Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2065
Peer-review started: October 28, 2023
First decision: December 12, 2023
Revised: December 22, 2023
Accepted: March 14, 2024
Article in press: March 14, 2024
Published online: April 26, 2024
Processing time: 170 Days and 19.4 Hours
Human immunodeficiency virus (HIV)-associated dementia (HAD) is a subcor
We report the case of a 54-year-old male who exhibited cognitive dysfunction and secondary behavioral changes following HIV infection and suspected prion ex
In the diagnostic process of rapidly progressive dementia, it is crucial to rule out as many potential causes as po
Core Tip: In the present case report, we excluded an extremely rare patient with human immunodeficiency virus (HIV) and cerebrospinal fluid 14-3-3 protein-positive. Unlike the previously reported 7 cases, our patient had sustained improvement with anti-HIV therapy and was also the only patient in this entity to survive. Consequently, our report provided a completely different reference for managing rapidly progressive dementia in particular cases.
- Citation: He YS, Qin XH, Feng M, Huang QJ, Zhang MJ, Guo LL, Bao MB, Tao Y, Dai HY, Wu B. Human immunodeficiency virus-associated dementia complex with positive 14-3-3 protein in cerebrospinal fluid: A case report. World J Clin Cases 2024; 12(12): 2065-2073
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2065.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2065
Human immunodeficiency virus (HIV)-associated dementia (HAD) is subcortical dementia characterized by memory deficits and psychomotor slowing, which occurs after the brain is infected with the HIV[1]. Cognitive dysfunction is a common symptom in patients with acquired immune deficiency syndrome (AIDS) and non-opportunistic infections caused by other viruses. Creutzfeldt-Jakob disease (CJD), also known as Cortico-striatum-myeloid degenerative disease, is characterized by mental disorders, dementia, Parkinson-like manifestations, ataxia, myoclonus, and muscle atrophy. CJD is a chronic and progressive disease caused by a rare infection with the prion protein[2]. Additionally, the cerebro
Here, a rare case is presented of a patient with AIDS and a positive 14-3-3 protein[2]. Although similar cases have been reported[3-7], this case provides new insights and is an important learning point for managing patients with rapidly pro
A 54-year-old male (Han ethnicity) presented to the neurology clinic of our institution with a 6 mo history of slurred speech that had worsened over the past 3 months.
The patient had suffered from memory disturbances for more than 1 year, with symptoms primarily including progre
Additionally, dizziness, and left ear tinnitus were occasionally noted but he did not present physical signs of dys
The patient’s past medical history was unremarkable.
The patient had no history of exposure to toxic substances or family history of specific genetic diseases.
The patient was alert and entered the ward with a normal gait. He exhibited slurred speech, uncontrolled frowning, and pursing of the lips. Neurological deficits were noted, including impairments in memory, orientation, reasoning, and emotional expression. Meningeal signs were absent. The pharyngeal reflex was diminished, limb muscle tone was hei
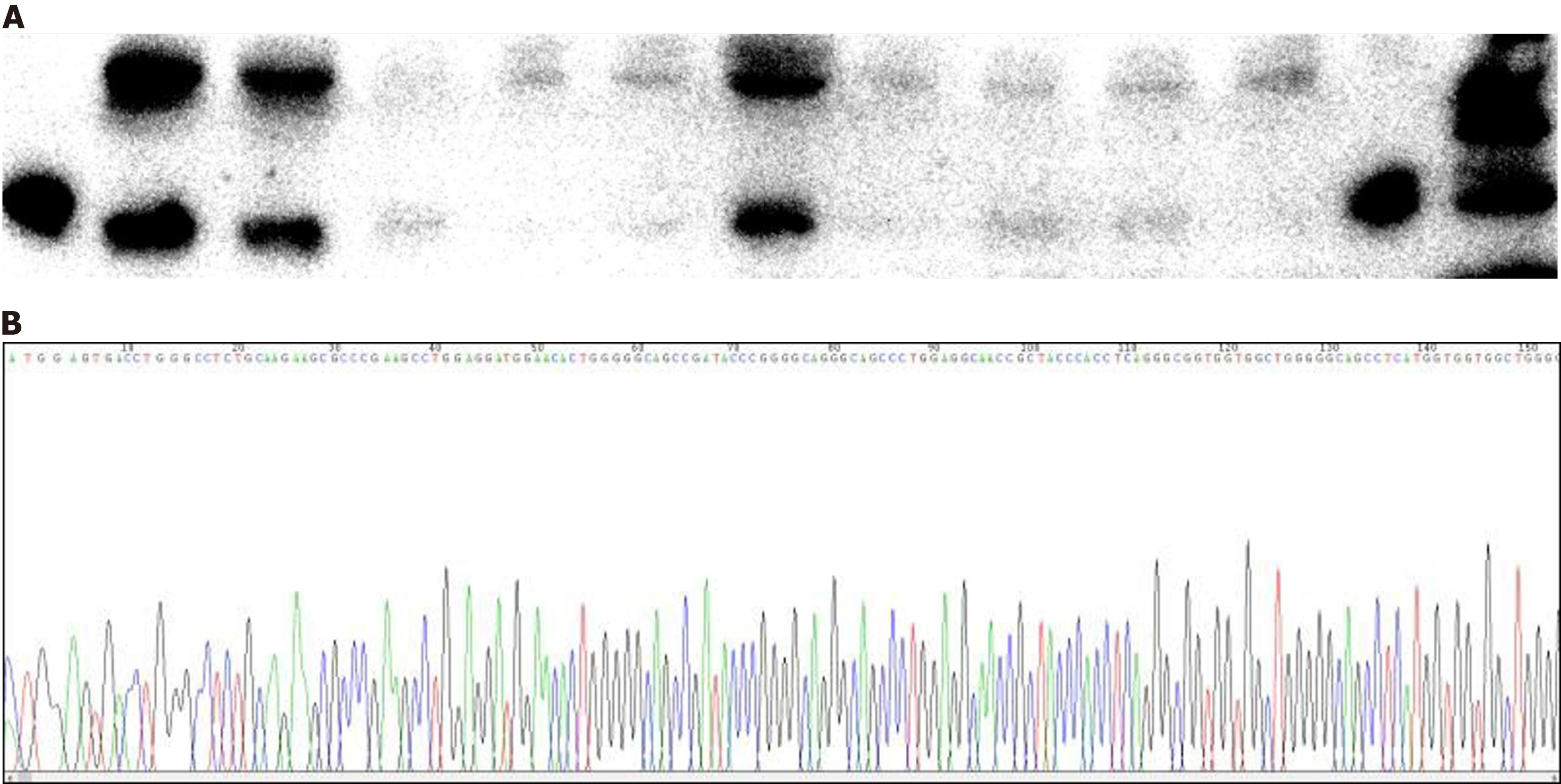
Upon his admission, the family reported abnormality on the Mini-Mental State Examination and Montreal Cognitive Assessment tests during a previous assessment at another hospital, although the medical records from that visit were not available to us. Following his admission, an initial HIV antibody screening returned positive results, prompting us to perform a confirmatory HIV antibody test on the patient’s blood (the final results were pending at that time). Laboratory tests indicated leukocytopenia with a white blood cell count of 3.2 × 109/L and lymphocytes at 0.96 × 109/L. Analysis of T cell subsets showed a T helper (TH)/T suppressor (TS) ratio of 0.1, with TH/inducer (cluster of differentiation 4 [CD4]) cells at 78/µL and TS/killer (CD8) cells at 1218/µL. In addition, the patient’s CSF protein concentration was elevated at 0.73 g/L. The CSF cell count was normal, and extensive CSF testing for biochemical markers, routine cultures (including bacteria, fungi, Mycobacterium tuberculosis, and Cryptococcus), and antibodies associated with autoimmune and paraneoplastic encephalitis all returned negative results. Liver and kidney functions were normal, as were tests for anti-thyroid peroxidase antibody, anti-thyroglobulin antibody, ceruloplasmin, anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, folic acid, and vitamin B12 levels. The CSF tested positive for the 14-3-3 protein, and genotyping confirmed 129 M/M and 219 E/E variants (Figure 1). An electroencephalogram (EEG) showed borderline abnormalities with periodic triphasic waves, which were not indicative of a typical disorder.
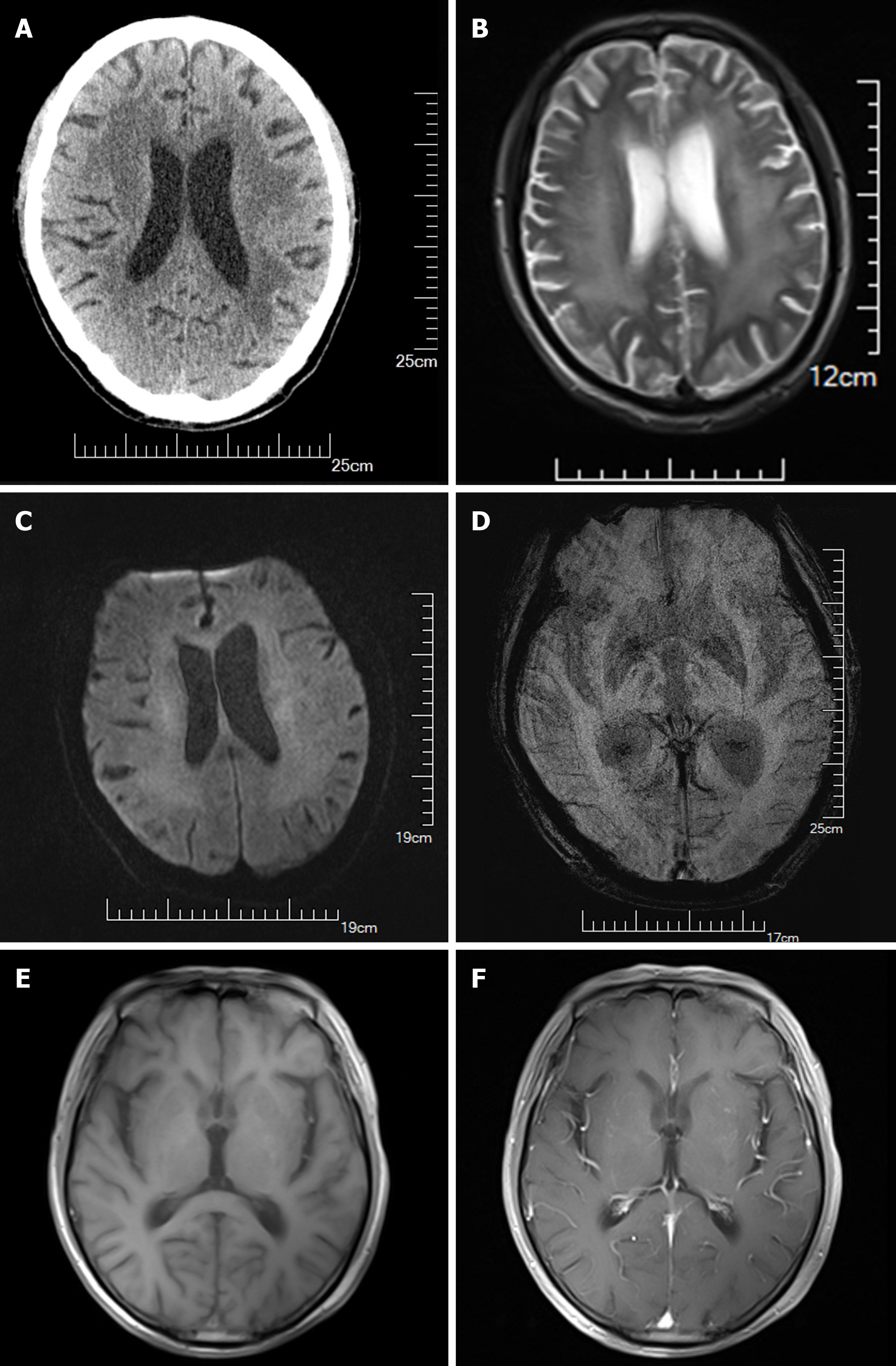
Cranial computed tomography (CT) revealed cerebral atrophy and demyelination abnormalities in the white matter (Figure 2), given the multiple pinpoint hypodensities within the white matter exhibited in the bilateral basal ganglia with non-enhancement in all lesions and was initially diagnosed with lacunar infarction (Figure 2A). Medium encepha
HIV-associated dementia.
The patient's condition worsened while awaiting a conclusive AIDS diagnosis. We treated the patient with symptomatic treatment and amantadine (Amantadine Hydrochloride Tablets, USP) for CJD, which was the initial diagnosis con
The patient was subsequently transferred to a specialized local center for infectious disease control to receive targeted treatment. Over the course of 4 years, the anti-HIV regimen provided by the center consisted of efavirenz (600 mg daily), tenofovir disoproxil (300 mg daily), lamivudine (100 mg daily), and compound sulfamethoxazole tablets (480 mg twice a day). The patient experienced rapid amelioration of symptoms following the commencement of antiretroviral therapy during his hospital stay.
Four years later, during a comprehensive outpatient follow-up assessment the patient exhibited clear consciousness and coherent speech; while recent memory and emotional expressiveness were mildly diminished, they were only marginally below the normal range; and the orientation and logical thinking functions were unremarkable. The limb muscle tension slightly increased, and the muscle strength was normal. The neurological signs and other symptoms were normal. The neuroradiological re-examination of the c-MRI (Figure 2E) revealed that the mild cerebral atrophy accompanying obvious demyelination in the white matter around the bilateral cerebral ventricle had improved than previous imaging. Addi
| Test items | Before | After |
| MoCA | 16 | 25 |
| ADL | 25 | 70 |
| Muscle strength | V | V |
| Hypertonia | (+) | Improvement |
| Pathological reflex | (-) | (-) |
| Neuroradiology | (+) | Improvement |
CJD is a degenerative central nervous system disease caused by prion proteins, mainly manifested as advancing de
In their comprehensive review of the literature from 1995 to 2011, Muayqil et al[10] analyzed 38 studies involving 1849 suspected cases of sCJD with 14-3-3 protein assays conducted. Their findings indicated that the 14-3-3 protein is a va
MRI sensitivity is 80% in CJD[9,11,13,14], Some studies put the sensitivity as high as 92% to 98%[15-17]. At the same time, its specificity is 74%–98%[9,13].
Periodic sharp-wave complexes (PSWCs) at a frequency of 1 Hz are a hallmark EEG pattern for CJD, demonstrating a sensitivity of 64% and a specificity of 91% in diagnosis[18]. The molecular classification of sporadic CJD hinges on poly
In such cases, the diagnosis of probable CJD should meet the criteria for symptomatology, ancillary tests, and ex
HAD is a common neurological complication after HIV infection and is mainly associated with memory impairment, motor coordination difficulties, cognitive deficits, difficulty performing complex tasks, and behavioral changes, including apathy and atypical reactions[1,20].
Most patients initially present with only short-term memory disorders in the early stages of AIDS; however, as the disease progresses, HIV-related chronic inflammation and immune activation may affect multiple brain regions. This can lead to dysfunctions in memory, cognition, language expression, and comprehension. With the widespread application of highly active antiretroviral therapy, the life expectancy of patients with HIV has significantly increased. Despite this, the incidence of moderate neurocognitive impairments remains high. A possible reason is that most anti-HIV drugs do not efficiently cross the blood-brain barrier to enter the central nervous system (CNS), resulting in insufficient drug concentrations in the CNS. Combined with the environmental factors within the CNS, HIV is prone to mutation, and the chronic accumulation of neurotoxicity leads to moderate neurocognitive dysfunction[20].
In this case, the diagnosis was considered infectious dementia combined with the medical history of the patient and auxiliary examination. The prime suspect was HIV, based on the following. Both HIV antibody screening test and HIV antibody confirmatory tests were positive. The apparent symptoms, including memory disorders, slowed mental proce
Four years after initiating anti-HIV treatment, we noted improved cognitive function and self-care abilities. However, memory remained worse than before; therefore, it is possible that prions may also play a role in the patient's rapid pro
Patients with co-infection of HIV and prions are very rare. To the best of our knowledge, only 5 cases have been diag
| Ref. | Pt | Sex | Age | Race/Region | Symptoms | Examination | Diagnosis | Management | Outcomes |
| Babi et al[4] | 2016 | Male | 66 | United States | Conceptual apraxia, apathy, memory impairment, and gait disturbance, ataxia with gait disturbance, chronic peripheral neuropathy | CSF: 14-3-3(+); T-Tau(+); RT-Qu IC(+); MRI: signal abnormalities in the bilateral caudate, putamen, and thalami, as well as gyriform cortical; EEG: (-); PRNP: N/A; Autopsy: CJD | Sporadic CJD | Palliative care | Passed away (3 months) |
| Eimer et al[7] | 2018 | Male | 59 | Caucasian | Mildly disoriented being insecure about the situation and location | CSF: 14-3-3(+); MRI: signal abnormalities in the caudate nuclei, frontal cortex, and parietal cortex bilaterally; EEG: periodic triphasic spike and wave complexes; PRNP: M129V; Autopsy: CJD | Sporadic CJD | Palliative care | Passed away (2 months) |
| Abu-Rumeileh et al[3] | 2018 | Male | 62 | Italy | Drowsy, with reduced verbal fluency, miotic reagent pupils, and a mask face. Axial and limb plastic hypertonia and dystonia of both hands | CSF: 14-3-3(+); MRI: cortical atrophy and multiple white matter lesions. EEG: pseudo-periodic slow spike discharges; PRNP: N/A; Autopsy: CJD | Sporadic CJD | Palliative care | Passed away (4 months) |
| De Carvalho Neto et al[6] | 2019 | Male | 52 | Caucasian | Progressive imbalance, motor and cognitive deterioration and hypersomnia | CSF: 14-3-3(+); MRI: cortical gyri_x005f form restriction on both hemispheres; EEG: triphasic PSWC; PRNP: N/A; Autopsy: N/A | Probable sporadic CJD | Palliative care | Passed away (greater than 2 months) |
| van de Ven et al[21] | 2019 | Male | 63 | Black Zimbabwean | Progressive difficulties with decision-making, obsessive compulsive disorder and visual hallucinations | CSF: 14-3-3 (weakly+); MRI: bilateral abnormal signal within the posterolateral thalami compatible with pulvinar sign; EEG: Diffuse excess of slow activity; PRNP: M129V; Autopsy: CJD | Variant CJD | Palliative care | Passed away (10 months) |
| Dahy et al[5] | 2021 | Male | 52 | Brazil | Global cerebellar syndrome, bilateral Babinski, 4-limb paratonia and release of face axial reflexes. The memory, attention and executive function deficits | CSF: 14-3-3(+); MRI: bilateral hyper intensity of images in caudal nuclei; EEG: (-); PRNP: M129V; Autopsy: N/A | Probable sporadic CJD | N/A | Passed away (13 months) |
| Dahy et al[5] | 2021 | Male | 61 | Brazil | Asthenia, lack of appetite, difficulty sleeping and occasional memory lapses, uncoordinated steps, visual delusions and bladder incontinence | CSF: 14-3-3(+); MRI: bilateral cortical ribboning in the cerebral cortex; PRNP: N/A; EEG: N/A; Autopsy: N/A | Probable sporadic CJD | N/A | Passed away (5 months) |
| Current report | 2022 | Male | 54 | Han/China | Progressive hypomnesis, paroxysmal anterograde amnesia, unsteady gait | CSF: 14-3-3 (weakly+); MRI: Bilateral abnormal signal within the posterolateral thalami compatible; EEG: Borderline abnormality of the periodic triphasic wave; PRNP: 129 M/M; Autopsy: N/A | Probable ADC | Anti-HIV | Improved and following-up |
The first patient was published by Babi et al[4] in 2016. The patient had well-controlled chronic AIDS. The elderly man passed away 3 months after a positive 14-3-3 protein test in the CSF, and a diagnosis of sCJD was confirmed histopathologically by autopsy. Subsequent reports indicate that all patients with similar conditions died within 2 months to 13 months[3,5-7,21]. In 3 of these cases, the diagnosis of sCJD was also confirmed by autopsy[3,7,21], and variant CJD in 1 case[21]. In the remaining 2 cases, autopsies were unavailable, but CJD was highly suspected[5,6]. The majority of these authors concur that there is no direct evidence linking HIV infection and prion diseases; however, further investigation is needed[4,6,7,21]. Abu-Rumeileh et al[3] concluded that RT-QuIC should be utilized as a specific screening tool for pro
In our case, the patient's symptoms improved following anti-AIDS treatment, reducing the likelihood to be diagnosed with CJD (Table 1). Although 7 patients documented in previous reports shared similarities with the current case, presenting with AIDS and positive 14-3-3 protein, they were ultimately confirmed to have CJD via autopsy (Table 2).
In patients without routine HIV screening tests, RPD and positive 14-3-3 protein in CSF may easily lead to a misdiagnosis of CJD. Neurologists should exert every effort to determine the cause of RPD during diagnosis. Positive 14-3-3 protein expression is of great value in CJD diagnosis, but it also has some limitations and presents interference. Re-evaluation of the CSF 14-3-3 protein or an RT-QuIC test should be considered to enhance diagnostic accuracy when additional examinations are not available for such rare cases.
HAD and CJD are easily misdiagnosed. In the etiological diagnosis of RPD, it is vital to exclude as many causes as po
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khosravi M, Iran S-Editor: Liu JH L-Editor: Filipodia P-Editor: Xu ZH
| 1. | McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 357] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Sitammagari KK, Masood W. Creutzfeldt Jakob Disease. 2024 Jan 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 3. | Abu-Rumeileh S, Capellari S, Parchi P. Rapidly Progressive Alzheimer's Disease: Contributions to Clinical-Pathological Definition and Diagnosis. J Alzheimers Dis. 2018;63:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Babi MA, Kraft BD, Sengupta S, Peterson H, Orgel R, Wegermann Z, Lugogo NL, Luedke MW. Related or not? Development of spontaneous Creutzfeldt-Jakob disease in a patient with chronic, well-controlled HIV: A case report and review of the literature. SAGE Open Med Case Rep. 2016;4:2050313X16672153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Dahy FE, Novaes CTG, Bandeira GA, Ramin LF, Oliveira ACP, Smid J. Sporadic Creutzfeldt-Jakob disease in two clinically and virologically controlled Brazilian HIV patients who progressed rapidly to dementia: case reports and literature review. Rev Inst Med Trop Sao Paulo. 2021;63:e23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 6. | De Carvalho Neto EG, Gomes MF, De Oliveira M, Guete MIN, Santos IP, Monteiro MD, Stelzer FG, Kowacs F, Barea LM. The worst is yet to come: probable sporadic Creutzfeldt-Jakob disease in a well-controlled HIV patient. Prion. 2019;13:156-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Eimer J, Vesterbacka J, Savitcheva I, Press R, Roshanisefat H, Nowak P. Nonopportunistic infection leading to rapidly progressive dementia in a patient with HIV/AIDS: A case report. Medicine (Baltimore). 2018;97:e0162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Geschwind MD. Rapidly Progressive Dementia. Continuum (Minneap Minn). 2016;22:510-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Hermann P, Appleby B, Brandel JP, Caughey B, Collins S, Geschwind MD, Green A, Haïk S, Kovacs GG, Ladogana A, Llorens F, Mead S, Nishida N, Pal S, Parchi P, Pocchiari M, Satoh K, Zanusso G, Zerr I. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021;20:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 10. | Muayqil T, Gronseth G, Camicioli R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2012;79:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Franceschini A, Baiardi S, Hughson AG, McKenzie N, Moda F, Rossi M, Capellari S, Green A, Giaccone G, Caughey B, Parchi P. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci Rep. 2017;7:10655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 12. | Lattanzio F, Abu-Rumeileh S, Franceschini A, Kai H, Amore G, Poggiolini I, Rossi M, Baiardi S, McGuire L, Ladogana A, Pocchiari M, Green A, Capellari S, Parchi P. Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol. 2017;133:559-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Abu-Rumeileh S, Steinacker P, Polischi B, Mammana A, Bartoletti-Stella A, Oeckl P, Baiardi S, Zenesini C, Huss A, Cortelli P, Capellari S, Otto M, Parchi P. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res Ther. 2019;12:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Fiorini M, Iselle G, Perra D, Bongianni M, Capaldi S, Sacchetto L, Ferrari S, Mombello A, Vascellari S, Testi S, Monaco S, Zanusso G. High Diagnostic Accuracy of RT-QuIC Assay in a Prospective Study of Patients with Suspected sCJD. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Bizzi A, Pascuzzo R, Blevins J, Grisoli M, Lodi R, Moscatelli MEM, Castelli G, Cohen ML, Schonberger LB, Foutz A, Safar JG, Appleby BS, Gambetti P. Evaluation of a New Criterion for Detecting Prion Disease With Diffusion Magnetic Resonance Imaging. JAMA Neurol. 2020;77:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Forner SA, Takada LT, Bettcher BM, Lobach IV, Tartaglia MC, Torres-Chae C, Haman A, Thai J, Vitali P, Neuhaus J, Bostrom A, Miller BL, Rosen HJ, Geschwind MD. Comparing CSF biomarkers and brain MRI in the diagnosis of sporadic Creutzfeldt-Jakob disease. Neurol Clin Pract. 2015;5:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Rudge P, Hyare H, Green A, Collinge J, Mead S. Imaging and CSF analyses effectively distinguish CJD from its mimics. J Neurol Neurosurg Psychiatry. 2018;89:461-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Steinhoff BJ, Zerr I, Glatting M, Schulz-Schaeffer W, Poser S, Kretzschmar HA. Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann Neurol. 2004;56:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Yu SL, Jin L, Sy MS, Mei FH, Kang SL, Sun GH, Tien P, Wang FS, Xiao GF. Polymorphisms of the PRNP gene in Chinese populations and the identification of a novel insertion mutation. Eur J Hum Genet. 2004;12:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Joseph J, Colosi DA, Rao VR. HIV-1 Induced CNS Dysfunction: Current Overview and Research Priorities. Curr HIV Res. 2016;14:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | van de Ven NS, Vera J, Jones JR, Vundavalli S, Ridha BH. Sporadic CJD in association with HIV. J Neurol. 2019;266:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |