Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2056
Peer-review started: September 22, 2023
First decision: December 15, 2023
Revised: January 23, 2024
Accepted: March 1, 2024
Article in press: March 1, 2024
Published online: April 26, 2024
Processing time: 206 Days and 16.3 Hours
Various non-steroidal anti-inflammatory drugs (NSAIDs) have been used for ju
To perform a systematic review and network meta-analysis to determine the op
We searched for randomized controlled trials (RCTs) from PubMed, EMBASE, Google Scholar, CNKI, and Wanfang without restriction for publication date or language at August, 2023. Any RCTs that comparing the effectiveness of NSAIDs with each other or placebo for JIA were included in this network meta-analysis. The surface under the cumulative ranking curve (SUCRA) analysis was used to rank the treatments. P value less than 0.05 was identified as statistically signifi
We included 8 RCTs (1127 patients) comparing 8 different instructions including meloxicam (0.125 qd and 0.250 qd), Celecoxib (3 mg/kg bid and 6 mg/kg bid), piroxicam, Naproxen (5.0 mg/kg/d, 7.5 mg/kg/d and 12.5 mg/kg/d), inuprofen (30-40 mg/kg/d), Aspirin (60-80 mg/kg/d, 75 mg/kg/d, and 55 mg/kg/d), To
In summary, celecoxib (6 mg/kg bid) was found to be the most effective NSAID for treating JIA. Rofecoxib, pi
Core Tip: In summary, celecoxib (6 mg/kg bid) was found to be the most effective non-steroidal anti-inflammatory drug for treating juvenile idiopathic arthritis. Rofecoxib, piroxicam, and meloxicam may be safer options, but further research is needed to confirm these findings in larger trials with higher quality studies.
- Citation: Zeng T, Ye JZ, Qin H, Xu QQ. Systematic review and network meta-analysis of different non-steroidal anti-inflammatory drugs for juvenile idiopathic arthritis. World J Clin Cases 2024; 12(12): 2056-2064
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2056.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2056
Juvenile idiopathic arthritis (JIA) refers to several types of chronic arthritis that appear before the age of 16[1-3]. JIA affects 294000 children in the United States, which characterized by chronic arthritis[4,5]. The pathogenesis of JIA was unknown. Clinical manifestations of JIA is joint pain, swelling, and morning stiffness[6,7]. Symptoms of JIA often persist into adulthood and are one of the leading causes of joint dysfunction in children[8]. At present, JIA is difficult to cure in the short term. The goal of treatment for JIA is to achieve sustained remission or low disease activity[9].
There are two forms of COX in the human body currently: COX-1 and COX-2[10,11]. Normally, COX-2 expression is low, but in inflammatory conditions, it is dramatically increased and thus causing a high level of inflammation[12]. Non-steroidal anti-inflammatory drugs (NSAIDs) work by blocking COX enzyme synthesis, which in turn inhibits prosta
NSAIDs, which include traditional non-selective NSAIDS and selective NSAIDs. There are no direct comparisons of NSAIDs in current research, so it’s important to evaluate their effectiveness and safety from the perspectives of healthcare providers and payers. Currently, there is a lack of systematic review and meta-analysis that comparing different NSAIDs for JIA. Network meta-analysis enables comparisons between drugs that have not been directly compared in head-to-head trials, using a common comparator like placebo[15,16]. We will use network meta-analysis to determine the best treatment for JIA and guide clinical decision-making. Our goal is to compare NSAIDs for JIA treatment through network meta-analysis.
Two authors independently searched the electronic literature database of PubMed, EMBASE, Google Scholar, CNKI and Wanfang without restriction for publication date or language at August, 2023. The key words for searching can be seen in Supplement material. Articles and references were searched to prevent overlooking important sources. Previous sys
The inclusion criteria were as follows: (1) Patients were diagnosed with JIA; (2) studies comparing NSAIDs therapies [meloxicam (0.125 qd and 0.250 qd), Celecoxib (3 mg/kg bid and 6 mg/kg bid)], piroxicam, Naproxen (5.0 mg/kg/d, 7.5 mg/kg/d, and 12.5 mg/kg/d), Inuprofen (30-40 mg/kg/d), Aspirin (60-80 mg/kg/d, 75 mg/kg/d, and 55 mg/kg/d), Tolmetin (15 mg/kg/d), Rofecoxib (0.3 mg/kg qd, 0.6 mg/kg), or with placebo; (3) randomized controlled trials (RCTs); and (4) studies reporting ACR Pedi 30 response and adverse events in patients.
The following studies were excluded: (1) Abstract only (insufficient data); (2) repeatedly published studies; (3) re
Two investigators independently extracted data from included trials using a standardized form, including author, pub
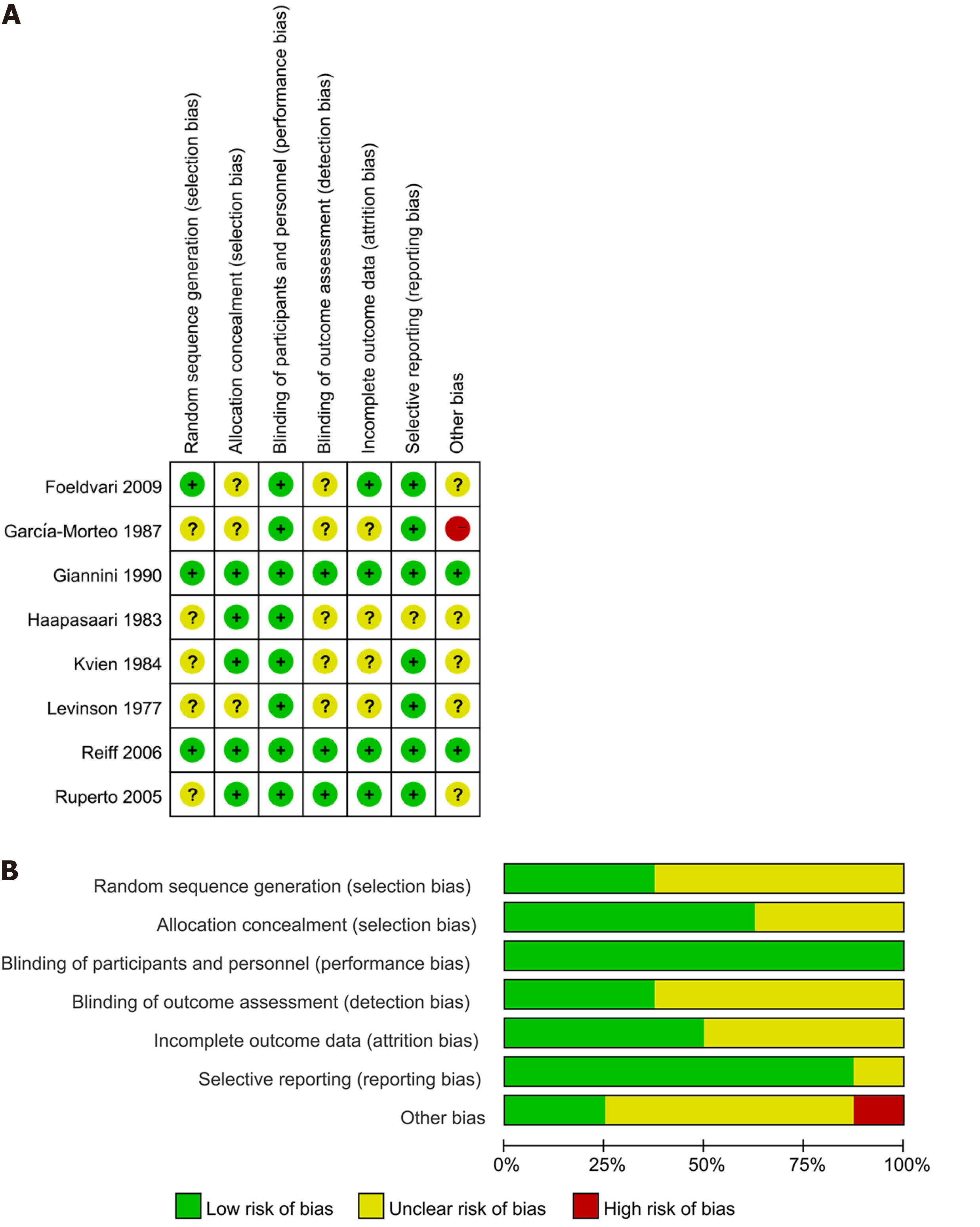
Two assessors evaluated the quality of individual trials based on the Cochrane Handbook, looking at factors like randomization, blinding, and reporting bias. Trials were categorized as “low risk”, “high risk”, or “unclear”.
A network meta-analysis was performed to compare various treatments utilizing a random-effect model within a Ba
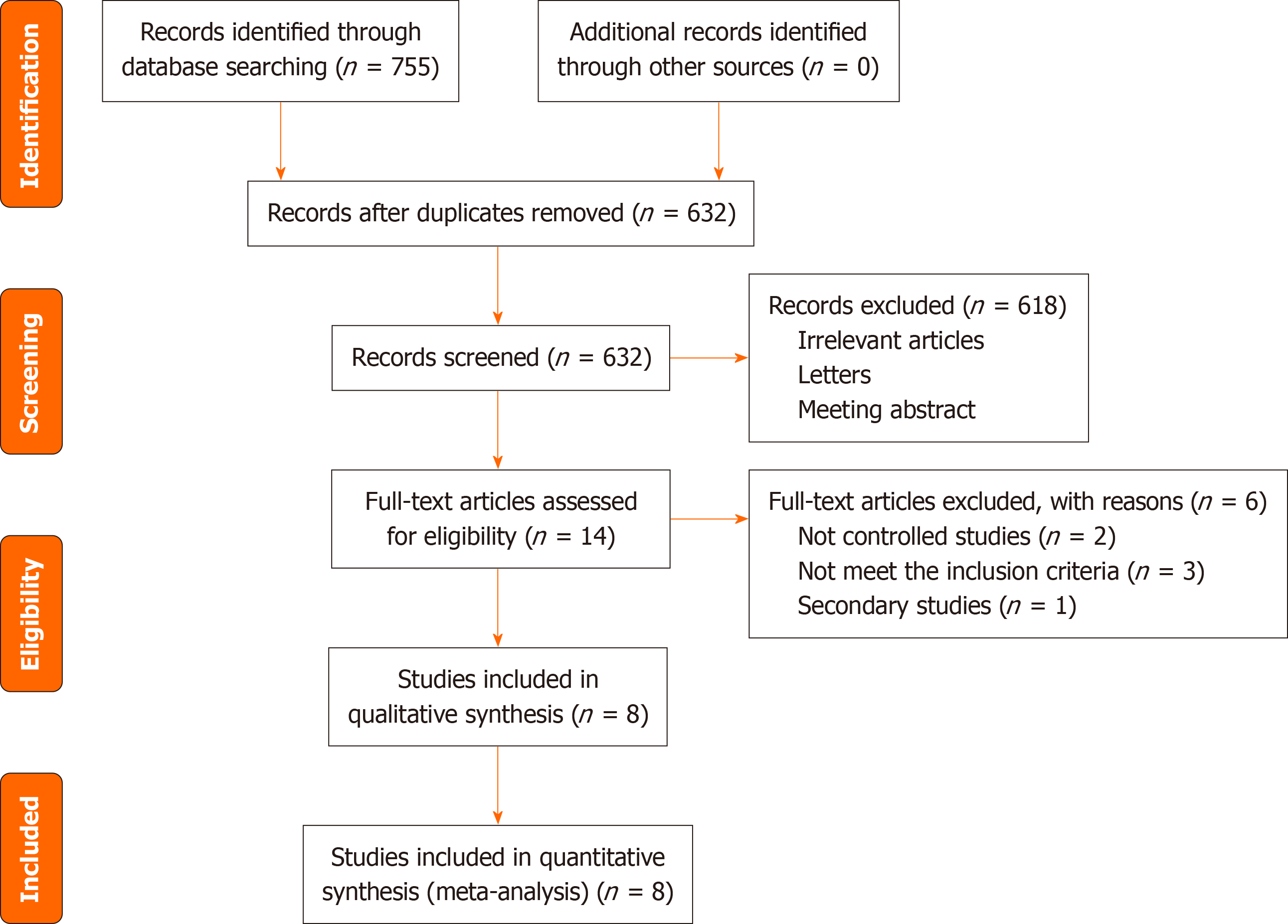
The search retrieved a total of 755 articles which were identified from PubMed (322), EMBASE (189), Google Scholar (215), CNKI (20), and Wanfang (9). Of these, 123 were removed as duplicates. Based on our review of the title and ab
Table 1 displayed the basic characteristics of the included studies. The total sample included 467 patients whose mean or median baseline age of participants ranged from 7.7 to 11.4 years and all of them were published after 1977. Subtype of JIA including polyarticular JIA, oligoarticular JIA and systemic JIA. NSAIDs including meloxicam (0.125 qd and 0.250 qd), Celecoxib (3 mg/kg bid and 6 mg/kg bid), piroxicam, Naproxen (5.0 mg/kg/d, 7.5 mg/kg/d, and 12.5 mg/kg/d), Inuprofen (30-40 mg/kg/d), Aspirin (60-80 mg/kg/d, 75 mg/kg/d, and 55 mg/kg/d), Tolmetin (15 mg/kg/d), Rofecoxib (0.3 mg/kg qd, 0.6 mg/kg), and placebo. Most trials included in the meta-analysis had unclear risk of bias, with 3 studies having adequate random sequence generation and 5 studies reporting adequate allocation concealment. Blinding of participants and personnel was adequate in all included studies, with details shown in Figure 2.
| Ref. | Sample size | Mean age | Subtype | t1 | t2 | t3 | DMARDs (%) | Biologic agents (%) | CS (%) | Treatment duration (wk) | ||||
| t1 | t2 | t3 | t1 | t2 | t3 | |||||||||
| Ruperto et al[17], 2005 | 73 | 74 | 78 | 8.9 | 9.0 | 7.5 | pJIA, oJIA | Meloxicam (0.125 qd) | Meloxicam (0.250 qd) | Naproxen (5.000 mg/kg) | 24.7/28.4/37.2 | NS | 19.3/22.0/14.9 | 12 |
| Foeldvari et al[18], 2009 | 77 | 82 | 83 | 10.4 | 10.2 | 10.4 | pJIA, oJIA | Celecoxib (3.0 mg/kg bid) | Celecoxib (6.0 mg/kg bid) | Naproxen (7.5 mg/kg) | 50.6/47.6/51.8 | 0/3.7/3.6 | NS | 12 |
| García-Morteo et al[19], 1987 | 12 | 14 | 8.5 | 8.5 | pJIA, oJIA | Piroxicam | Naproxen (12.5 mg/kg/d) | NS | NS | 11.5 | 12 | |||
| Giannini et al[20], 1990 | 45 | 47 | 7.7 | 7.7 | pJIA, oJIA, sJIA | Inuprofen (30-40 mg/kg/d) | Aspirin (60-80 mg/kg/d) | 0 | 0 | NS | 12 | |||
| Haapasaari et al[21], 1983 | 15 | 15 | 15 | NS | NS | NS | pJIA, oJIA, sJIA | Diclofenac (2-3 mg/kg/d) | Aspirin (50-100 mg/kg/d) | Placebo | NS | NS | NS | 2 |
| Kvien et al[22], 1984 | 40 | 40 | 11.4 | 9.0 | pJIA, oJIA | Naproxen (10 mg/kg/d) | Aspirin (75 mg/kg/d) | 0 | 0 | 0 | 24 | |||
| Levinson et al[23], 1977 | 53 | 54 | 9.4 | 9.0 | pJIA, oJIA, sJIA | Tolmetin (15 mg/kg/d) | Aspirin (55 mg/kg/d) | NS | 0 | 0 | 12 | |||
| Reiff et al[24], 2006 | 109 | 100 | 101 | 9.7 | 9.4 | 10.7 | pJIA, oJIA | Rofecoxib (0.3 mg/kg qd) | Rofecoxib (0.6 mg/kg) | Naproxen (7.5 mg/kg/d) | 53.2/51.0/45.5 | NS | 19.3/22.0/14.9 | 12 |
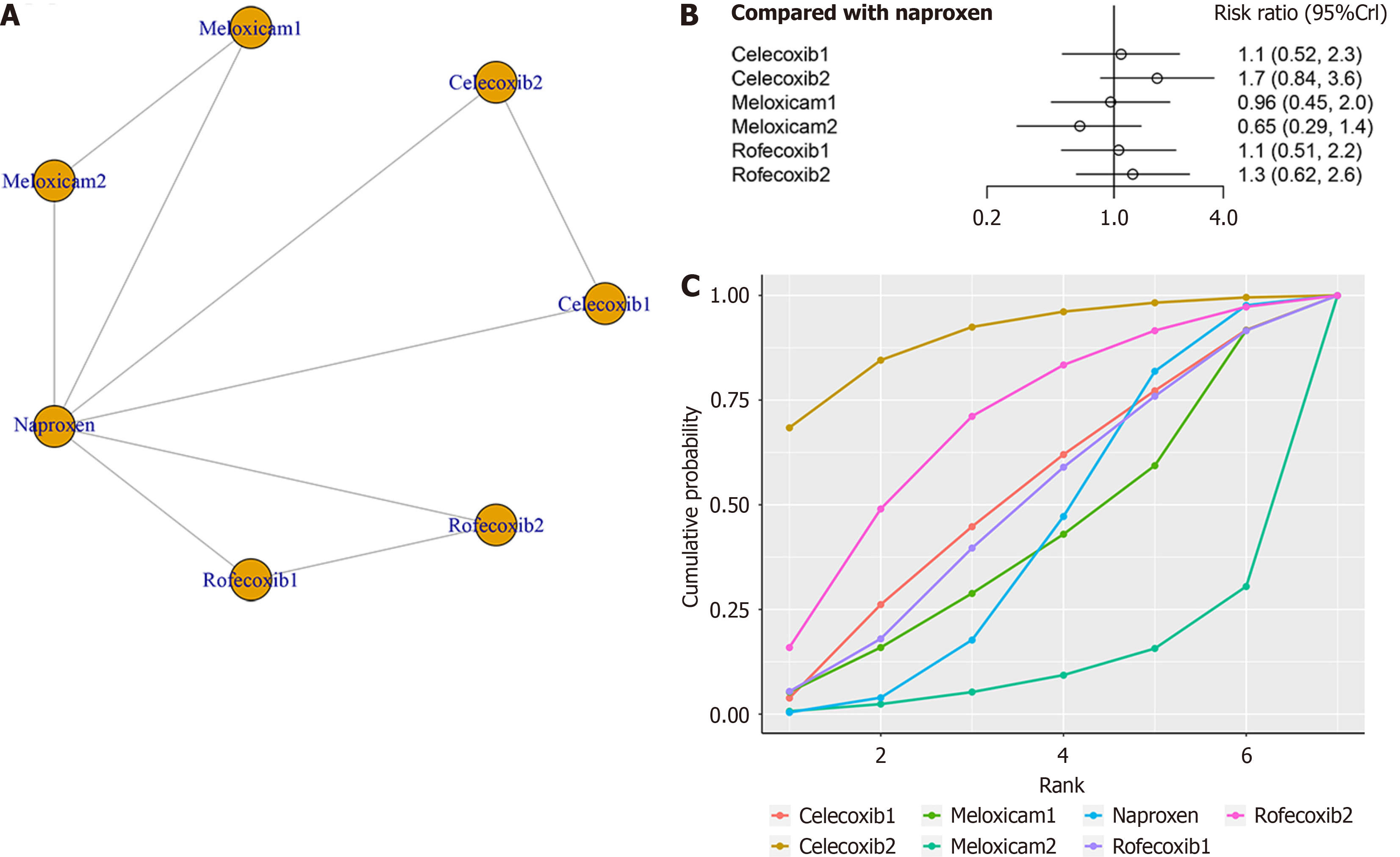
Three studies, involving a total of 770 patients, evaluated the clinical efficacy of four treatments (meloxicam, celecoxib, naproxen, and rofecoxib) in relation to the ACR Pedi 30 response. The network structure diagrams in Figure 3A illustrate the direct comparisons between these drugs in terms of their impact on the ACR Pedi 30 response. There were no notable differences in ACR Pedi 30 response between NSAIDs (Figure 3B). Celecoxib (6 mg/kg bid) had the highest ranking in SUCRA at 88.9%, followed by rofecoxib at 68.1% and Celecoxib (3 mg/kg bid) at 51.0% (Figure 3C).
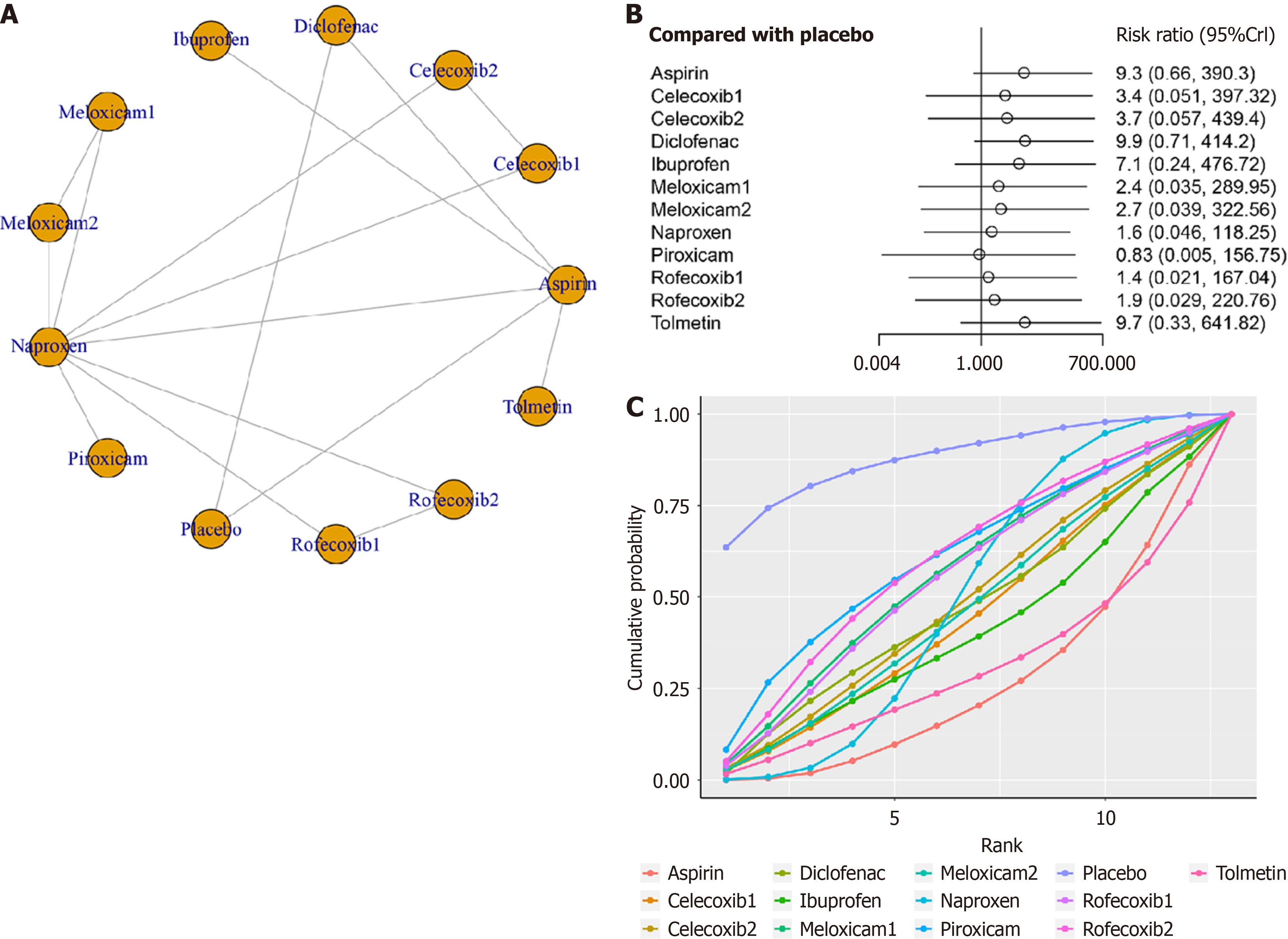
Eight studies with ten treatments (meloxicam, naproxen, piroxicam, placebo, rofecoxib, tolmetin, aspirin, celecoxib, and diclofenac) were analyzed for adverse events (Figure 4A). The network structure diagrams showed direct comparisons between the drugs, revealing no significant differences in adverse events among any two NSAIDs (Figure 4B). The results of the SUCRA indicate that the placebo intervention achieved the highest ranking with a SUCRA value of 88.2%, followed by piroxicam with a SUCRA of 60.5%. Rofecoxib at a dosage of 0.600 mg/kg per day ranked third with a SUCRA of 56.1%, while meloxicam at a dosage of 0.125 mg/kg per day and rofecoxib at a dosage of 0.3 mg/kg per day both achi
The systematic review found no significant differences in efficacy or safety among NSAIDs. Celecoxib and rofecoxib were ranked highest in terms of efficacy, while piroxicam and rofecoxib were deemed safer compared to other NSAIDs.
Two relevant pair-wise meta-analyses on the topic have been published[25,26]. Our meta-analysis aligns with previous studies, but offers unique contributions. It is the first network meta-analysis comparing NSAIDs for JIA and includes a protocol for optimal treatment. Thus, our results from this network meta-analysis could help health-care professionals make clinical decisions. NSAIDs remain essential for relieving joint symptoms in JIA patients, despite the shift towards biologics-targeted therapy.
This review is the first to systematically analyze and compare NSAIDs in JIA patients, providing a more comprehensive assessment than direct comparisons. At the same time, SUCRA value from network meta-analysis realize the efficacy and safety of each drug global sorting. The study did not find a statistically significant difference in lowering ACR Pedi 30 response between the two drugs. Based on the SUCRA values derived from trials included in our network meta-analysis, celecoxib (6 mg/kg bid) seem to be the most efficacious drug in lowering ACR Pedi 30 response. This was similar to the finding of the previous study, where a no significant difference between NSAIDs for osteoarthritis[27].
Furthermore, NSAIDs are well tolerated and has a good safety record. The most common adverse reactions are gas
Our meta-analysis has limitations, including the lack of randomized controlled trials and a small number of par
In summary, celecoxib (6 mg/kg bid) was found to be the most effective NSAID for treating JIA. Rofecoxib, piroxicam, and meloxicam may be safer options, but further research is needed to confirm these findings in larger trials with higher quality studies.
Different non-steroidal anti-inflammatory drugs (NSAIDs) have been used for juvenile idiopathic arthritis (JIA), but the best method has not been determined.
To perform a systematic review and network meta-analysis to identify the most effective NSAID for JIA patients.
To perform a systematic review and network meta-analysis to determine the optimal instructions.
We searched for randomized controlled trials (RCTs) from PubMed, EMBASE, Google Scholar, CNKI, and Wanfang without restriction for publication date or language at August, 2022. Any RCTs that comparing the effectiveness of NSAIDs with each other or placebo for JIA were included in this network meta-analysis. The surface under the cumu
Eight RCTs (1127 patients) compared different instructions for NSAIDs, including meloxicam, Celecoxib, piroxicam, Naproxen, inuprofen, Aspirin, Tolmetin, Rofecoxib, and placebo. No significant differences were found in ACR Pedi 30 response between any two NSAIDs. Celecoxib (6 mg/kg bid) had the highest SUCRA ranking at 88.9%, followed by rofecoxib at 68.1% and Celecoxib (3 mg/kg bid) at 51.0%. There were no notable differences in adverse events between NSAIDs. Placebo had the highest ranking, followed by piroxicam, rofecoxib (0.600 mg/kg qd), meloxicam (0.125 mg/kg qd), and rofecoxib (0.300 mg/kg qd).
In summary, celecoxib (6 mg/kg bid) was found to be the most effective NSAID for treating JIA.
Rofecoxib, piroxicam, and meloxicam may be safer options, but further research is needed to confirm these findings in larger trials with higher quality studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Glumac S, Croatia S-Editor: Chen YL L-Editor: A P-Editor: Xu ZH
| 1. | Zaripova LN, Midgley A, Christmas SE, Beresford MW, Baildam EM, Oldershaw RA. Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Pediatr Rheumatol Online J. 2021;19:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 2. | Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2011;377:2138-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 588] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 3. | Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. 2007;369:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 1056] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 4. | Okamoto N, Yokota S, Takei S, Okura Y, Kubota T, Shimizu M, Nozawa T, Iwata N, Umebayashi H, Kinjo N, Kunishima T, Yasumura J, Mori M. Clinical practice guidance for juvenile idiopathic arthritis (JIA) 2018. Mod Rheumatol. 2019;29:41-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Gowdie PJ, Tse SM. Juvenile idiopathic arthritis. Pediatr Clin North Am. 2012;59:301-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Boros C, Whitehead B. Juvenile idiopathic arthritis. Aust Fam Physician. 2010;39:630-636. [PubMed] |
| 7. | Bovid KM, Moore MD. Juvenile Idiopathic Arthritis for the Pediatric Orthopedic Surgeon. Orthop Clin North Am. 2019;50:471-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Akioka S. Interleukin-6 in juvenile idiopathic arthritis. Mod Rheumatol. 2019;29:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Jacobson JL, Pham JT. Juvenile Idiopathic Arthritis: A Focus on Pharmacologic Management. J Pediatr Health Care. 2018;32:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol. 2020;180:114147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 884] [Article Influence: 176.8] [Reference Citation Analysis (0)] |
| 11. | Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: Clinical importance, pathogenesis, and risk mitigation. Surv Ophthalmol. 2020;65:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Schjerning AM, McGettigan P, Gislason G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat Rev Cardiol. 2020;17:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 13. | Dodwell ER, Latorre JG, Parisini E, Zwettler E, Chandra D, Mulpuri K, Snyder B. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int. 2010;87:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Miller LG, Prichard JG. Current issues in NSAID therapy. Prim Care. 1990;17:589-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Zhao Z, Ma JX, Ma XL. Different Intra-articular Injections as Therapy for Hip Osteoarthritis: A Systematic Review and Network Meta-analysis. Arthroscopy. 2020;36:1452-1464.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Zhao Z, Ma J, Ma X. Comparative efficacy and safety of different hemostatic methods in total hip arthroplasty: a network meta-analysis. J Orthop Surg Res. 2019;14:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Ruperto N, Nikishina I, Pachanov ED, Shachbazian Y, Prieur AM, Mouy R, Joos R, Zulian F, Schwarz R, Artamonova V, Emminger W, Bandeira M, Buoncompagni A, Foeldvari I, Falcini F, Baildam E, Kone-Paut I, Alessio M, Gerloni V, Lenhardt A, Martini A, Hanft G, Sigmund R, Simianer S; Pediatric Rheumatology International Trials Organization. A randomized, double-blind clinical trial of two doses of meloxicam compared with naproxen in children with juvenile idiopathic arthritis: short- and long-term efficacy and safety results. Arthritis Rheum. 2005;52:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Foeldvari I, Szer IS, Zemel LS, Lovell DJ, Giannini EH, Robbins JL, West CR, Steidle G, Krishnaswami S, Bloom BJ. A prospective study comparing celecoxib with naproxen in children with juvenile rheumatoid arthritis. J Rheumatol. 2009;36:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | García-Morteo O, Maldonado-Cocco JA, Cuttica R, Garay SM. Piroxicam in juvenile rheumatoid arthritis. Eur J Rheumatol Inflamm. 1987;8:49-53. [PubMed] |
| 20. | Giannini EH, Brewer EJ, Miller ML, Gibbas D, Passo MH, Hoyeraal HM, Bernstein B, Person DA, Fink CW, Sawyer LA. Ibuprofen suspension in the treatment of juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. J Pediatr. 1990;117:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Haapasaari J, Wuolijoki E, Ylijoki H. Treatment of juvenile rheumatoid arthritis with diclofenac sodium. Scand J Rheumatol. 1983;12:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Kvien TK, Høyeraal HM, Sandstad B. Naproxen and acetylsalicylic acid in the treatment of pauciarticular and polyarticular juvenile rheumatoid arthritis. Assessment of tolerance and efficacy in a single-centre 24-week double-blind parallel study. Scand J Rheumatol. 1984;13:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Levinson JE, Baum J, Brewer E Jr, Fink C, Hanson V, Schaller J. Comparison of tolmetin sodium and aspirin in the treatment of juvenile rheumatoid arthritis. J Pediatr. 1977;91:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 61] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Reiff A, Lovell DJ, Adelsberg JV, Kiss MH, Goodman S, Zavaler MF, Chen PY, Bolognese JA, Cavanaugh P Jr, Reicin AS, Giannini EH. Evaluation of the comparative efficacy and tolerability of rofecoxib and naproxen in children and adolescents with juvenile rheumatoid arthritis: a 12-week randomized controlled clinical trial with a 52-week open-label extension. J Rheumatol. 2006;33:985-995. [PubMed] |
| 25. | Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F, Sturkenboom M, Perez-Gutthann S; Safety of Non-Steroidal Anti-Inflammatory Drugs (SOS) Project. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf. 2012;35:1127-1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 26. | DeWitt EM, Sherry DD, Cron RQ. Pediatric rheumatology for the adult rheumatologist I: therapy and dosing for pediatric rheumatic disorders. J Clin Rheumatol. 2005;11:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Song GG, Seo YH, Kim JH, Choi SJ, Ji JD, Lee YH. Relative efficacy and tolerability of etoricoxib, celecoxib, and naproxen in the treatment of osteoarthritis : A Bayesian network meta-analysis of randomized controlled trials based on patient withdrawal. Z Rheumatol. 2016;75:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Grosser T, Ricciotti E, FitzGerald GA. The Cardiovascular Pharmacology of Nonsteroidal Anti-Inflammatory Drugs. Trends Pharmacol Sci. 2017;38:733-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Tielemans MM, Eikendal T, Jansen JB, van Oijen MG. Identification of NSAID users at risk for gastrointestinal complications: a systematic review of current guidelines and consensus agreements. Drug Saf. 2010;33:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16:821-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (0)] |