Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2040
Peer-review started: November 12, 2023
First decision: January 30, 2024
Revised: February 25, 2024
Accepted: March 15, 2024
Article in press: March 15, 2024
Published online: April 26, 2024
Processing time: 154 Days and 18.4 Hours
This study was designed to investigate the clinical outcomes of enhanced recovery after surgery (ERAS) in the perioperative period in elderly patients with non-small cell lung cancer (NSCLC).
To investigate the potential enhancement of video-assisted thoracic surgery (VATS) in postoperative recovery in elderly patients with NSCLC.
We retrospectively analysed the clinical data of 85 elderly NSCLC patients who underwent ERAS (the ERAS group) and 327 elderly NSCLC patients who re
After propensity score matching, ERAS significantly reduced the postoperative hospital stay (6.96 ± 4.16 vs 8.48 ± 4.18 d, P = 0.001) and total hospital expenses (48875.27 ± 18437.5 vs 55497.64 ± 21168.63 CNY, P = 0.014) and improved the sa
ERAS effectively reduced the postoperative hospital stay and total hospital expenses and improved the satisfaction score in the perioperative period for elderly NSCLC patients who underwent lobectomy but not for patients who underwent sublobar resection.
Core Tip: This study was designed to investigate the clinical outcomes of enhanced recovery after surgery (ERAS) in the perioperative period in elderly patients with non-small cell lung cancer (NSCLC). ERAS significantly reduced the postoperative hospital stay (6.96 ± 4.16 vs 8.48 ± 4.18 d, P = 0.001) and total hospital expenses (48875.27 ± 18437.5 vs 55497.64 ± 21168.63 CNY, P = 0.014) and improved the satisfaction score (79.8 ± 7.55 vs 77.35 ± 7.72, P = 0.029) relative to those for routine care. ERAS effectively reduced the postoperative hospital stay and total hospital expenses and improved the satisfaction score in the perioperative period for elderly NSCLC patients who underwent lobectomy but not for patients who underwent sublobar resection.
- Citation: Sun MH, Wu LS, Qiu YY, Yan J, Li XQ. Enhanced recovery after surgery in elderly patients with non-small cell lung cancer who underwent video-assisted thoracic surgery. World J Clin Cases 2024; 12(12): 2040-2049
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2040.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2040
Lung cancer ranks first in all malignant tumours with respect to morbidity and mortality[1]. Surgery is the main treat
This study was designed to retrospectively analyse the clinical outcomes of ERAS in elderly patients with lung cancer who underwent VATS at Peking University Shenzhen Hospital over a 5-year period and to investigate the role of ERAS (after propensity score matching) in improving postoperative recovery.
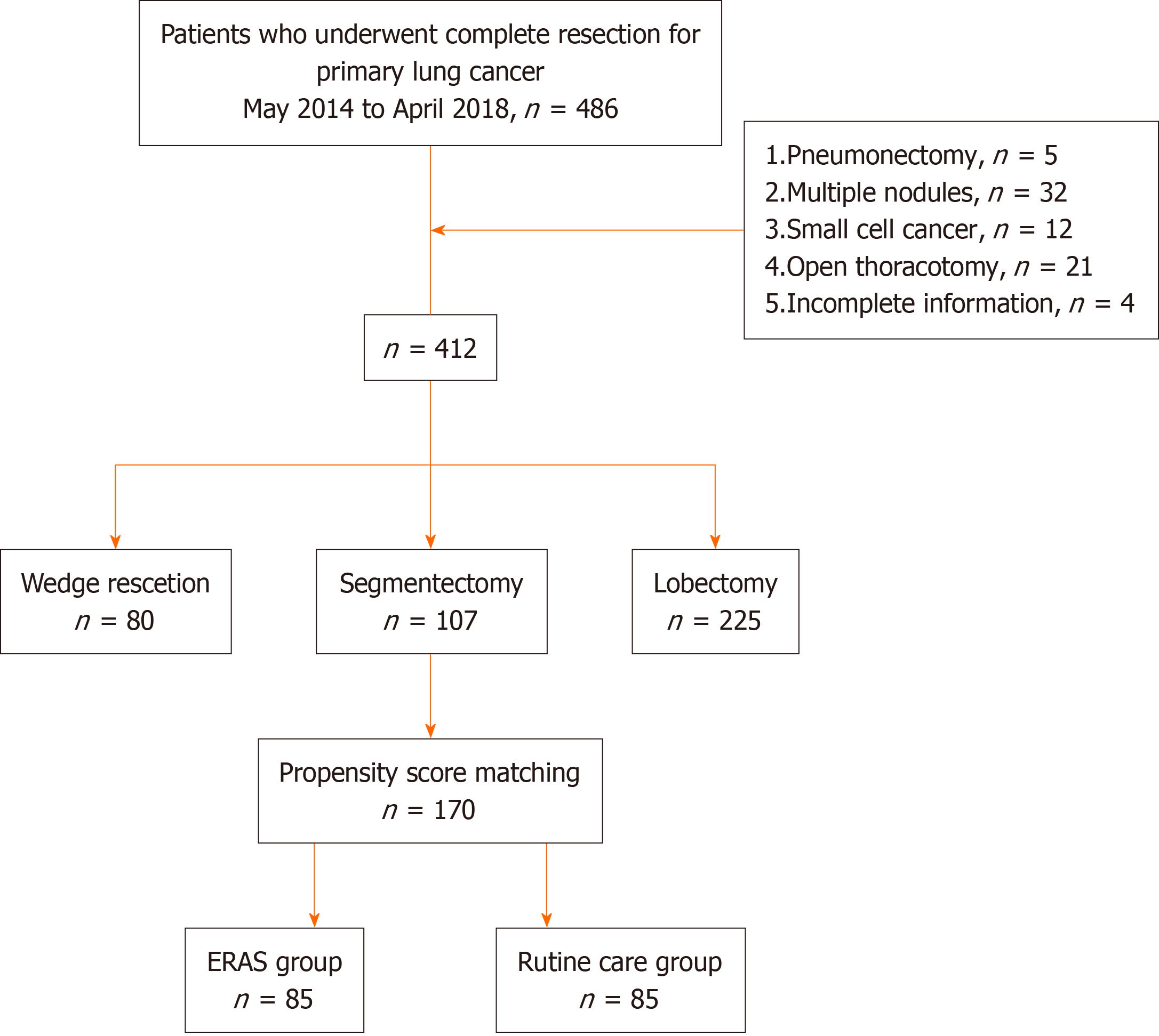
We retrospectively analysed the clinical data of 412 elderly patients with lung cancer who underwent VATS at the De
The inclusion criteria were as follows: (1) Patients who underwent VATS and were pathologically confirmed to have non-small cell lung cancer (NSCLC) after surgery; (2) patients aged 65-80 years old; (3) patients with NSCLC in TNM stage I to II confirmed by postoperative pathology; and (4) patients with complete clinical data. The exclusion criteria were as follows: (1) Patients with pneumonectomy; or (2) patients with pathologically confirmed small cell lung cancer.
Perioperative management: The patients were divided into the control group and the ERAS group. The control group received routine care, and the ERAS group underwent ERAS (Table 1).
| Measures | Routine care | ERAS | |
| Preoperative | Education | Routine preoperative education | ERAS education |
| Diet | Fasting for 6 h | Drink 1000 mL of 10% glucose the night before surgery; drink 200 mL of 10% glucose 2 h before surgery | |
| Sedatives (to improve sleep) | Yes | Yes | |
| Intraoperative | Indwelling catheter after anaesthesia | Yes | Yes |
| Temperature maintenance | No | Yes | |
| Postoperative | Analgesia | Patient-controlled epidural analgesia | Use of NSAIDs for 48 h |
| Infusion volume | Total intravenous infusion during the first 24 h after the operation < 1500 mL, infusion rate 20-30 mL/min; vasoconstrictors may be used in the case of hypotension or urine output < 20 mL/h | Rapid intravenous drip of 250 mL of saline within 1 h; the remaining parameters were the same as those in the routine care group | |
| Diet during the first 6 hours after the operation | A small amount of water | 400 mL of liquid food | |
| Promote bowel movements | No | Chewing gum | |
| Catheter removal | 24 h after the operation | 12 h after the operation | |
| Early exercise | Patient choice | Lower limb movements |
Preoperative management: All patients underwent a one-stop preoperative assessment by surgeons, anaesthesiologists, and nurses to facilitate optimal preoperative preparation and were closely monitored during and after the operation for any complications. The ERAS group was given a copy of an ERAS education brochure with detailed descriptions about daily goals and was asked to complete a diary. Intraoperative management: All patients were given prophylactic anti
| Patient preoperative education | |
| Pre-operative | Be familiar with the environment and hospitalization process |
| Preoperative nutritional risk screening | |
| Eat a healthy diet & stay active (1-2 wk before surgery) | |
| Normal diet the day before surgery | |
| Drink moderate glucose 2 h before surgery | |
| Preventive use of antibiotics | |
| Postoperative | Eating liquid food moderately within six hours after surgery & infusion |
| Receive any necessary medications | |
| Removed catheter at 12 h after operation | |
| Day after surgery | Normal diet |
| Use mixture of non-narcotic pain medication to keep comfortable | |
| Get out of bed as soon as possible | |
| Try to cough and expectorate | |
Criteria for discharge and follow-up: Discharge criteria were as follows: (1) Removal of the closed thoracic drainage tube; physical mobility; (2) no difficulty breathing (no shortness of breath, wheezing or stridor; oxygen saturation > 94%); and (3) no serious complications; complications (if any) were under control.
Calculation of medical expenses: The hospital medical records were used to record and calculate the total medical ex
Satisfaction: A homemade satisfaction questionnaire was used during the week after discharge to evaluate patient satisfaction. The contents included staff attitude, operating techniques, timeliness of nursing, overall hospital experience, and pain score. Quality of life was analysed, including physical performance, physical pain, mental state, and general health.
Statistical analysis: R language 3.5.3 was used for propensity score matching of pathological classification, TNM stage, and surgical approach at 1:1 between the ERAS group and the control group.
SPSS v25.0 was used for statistical analysis. Measurement data are expressed as the mean value ± SD and analysed with the independent sample t-test or Mann-Whitney U test; count data are expressed as the frequency and were ana
Among the 412 elderly patients with lung cancer who underwent VATS, 327 patients were in the control group and 85 patients were in the ERAS group. No significant between-group differences were observed regarding age (P = 0.220), sex (P = 0.982), body mass index (P = 0.540), or forced expiratory volume in the first second (P = 0.615) (Figure 1). Moreover, 330 patients had adenocarcinoma, and 82 patients had squamous cell carcinoma; 290 patients were in TNM stage I, and 122 patients were in stage II; 187 patients underwent sublobar resection, and 225 patients underwent lobectomy (Table 3). After matching, the control group and the ERAS group each included 85 patients.
| Baseline data | Before the match (n = 412) | After match (n = 170) | |||||
| Routine care (n = 327) | ERAS (n = 85) | P value | Routine care (n = 85) | ERAS (n = 85) | P value | ||
| Age | 72.18 ± 4.53 | 72.91 ± 4.94 | 0.22 | 72.55 ± 5 | 72.91 ± 4.94 | 0.643 | |
| Sex | Male | 215 | 56 | 0.982 | 59 | 56 | 0.624 |
| Female | 112 | 29 | 26 | 29 | |||
| BMI (kg/m2) | 22.54 ± 2.69 | 22.73 ± 2.62 | 0.54 | 22.51 ± 2.4 | 22.73 ± 2.62 | 0.565 | |
| FEV1 (L) | 3.21 ± 0.45 | 3.24 ± 0.41 | 0.615 | 3.24 ± 0.41 | 3.24 ± 0.41 | 0.983 | |
| Pathological classification | Adenocarcinoma | 261 | 69 | 0.78 | 69 | 69 | 1 |
| Squamous cell carcinoma | 66 | 16 | 16 | 16 | |||
| TNM stage | I | 235 | 55 | 0.198 | 55 | 55 | 1 |
| II | 92 | 30 | 30 | 30 | |||
| Surgical approach | Uniportal VATS | 282 | 69 | 0.242 | 75 | 69 | 0.201 |
| Three ports VATS | 45 | 16 | 10 | 16 | |||
| Scope of resection | Pulmonary wedge | 66 | 14 | 0.64 | 14 | 14 | 1 |
| Lung segment | 86 | 21 | 21 | 21 | |||
| Lobectomy | 175 | 50 | 50 | 50 | |||
No patient died during the perioperative period or required blood transfusion. At the end of surgery, the tracheal intubation was removed in the operating room, and the patients were able to breathe spontaneously with normal blood gas analysis results. All patients were sent back to the general ward, and no patient required mechanical ventilation in the intensive care unit. Before discharge, any postoperative complications were alleviated and resolved with treatment.
ERAS significantly improved postoperative hospital stay (6.98 ± 4.3 vs 8.92 ± 4.42 d, P = 0.002), total hospital expenses (52041.86 ± 19062.33 vs 60760.79 ± 20511.58, P = 0.016), and overall satisfaction (79.66 ± 7.5 vs 76.26 ± 7.42, P = 0.013) in the lobectomy subgroup (Table 4). Postoperative hospital stay also improved in the sublobar resection subgroup (6.94 ± 4.03 vs 7.86 ± 3.78 d, P = 0.09), but the differences of total hospital expenses (P = 0.247) and overall satisfaction (P = 0.621) did not reach statistical significance. In the ERAS group (n = 85), 3 patients had atelectasis, 9 had pulmonary infection, 4 had atrial fibrillation, and 2 had arrhythmia; the postoperative complication rate was 21.2%. In the control group (n = 85), 4 patients had atelectasis, 12 had pulmonary infection, 4 had atrial fibrillation, and 3 had arrhythmia; the postoperative complication rate was 27.1%. The difference did not reach statistical significance (Table 5).
| Outcome Measures | Total (n = 170) | Pulmonary wedge (n = 28) | Segmentectomy (n = 42) | Pulmonary lobe (n = 100) | ||||||||
| ERAS (n = 85) | Routine care (n = 85) | P value | ERAS (n = 14) | Routine care (n = 14) | P value | ERAS (n = 21) | Routine care (n = 21) | P value | ERAS (n = 50) | Routine care (n = 50) | P value | |
| Postoperative hospital stay (d) | 6.06 ± 2.07 | 6.61 ± 1.68 | 0.024 | 5.43 ± 1.91 | 6.14 ± 1.99 | 0.352 | 5.9 ± 2.51 | 6.29 ± 1.65 | 0.325 | 6.3 ± 1.91 | 6.88 ± 1.59 | 0.040 |
| Total hospital expenses (CNY) | 42757.63 ± 14963.16 | 53748.72 ± 18356.11 | 0.000 | 37812.08 ± 13327.54 | 41836.7 ± 13282.69 | 0.454 | 39187.44 ± 18933.83 | 51245.25 ± 16865.5 | 0.007 | 45641.86 ± 13016.75 | 58135.55 ± 18757.68 | 0.001 |
| Postoperative 48-h pain score | 2.38 ± 0.91 | 2.59 ± 0.88 | 0.109 | 2.29 ± 0.83 | 2.43 ± 0.76 | 0.667 | 2.33 ± 1.11 | 2.48 ± 0.87 | 0.560 | 2.42 ± 0.86 | 2.68 ± 0.91 | 0.135 |
| Satisfaction score | 80.65 ± 7.74 | 76.67 ± 7.1 | 0.001 | 80 ± 7.99 | 77 ± 6.86 | 0.427 | 80.29 ± 7.12 | 76.9 ± 5.66 | 0.130 | 80.98 ± 8.04 | 76.48 ± 7.79 | 0.003 |
| Readmission within 30 d | 0 | 1 | 1.000 | 0 | 0 | 0 | 0 | 0 | 1 | 1.000 | ||
| Complications (n) | 14 | 26 | 0.030 | 2 | 2 | 1.000 | 5 | 12 | 0.028 | 7 | 12 | 0.065 |
| Air leakage | 7 | 13 | 0.153 | 1 | 1 | 1.000 | 3 | 7 | 0.277 | 3 | 5 | 0.712 |
| Atelectasis | 2 | 4 | 0.678 | 0 | 0 | 1 | 2 | 1.000 | 1 | 2 | 1.000 | |
| Pulmonary infection | 3 | 6 | 0.493 | 1 | 1 | 1.000 | 1 | 1 | 1.000 | 1 | 4 | 0.359 |
| Atrial fibrillation | 1 | 2 | 1.000 | 0 | 0 | 0 | 1 | 1.000 | 1 | 1 | 1.000 | |
| Arrhythmia | 1 | 1 | 1.000 | 0 | 0 | 0 | 1 | 1.000 | 1 | 0 | 1.000 | |
| Outcome measures | Total (n = 170) | Age 60-73 (n = 28) | Age 74-80 (n = 42) | ||||||
| ERAS (n = 85) | Routine (n = 85) | P value | ERAS (n = 14) | Routine care | P value | ERAS (n = 21) | Routine | P value | |
| Postoperative hospital stay (d) | 6.06 ± 2.07 | 6.61 ± 1.68 | 0.024 | 5.96 ± 2 | 6.57 ± 1.7 | 0.057 | 6.18 ± 2.17 | 6.67 ± 1.69 | 0.188 |
| Total hospital expenses (CNY) | 42757.63 ± 14963.16 | 53748.72 ± 18356.11 | 0.000 | 42122.76 ± 13923.83 | 52334 ± 18206.28 | 0.008 | 43471.85 ± 16202.51 | 55417.37 ± 18628.5 | 0.001 |
| Postoperative 48-h pain score | 2.38 ± 0.91 | 2.59 ± 0.88 | 0.109 | 2.33 ± 0.83 | 2.8 ± 0.83 | 0.006 | 2.43 ± 1.01 | 2.33 ± 0.87 | 0.687 |
| Satisfaction score | 80.65 ± 7.74 | 76.67 ± 7.1 | 0.001 | 81.16 ± 7.52 | 76.78 ± 6.31 | 0.004 | 80.08 ± 8.04 | 76.54 ± 8.01 | 0.055 |
| Readmission within 30 d | 0 | 1 | 1.000 | 0 | 0 | 0 | 1 | 1.000 | |
| Complications (n) | 14 | 26 | 0.030 | 5 | 11 | 0.109 | 8 | 15 | 0.071 |
| Air leakage | 7 | 13 | 0.153 | 4 | 5 | 1.000 | 3 | 8 | 0.179 |
| Atelectasis | 2 | 4 | 0.678 | 1 | 2 | 1.000 | 1 | 2 | 0.982 |
| Pulmonary infection | 3 | 6 | 0.493 | 1 | 3 | 0.625 | 2 | 3 | 0.977 |
| Atrial fibrillation | 1 | 2 | 1.000 | 0 | 1 | 1.000 | 1 | 1 | 1.000 |
| Arrhythmia | 1 | 1 | 1.000 | 0 | 0 | 1 | 1 | 1.000 | |
ERAS is a multimodal perioperative protocol based on best medical evidence. In the 1990s, Kehlet et al[11] first used it for patients undergoing colectomy to enhance postoperative recovery[11]. It includes preoperative optimization, intraoperative stress management, and enhanced postoperative recovery, with the goal of accelerating the recovery and re
In recent years, a large body of evidence has demonstrated that VATS reduces complications and improves the prognosis of patients with lung cancer[5,21]. At present, however, evidence of the effectiveness of ERAS following VATS is inadequate, especially evidence on the role of ERAS following VATS in elderly patients with lung cancer. This was the first study to investigate the role of ERAS in the perioperative period in elderly patients with lung cancer. We performed propensity score matching to optimize the control group and comprehensively analysed perioperative outcome mea
This is the first study to perform propensity score matching to demonstrate the effectiveness of ERAS for elderly patients with lung cancer. Further subgroup analysis showed that ERAS had significant effects in the lobectomy subgroup. In summary, ERAS may be used as an effective treatment for elderly patients with lung cancer, especially patients under
Lung cancer is the leading cause of death worldwide, and non-small cell lung cancer (NSCLC) in the elderly accounts for a significant proportion. With the significant growth of the aging population, the need for surgical treatment of elderly patients has gradually become more prominent. Video-assisted thoracic surgery (VATS) has become an important choice for the treatment of senile NSCLC due to its characteristics of less trauma and rapid recovery. However, current sys
The aim of this study was to investigate the potential enhancement of VATS in postoperative recovery in elderly patients with NSCLC.
This study was designed to investigate the clinical outcomes of enhanced recovery after surgery (ERAS) in the perioperative period in elderly patients with NSCLC.
We retrospectively analysed the clinical data of 85 elderly NSCLC patients who underwent ERAS (the ERAS group) and 327 elderly NSCLC patients who received routine care (the control group) after VATS at the Department of Thoracic Surgery of Peking University Shenzhen Hospital between May 2015 and April 2017. After propensity score matching of baseline data, we analysed the postoperative stay, total hospital expenses, postoperative 48-hour pain score, and post
After propensity score matching, ERAS significantly reduced the postoperative hospital stay (6.96 ± 4.16 vs 8.48 ± 4.18 d, P = 0.001) and total hospital expenses (48875.27 ± 18437.5 vs 55497.64 ± 21168.63 CNY, P = 0.014) and improved the satisfaction score (79.8 ± 7.55 vs 77.35 ± 7.72, P = 0.029) relative to those for routine care. No significant between-group difference was observed in postoperative 48-h pain score (4.68 ± 1.69 vs 5.28 ± 2.1, P = 0.090) or postoperative compli
ERAS effectively reduced the postoperative hospital stay and total hospital expenses and improved the satisfaction score in the perioperative period for elderly NSCLC patients who underwent lobectomy but not for patients who underwent sublobar resection.
We look forward to more large-sample, multicenter studies to validate the recovery benefits of VATS in elderly patients with NSCLC and to further clarify the safety and effectiveness of the surgical technique. At the same time, combined with biological markers and imaging techniques, the specific mechanism of VATS on postoperative inflammatory response, immune function, and quality of life in elderly patients was further studied. With the help of advanced technical means, the individual differences of elderly patients were finely delineated to provide a more accurate basis for personalized surgical treatment. In addition, the long-term efficacy and survival rate of VATS in elderly patients were evaluated through long-term follow-up to comprehensively understand the long-term impact of surgery. These future research directions will provide an in-depth and comprehensive understanding for further promoting the development of surgical treatment for elderly NSCLC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Perez-Holanda S, Spain S-Editor: Li L L-Editor: A P-Editor: Xu ZH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55701] [Article Influence: 7957.3] [Reference Citation Analysis (132)] |
| 2. | Osarogiagbon RU, Veronesi G, Fang W, Ekman S, Suda K, Aerts JG, Donington J. Early-Stage NSCLC: Advances in Thoracic Oncology 2018. J Thorac Oncol. 2019;14:968-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Chen FF, Zhang D, Wang YL, Xiong B. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage Ⅰ non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol. 2013;39:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Vannucci F, Gonzalez-Rivas D. Is VATS lobectomy standard of care for operable non-small cell lung cancer? Lung Cancer. 2016;100:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Falcoz PE, Puyraveau M, Thomas PA, Decaluwe H, Hürtgen M, Petersen RH, Hansen H, Brunelli A; ESTS Database Committee and ESTS Minimally Invasive Interest Group. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg. 2016;49:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 6. | Birim O, Zuydendorp HM, Maat AP, Kappetein AP, Eijkemans MJ, Bogers AJ. Lung resection for non-small-cell lung cancer in patients older than 70: mortality, morbidity, and late survival compared with the general population. Ann Thorac Surg. 2003;76:1796-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Kawaguchi Y, Hanaoka J, Ohshio Y, Igarashi T, Kataoka Y, Okamoto K, Kaku R, Hayashi K. A risk score to predict postoperative complications after lobectomy in elderly lung cancer patients. Gen Thorac Cardiovasc Surg. 2018;66:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Im Y, Park HY, Shin S, Shin SH, Lee H, Ahn JH, Sohn I, Cho JH, Kim HK, Zo JI, Shim YM, Lee HY, Kim J. Prevalence of and risk factors for pulmonary complications after curative resection in otherwise healthy elderly patients with early stage lung cancer. Respir Res. 2019;20:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Gonfiotti A, Viggiano D, Bongiolatti S, Bertolaccini L, Solli P, Bertani A, Voltolini L, Crisci R, Droghetti A. Enhanced Recovery After Surgery (ERAS (®)) in thoracic surgical oncology. Future Oncol. 2018;14:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Rogers LJ, Bleetman D, Messenger DE, Joshi NA, Wood L, Rasburn NJ, Batchelor TJP. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. 2018;155:1843-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 11. | Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1737] [Article Influence: 62.0] [Reference Citation Analysis (2)] |
| 12. | Van Haren RM, Mehran RJ, Mena GE, Correa AM, Antonoff MB, Baker CM, Woodard TC, Hofstetter WL, Roth JA, Sepesi B, Swisher SG, Vaporciyan AA, Walsh GL, Rice DC. Enhanced Recovery Decreases Pulmonary and Cardiac Complications After Thoracotomy for Lung Cancer. Ann Thorac Surg. 2018;106:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, Ljungqvist O, Soop M, Ramirez J; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:801-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 14. | Cerfolio RJ, Pickens A, Bass C, Katholi C. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg. 2001;122:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Muehling BM, Halter GL, Schelzig H, Meierhenrich R, Steffen P, Sunder-Plassmann L, Orend KH. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg. 2008;34:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Salati M, Brunelli A, Xiumè F, Refai M, Pompili C, Sabbatini A. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardiothorac Surg. 2012;41:1083-7; discussion 1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Madani A, Fiore JF Jr, Wang Y, Bejjani J, Sivakumaran L, Mata J, Watson D, Carli F, Mulder DS, Sirois C, Ferri LE, Feldman LS. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery. 2015;158:899-908; discussion 908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2:CD009121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2014;18:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Puri V, Patel AP, Crabtree TD, Bell JM, Broderick SR, Kreisel D, Krupnick AS, Patterson GA, Meyers BF. Unexpected readmission after lung cancer surgery: A benign event? J Thorac Cardiovasc Surg. 2015;150:1496-1504, 1505.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |