu M, Chen J, Xu Y, Guo L, Qu R, Wang X, Sun Z, Liu M, Shi H, Wang H, Feng Y, Shao R, Chai R, Li Q, Xing Q, Zhang R, Nogales E, Jin L, He L, Gupta ML Jr, Cowan NJ, Wang L. Mutations in TUBB8 and Human Oocyte Meiotic Arrest. N Engl J Med. 2016;374:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, Xu Y, Chen B, Qu R, Sun Z, Sun X, Jin L, He L, Kuang Y, Cowan NJ, Wang L. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53:662-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Yuan P, Zheng L, Liang H, Li Y, Zhao H, Li R, Lai L, Zhang Q, Wang W. A novel mutation in the TUBB8 gene is associated with complete cleavage failure in fertilized eggs. J Assist Reprod Genet. 2018;35:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Kuang Y, Sang Q, Wang L. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod. 2017;32:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Mu J, Wang W, Chen B, Wu L, Li B, Mao X, Zhang Z, Fu J, Kuang Y, Sun X, Li Q, Jin L, He L, Sang Q, Wang L. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J Med Genet. 2019;56:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 10. | Wang AC, Zhang YS, Wang BS, Zhao XY, Wu FX, Zhai XH, Sun JX, Mei SY. Mutation analysis of the TUBB8 gene in primary infertile women with arrest in oocyte maturation. Gynecol Endocrinol. 2018;34:900-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Chen B, Wang W, Peng X, Jiang H, Zhang S, Li D, Li B, Fu J, Kuang Y, Sun X, Wang X, Zhang Z, Wu L, Zhou Z, Lyu Q, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L, Sang Q, Wang L. The comprehensive mutational and phenotypic spectrum of TUBB8 in female infertility. Eur J Hum Genet. 2019;27:300-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Jia Y, Li K, Zheng C, Tang Y, Bai D, Yin J, Chi F, Zhang Y, Li Y, Tu Z, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kong P, Wu B, Hu Y, Wang H, Liu W, Teng X, Gao S. Identification and rescue of a novel TUBB8 mutation that causes the first mitotic division defects and infertility. J Assist Reprod Genet. 2020;37:2713-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Zhao L, Guan Y, Wang W, Chen B, Xu S, Wu L, Yan Z, Li B, Fu J, Shi R, Shi J, Du J, Li Q, Zhang Z, Mu J, Zhou Z, Dong J, Jin L, He L, Sun X, Kuang Y, Wang L, Sang Q. Identification novel mutations in TUBB8 in female infertility and a novel phenotype of large polar body in oocytes with TUBB8 mutations. J Assist Reprod Genet. 2020;37:1837-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 673] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 15. | Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005;282:39-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | He GF, Yang LL, Luo SM, Ma JY, Ge ZJ, Shen W, Yin S, Sun QY. The role of L-type calcium channels in mouse oocyte maturation, activation and early embryonic development. Theriogenology. 2017;102:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Fragouli E, Wells D, Delhanty JD. Chromosome abnormalities in the human oocyte. Cytogenet Genome Res. 2011;133:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |