Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2023
Peer-review started: February 23, 2024
First decision: March 9, 2024
Revised: March 9, 2024
Accepted: March 22, 2024
Article in press: March 22, 2024
Published online: April 26, 2024
Processing time: 52 Days and 11.9 Hours
In this editorial, we comment on the article by Wang and Long, published in a recent issue of the World Journal of Clinical Cases. The article addresses the challenge of predicting intensive care unit-acquired weakness (ICUAW), a neuromuscular disorder affecting critically ill patients, by employing a novel processing strategy based on repeated machine learning. The editorial presents a dataset comprising clinical, demographic, and laboratory variables from intensive care unit (ICU) patients and employs a multilayer perceptron neural network model to predict ICUAW. The authors also performed a feature importance analysis to identify the most relevant risk factors for ICUAW. This editorial contributes to the growing body of literature on predictive modeling in critical care, offering insights into the potential of machine learning approaches to improve patient outcomes and guide clinical decision-making in the ICU setting.
Core Tip: Predicting intensive care unit-acquired weakness (ICUAW) is crucial for improving patient outcomes. This editorial presents the potential of machine learning, specifically the multilayer perceptron neural network model, in predicting ICUAW. Insights into ICUAW risk factors and guides clinical decision-making in critical care are offered. The importance of developing accurate and reliable predictive models to improve patient outcomes in the intensive care unit setting is also emphasized.
- Citation: Ardila CM, González-Arroyave D, Zuluaga-Gómez M. Predicting intensive care unit-acquired weakness: A multilayer perceptron neural network approach. World J Clin Cases 2024; 12(12): 2023-2030
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2023.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2023
Intensive care unit-acquired weakness (ICUAW) is a neuromuscular disorder that affects patients who have been admitted to an intensive care unit (ICU) for an extended period[1]. It is characterized by a generalized weakness that can affect both the respiratory and limb muscles, leading to difficulties in breathing, moving, and performing activities of daily living[1,2]. ICUAW can result from a combination of factors, including immobility, prolonged use of mechanical ventilation, and systemic inflammation[1].
ICUAW is a significant concern in critical care medicine for several reasons including prognostic indicators, impact on functional outcomes, resource utilization, and clinical decision-making[1-3].
The development of ICUAW is associated with increased morbidity and mortality rates among ICU patients. Patients with ICUAW are at higher risk of complications such as pneumonia, sepsis, and prolonged hospital stays. Predicting the development of ICUAW can help clinicians identify high-risk patients early and implement preventive measures to mitigate its impact[1,2]. ICUAW can have long-term consequences on a patient's functional status and quality of life. It can lead to muscle wasting, weakness, and difficulty in performing basic activities, which can impair the patient's ability to return to their pre-ICU level of functioning. Predicting ICUAW can help clinicians develop targeted rehabilitation programs to improve patient outcomes[1,3]. ICUAW can increase the need for prolonged mechanical ventilation, rehabilitation services, and long-term care, leading to increased healthcare costs and resource utilization. Predicting ICUAW can help healthcare providers allocate resources more efficiently and improve the cost-effectiveness of care delivery[1-3]. Predicting ICUAW can inform clinical decision-making regarding the use of sedation, mechanical ventilation, and physical therapy interventions. Early identification of patients at risk of developing ICUAW can guide the imple
Overall, predicting the performance of ICUAW is important for improving patient outcomes, optimizing resource utilization, and guiding clinical decision-making in the critical care setting. It allows healthcare providers to identify high-risk patients early and implement targeted interventions to mitigate the impact of ICUAW on patient morbidity and mortality. However, predicting ICUAW is challenging due to its multifactorial nature and the lack of a gold standard diagnostic test[1-3].
However, several methods have been used to assess the risk of ICUAW and predict its development including clinical assessment, electrophysiological testing, biomarkers, muscle ultrasound, and machine learning models[1,4-6]. Clinicians often use a combination of clinical signs and symptoms to assess the risk of ICUAW. These may include muscle weakness, difficulty weaning from mechanical ventilation, and prolonged ICU stay. However, clinical assessment alone may not be sensitive or specific enough to accurately predict ICUAW[1-4]. Electrophysiological tests, such as electromyography and nerve conduction studies, can assess the function of the peripheral nerves and muscles. These tests can detect abnormalities in nerve conduction and muscle activation, which may indicate the presence of ICUAW. However, these tests are invasive, time-consuming, and may not be feasible in critically ill patients[1-3,5-7]. Biomarkers, such as creatine kinase and myosin light chain, have been investigated as potential indicators of muscle injury and ICUAW. Elevated levels of these biomarkers may suggest muscle damage, but their specificity for ICUAW is limited, and they may also be elevated in other conditions[1-3,6,8]. Muscle ultrasound can assess muscle thickness and echogenicity, which may be altered in patients with ICUAW. However, the interpretation of ultrasound findings can be subjective, and the technique may be operator-dependent[1-3,9]. Table 1 illustrates the strengths and weaknesses of these methods for predicting ICUAW.
| Approach | Strengths | Weaknesses |
| Clinical assessment | Clinicians can use clinical signs and symptoms to assess the risk of ICUAW, which is a non-invasive and readily available method | Clinical signs and symptoms may not be sensitive or specific enough to accurately predict ICUAW |
| Electrophysiological Testing | Electrophysiological tests, such as electromyography and nerve conduction studies, can provide objective measures of muscle function and help diagnose ICUAW | Electrophysiological tests are invasive, time-consuming, and may not be feasible in critically ill patients |
| Biomarkers | Biomarkers, such as creatine kinase and myosin light chain, can indicate muscle damage and may be useful for diagnosing ICUAW | Biomarkers are not specific to ICUAW and may be elevated in other conditions |
| Muscle ultrasound | Muscle ultrasound can provide information about muscle thickness and echogenicity, which can be altered in patients with ICUAW | The interpretation of ultrasound findings can be subjective, and the technique may be operator-dependent |
Recent studies have explored the use of machine learning models, such as artificial neural networks, to predict ICUAW. These models can analyze large datasets and identify patterns that may be predictive of ICUAW. However, the performance of these models may vary depending on the quality and size of the dataset used for training[1-3,8,9].
The multilayer perceptron (MLP) neural network model is a type of artificial neural network that has been widely used in various fields, including healthcare, for predictive modeling tasks[10]. It is a feedforward neural network with multiple layers of nodes (neurons) that are interconnected by weighted edges. Each node in the input layer represents a feature of the input data, and each node in the output layer represents a prediction or classification label. The nodes in the hidden layers perform nonlinear transformations of the input data, allowing the model to capture complex patterns and relationships in the data[11].
The MLP model has several advantages that make it a potential solution to improve prediction accuracy for ICUAW. The MLP model can capture nonlinear relationships between input features and the target variable[11], which is essential for predicting complex medical conditions like ICUAW that may involve multiple interacting factors. The MLP model can automatically learn relevant features from the input data, reducing the need for manual feature engineering and potentially capturing subtle patterns that may be missed by traditional statistical models[12]. The MLP model can be easily scaled to handle large datasets with many features, making it suitable for analyzing electronic health record data and other healthcare datasets[13]. The MLP model can generalize well to new data, making it suitable for predicting ICUAW in different patient populations or healthcare settings[10]. Although MLP models are often considered "black box" models, techniques such as feature importance analysis and model visualization can help interpret the model's predictions[14] and understand the factors that contribute to ICUAW risk. There are several open-source libraries and tools available for building and training MLP models, making them accessible to researchers and clinicians without extensive machine-learning expertise[15].
Overall, the MLP neural network model is a promising approach for predicting ICUAW, and its flexibility, scalability, and ability to capture complex patterns in the data make it a potential solution to improve prediction accuracy for this condition. However, further research is needed to validate the model's performance in larger patient populations and to identify the most effective predictive variables.
Several studies have investigated the use of prediction models, including those based on neural networks and machine learning models to assess the risk of ICU and improve patient outcomes.
The study by Benyó et al[16] focuses on computational glycemic mechanism (GM) used to manage stress-caused hyperg
The study by Pappada et al[17] emphasizes the critical importance of achieving glycemic control in patients in ICUs, as it has been associated with reduced mortality, shorter ICU stays, and lower risks of complications such as infection. However, maintaining glycemic control in this setting is challenging due to the diverse range of illnesses and patient conditions. The study collected continuous glucose monitoring (CGM) data and other relevant measures from the electronic medical records of 127 patients for the first 72 h of ICU care. These patients had either type 1 or type 2 diabetes or had a glucose value > 150 mg/dL upon admission to the ICU. The researchers developed a neural network-based model to predict a complete trajectory of glucose values up to 135 min in advance. The model's accuracy was validated using data from 15 patients not included in the training set, simulating real-world healthcare settings. The predictive models showed improved accuracy and performance compared to previous models developed by the research team. The model error, expressed as the mean absolute difference percent, was 10.6% for interstitial glucose values and 15.9% for serum blood glucose values collected 135 min in the future. A Clarke Error Grid Analysis of model predictions concerning the reference CGM, and blood glucose measurements revealed that over 99% of model predictions could be considered clinically acceptable and would not lead to inaccurate insulin therapy or treatment recommendations. This high level of clinical acceptability suggests that these models could be valuable tools within a clinical decision support system to assist healthcare providers in optimizing glycemic management in critical care patients[17].
The study by Wang and Long[9] recently published in the World Journal of Clinical Cases, focuses on identifying significant risk factors for ICUAW and offering recommendations for its prevention and treatment. The study utilized a MLP neural network model to analyze data from the initial 14 d of ICU stay, including age, comorbidities, sedative and vasopressor dosages, duration of mechanical ventilation, length of ICU stays, and rehabilitation therapy. The rela
The study by Chang et al[18] focuses on predicting the need for ICU admission in patients with myasthenia gravis (MG), an autoimmune neuromuscular disorder characterized by muscle weakness. Although specialized neuro-intensive care can lead to good long-term outcomes, predicting the need for ICU care is critical for optimizing patient management. The study used three machine learning-based decision tree algorithms to predict ICU admission in 228 MG patients admitted between 2015 and 2018. The C5.0 decision tree outperformed the other models and identified several significant risk factors for ICU admission, including the Myasthenia Gravis Foundation of America clinical classification at admission, thymoma history, azathioprine treatment history, disease duration, sex, and onset age. The developed decision tree can serve as a supportive tool for clinicians to identify MG patients who require intensive care, thereby improving the quality of care and potentially reducing morbidity and mortality.
The study by Tran et al[8] concentrates on crafting a clinical tool grounded in machine learning to anticipate muscle ailment subcategories utilizing multi-cohort microarray expression information. The information was curated from 42 separate cohorts with expression outlines in publicly available gene sources, encompassing a diverse spectrum of subject ages and muscle tissue samples from non-central regions. The research classified cohorts into five categories of muscle disorders: Limited mobility, inflammatory muscle diseases, ICU-acquired weakness, congenital conditions, and chronic systemic illnesses. The dataset includes evidence on 34.099 genes, and procedures to augment the information was employed to rectify imbalances in subtype representation within muscle disorders. Support direction mechanism algorithms were trained on two-thirds of the 1260 samples using the most significant gene signatures identified through statistical tests. Validation of the model was conducted on the residual testers utilizing the area under the receiver operator curve (AUC). The study found that chronic systemic disease was the best-predicted class with an AUC of 0.872, while ICUAW and immobility were the least discriminated classes with AUCs of 0.777 and 0.789, respectively. Condition-particular gene set enhancement findings revealed that the genetic profile exhibited improvement in biological pathways such as proliferation of neural progenitor cells for ICU-acquired weakness and aerobic metabolism for congenital conditions. The research concludes that the devised molecular categorization instrument featuring the chosen genetic indicators for categorizing muscle disorders fills a notable void in the literature on muscular ailments and introduces a potentially valuable diagnostic aid for discerning muscle disorder variety in clinical practice.
In summary, these investigations underscore the promise of prediction models in evaluating risk and enhancing patient outcomes. Nonetheless, additional research is required to validate these models across larger patient cohorts and to pinpoint the most efficacious predictive variables.
The dataset used to train and test the MLP model for predicting ICUAW would typically consist of a variety of clinical and demographic variables collected from patients admitted to the ICU. The variables that could be included in the model are presented in Table 2.
| Patient profile and assessment | Variable |
| Demographic information | Age |
| Sex | |
| Race | |
| Other demographic characteristics of the patient | |
| Clinical characteristics | Comorbidities |
| Severity of illness scores ( | |
| Reason for ICU admission | |
| Laboratory values | Creatinine |
| Liver function tests | |
| Complete blood count | |
| Inflammatory markers | |
| Vital signs | Heart rate |
| Blood pressure | |
| Respiratory rate | |
| Temperature | |
| Medication and treatment | Sedatives |
| Analgesics | |
| Neuromuscular blocking agents | |
| Other medications | |
| Mechanical ventilation | Duration of mechanical ventilation |
| Mode of ventilation | |
| Ventilator settings | |
| Muscle strength and function | Assessment of muscle strength ( |
| Neurological status | Glasgow coma scale score |
| Neurological examination findings | |
| Presence of delirium | |
| Functional status | Pre-ICU functional status ( |
| Outcomes | Development of ICUAW |
| Duration of ICU stay | |
| Duration of mechanical ventilation | |
| Mortality |
The dataset would typically be divided into two subsets: A training set and a test set. The training set would be used to train the MLP model, while the test set would be used to evaluate the model's performance. The dataset may also be divided into a validation set, which is used to tune the model's hyperparameters and prevent overfitting. It is important to note that the dataset should be large enough to adequately represent the patient population and include enough patients who develop ICUAW to allow for meaningful analysis. Additionally, missing data and outliers should be carefully handled to ensure the reliability of the model's predictions.
The specific features and parameters of the MLP model for predicting ICUAW can vary depending on the dataset and the specific implementation of the model. However, some common features and parameters must be included, such as the number of layers, activation functions, optimization algorithm, regularization, batch size, learning rate, and dropout rate.
The MLP model typically consists of an input layer, one or more hidden layers, and an output layer. The number of hidden layers and the number of nodes (neurons) in each layer are hyperparameters that need to be determined based on the complexity of the dataset and the desired level of prediction accuracy[11-13,19].
Activation functions are used to introduce nonlinearity into the model, allowing it to capture complex patterns in the data. Common activation functions used in MLP models include the sigmoid function, the hyperbolic tangent function, and the rectified linear unit function[11-14].
The optimization algorithm is used to update the weights of the model during training to minimize the loss function. Common optimization algorithms used in MLP models include stochastic gradient descent (SGD), Adam, and RMSprop[11-14].
Regularization techniques, such as L1 and L2 regularization, are used to prevent overfitting by penalizing large weights in the model. Dropout is another regularization technique that randomly drops a fraction of the nodes in each layer during training to prevent co-adaptation of neurons[11-13].
The batch size is the number of samples used to compute the gradient of the loss function during each iteration of training. A smaller batch size may lead to faster convergence but may result in noisy updates, while a larger batch size may lead to more stable updates but may require more memory[11-14].
The learning rate is a hyperparameter that determines the size of the step taken by the optimization algorithm during each iteration of training. A higher learning rate may lead to faster convergence but may result in overshooting the minimum of the loss function, while a lower learning rate may lead to slower convergence but may result in more stable updates[11-14].
The dropout rate is the fraction of nodes that are randomly dropped during training. A higher dropout rate may lead to more regularization but may result in slower convergence, while a lower dropout rate may lead to faster convergence but may result in overfitting[12-15].
These are just some of the features and parameters that can be used in an MLP model for predicting ICUAW. The specific choices of features and parameters should be based on the characteristics of the dataset and the desired level of prediction accuracy.
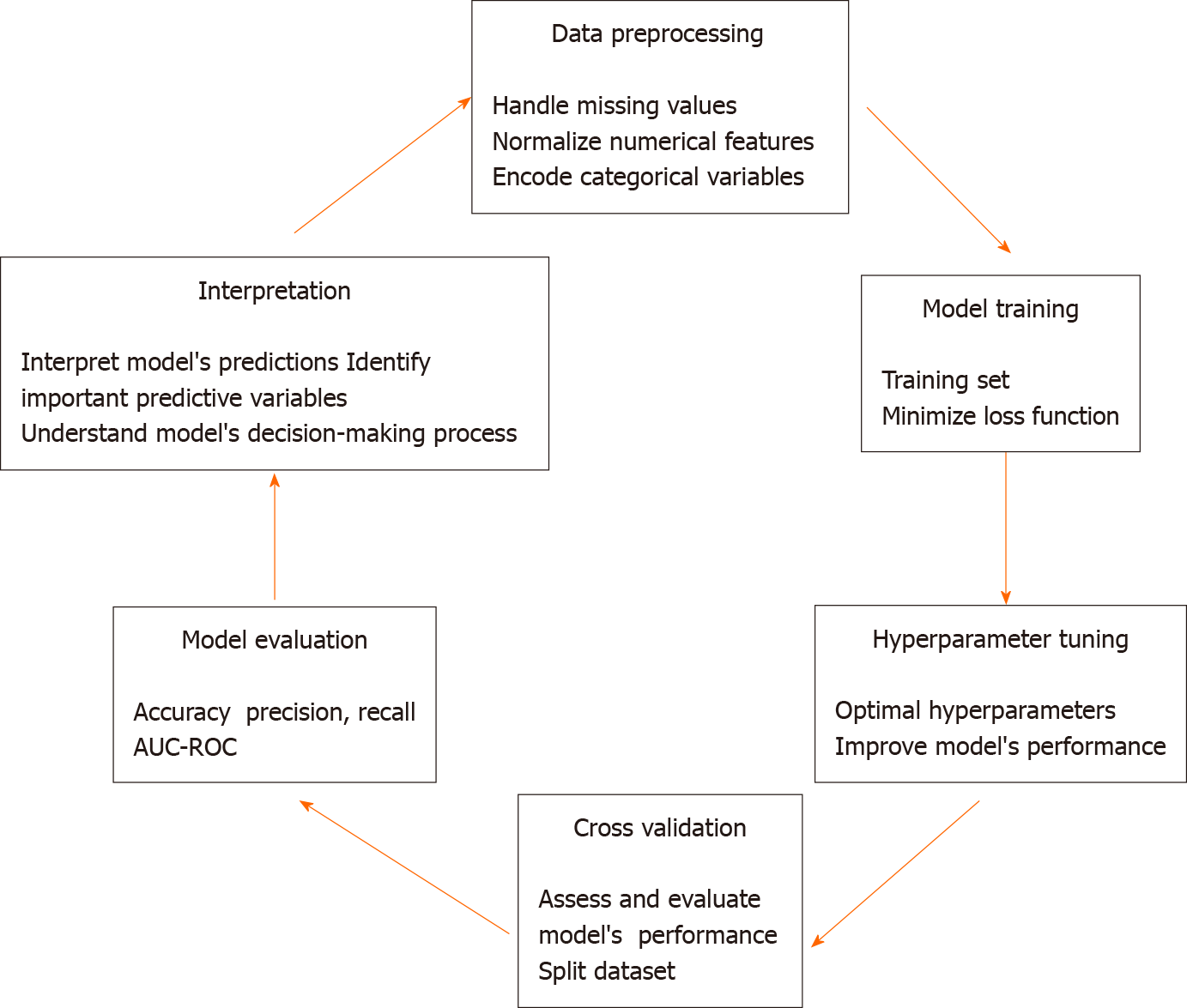
The process of training and validating the MLP model for predicting ICUAW involves several steps, including data preprocessing, model training, hyperparameter tuning, cross-validation, model evaluation, and interpretation (Figure 1). The step-by-step process is described below.
The first step is to preprocess the dataset by handling missing values, normalizing numerical features, and encoding categorical variables. This ensures that the data is in a suitable format for training the model. Next, the MLP model is trained using the training set. During training, the model's weights are updated iteratively using an optimization algorithm (e.g., SGD) to minimize the loss function. The loss function measures the difference between the model's predictions and the actual outcomes[20]. Hyperparameters are parameters that are not learned during training but are set before training begins. Examples of hyperparameters include the number of hidden layers, the number of nodes in each layer, the learning rate, and the dropout rate. Hyperparameter tuning involves selecting the optimal values for these hyperparameters to improve the model's performance. This can be done using techniques such as grid search, random search, or Bayesian optimization[21]. Cross-validation is a technique used to assess the generalization performance of the model. It involves splitting the dataset into multiple subsets (folds), training the model on some of the folds, and evaluating its performance on the remaining folds. This process is repeated multiple times, with different subsets used for training and evaluation each time. The average performance across all folds is used as an estimate of the model's generalization performance[10]. Once the model has been trained and validated, its performance is evaluated using the test set, which was not used during training or validation. The evaluation metrics used to assess the model's performance may include accuracy, precision, recall, F1 score, and area under the receiver operating characteristic curve[22]. These metrics provide insights into the model's ability to correctly classify patients with and without ICUAW. After evaluating the model, it is important to interpret its predictions and understand the factors that contribute to ICUAW risk. Techniques such as feature importance analysis and model visualization can help identify the most important predictive variables and understand the model's decision-making process[10,21,22]. The process is iterative and may involve multiple rounds of data preprocessing, model training, hyperparameter tuning, cross-validation, model evaluation, and interpretation.
By following these steps, researchers and clinicians can develop and validate an MLP model for predicting ICUAW that is accurate, reliable, and interpretable.
This editorial on predicting ICUAW using an MLP neural network model presents a comprehensive approach to addressing the challenges associated with predicting ICUAW. By leveraging the capabilities of the MLP model, researchers and clinicians can develop a predictive model that is accurate, reliable, and interpretable. The editorial highlights the importance of predicting ICUAW for improving patient outcomes, optimizing resource utilization, and guiding clinical decision-making in the critical care setting. The editorial presents the strengths and weaknesses of existing approaches to predicting ICUAW, including clinical assessment, electrophysiological testing, biomarkers, and muscle ultrasound. It emphasizes the limitations of these approaches and how the MLP model addresses these limitations by providing a nonlinear modeling approach, feature learning capabilities, scalability, generalization, and interpretability.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Colombia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soreq L, United Kingdom S-Editor: Zheng XM L-Editor: A P-Editor: Yu HG
| 1. | Chen J, Huang M. Intensive care unit-acquired weakness: Recent insights. J Intensive Med. 2024;4:73-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 2. | Tortuyaux R, Davion JB, Jourdain M. Intensive care unit-acquired weakness: Questions the clinician should ask. Rev Neurol (Paris). 2022;178:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Piva S, Fagoni N, Latronico N. Intensive care unit-acquired weakness: unanswered questions and targets for future research. F1000Res. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Wu TT, Chen QL, Lin XX, Xu ML, Chen XX, Luo CJ, Zhuang YN, Wei YQ, Wu JB, Xiong J, Chen LL, Li H. Effects of a multilevel intervention of resistance training with or without beta-hydroxy-beta-methylbutyrate in medical ICU patients during entire hospitalisation: a four-arm multicentre randomised controlled trial. Crit Care. 2023;27:493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Kasinathan A, Sharawat IK, Singhi P, Jayashree M, Sahu JK, Sankhyan N. Intensive Care Unit-Acquired Weakness in Children: A Prospective Observational Study Using Simplified Serial Electrophysiological Testing (PEDCIMP Study). Neurocrit Care. 2021;34:927-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Lugg ST, Howells PA, Thickett DR. The increasing need for biomarkers in intensive care unit-acquired weakness--are microRNAs the solution? Crit Care. 2015;19:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Zhang W, Wu J, Gu Q, Gu Y, Zhao Y, Ge X, Sun X, Lian J, Zeng Q. Changes in muscle ultrasound for the diagnosis of intensive care unit acquired weakness in critically ill patients. Sci Rep. 2021;11:18280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Tran A, Walsh CJ, Batt J, Dos Santos CC, Hu P. A machine learning-based clinical tool for diagnosing myopathy using multi-cohort microarray expression profiles. J Transl Med. 2020;18:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Wang L, Long DY. Significant risk factors for intensive care unit-acquired weakness: A processing strategy based on repeated machine learning. World J Clin Cases. 2024;12:1235-1242. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (38)] |
| 10. | Albaradei S, Thafar M, Alsaedi A, Van Neste C, Gojobori T, Essack M, Gao X. Machine learning and deep learning methods that use omics data for metastasis prediction. Comput Struct Biotechnol J. 2021;19:5008-5018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Lu J, Zou T, Jiang X. A Neural Network Based Approach to Inverse Kinematics Problem for General Six-Axis Robots. Sensors (Basel). 2022;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Zhao G, Jiang D, Liu X, Tong X, Sun Y, Tao B, Kong J, Yun J, Liu Y, Fang Z. A Tandem Robotic Arm Inverse Kinematic Solution Based on an Improved Particle Swarm Algorithm. Front Bioeng Biotechnol. 2022;10:832829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Xi J, Ersoy OK, Fang J, Wu T, Wei X, Zhao C. Parallel Multistage Wide Neural Network. IEEE Trans Neural Netw Learn Syst. 2023;34:4019-4032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Pomyen Y, Wanichthanarak K, Poungsombat P, Fahrmann J, Grapov D, Khoomrung S. Deep metabolome: Applications of deep learning in metabolomics. Comput Struct Biotechnol J. 2020;18:2818-2825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 15. | Wu J. A Product Styling Design Evaluation Method Based on Multilayer Perceptron Genetic Algorithm Neural Network Algorithm. Comput Intell Neurosci. 2021;2021:2861292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Benyó B, Paláncz B, Szlávecz Á, Szabó B, Kovács K, Chase JG. Classification-based deep neural network vs mixture density network models for insulin sensitivity prediction problem. Comput Methods Programs Biomed. 2023;240:107633. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Pappada SM, Owais MH, Cameron BD, Jaume JC, Mavarez-Martinez A, Tripathi RS, Papadimos TJ. An Artificial Neural Network-based Predictive Model to Support Optimization of Inpatient Glycemic Control. Diabetes Technol Ther. 2020;22:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Chang CC, Yeh JH, Chiu HC, Chen YM, Jhou MJ, Liu TC, Lu CJ. Utilization of Decision Tree Algorithms for Supporting the Prediction of Intensive Care Unit Admission of Myasthenia Gravis: A Machine Learning-Based Approach. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Silva F, Sanz M, Seixas J, Solano E, Omar Y. Perceptrons from memristors. Neural Netw. 2020;122:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Denysyuk HV, Pinto RJ, Silva PM, Duarte RP, Marinho FA, Pimenta L, Gouveia AJ, Gonçalves NJ, Coelho PJ, Zdravevski E, Lameski P, Leithardt V, Garcia NM, Pires IM. Algorithms for automated diagnosis of cardiovascular diseases based on ECG data: A comprehensive systematic review. Heliyon. 2023;9:e13601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Ali H, Muthudoss P, Ramalingam M, Kanakaraj L, Paudel A, Ramasamy G. Machine Learning-Enabled NIR Spectroscopy. Part 2: Workflow for Selecting a Subset of Samples from Publicly Accessible Data. AAPS PharmSciTech. 2023;24:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Debnath T, Nakamoto T. Predicting individual perceptual scent impression from imbalanced dataset using mass spectrum of odorant molecules. Sci Rep. 2022;12:3778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |