Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2016
Peer-review started: December 25, 2023
First decision: February 9, 2024
Revised: February 10, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: April 26, 2024
Processing time: 112 Days and 22.3 Hours
Pain in chronic pancreatitis (CP) is difficult to manage. Many patients suffer from inadequate pain relief, completely incapacitating them in their daily activities. Historically, despite their well-known adverse effects, opioids have been the pillar of treatment regimens in painful CP. The management is now gradually evolving with a better understanding of the underlying pathophysiology of CP-related pain. Clinicians should follow a holistic approach to the management of CP-associated pain, which must involve lifestyle changes that are coupled with analgesic medications and other pain-relieving interventions. Furthermore, there is no easy cure for vanquishing CP-associated pain. Each patient must be evaluated on a case-by-case basis by a multidisciplinary team to decide which treatment option is best suited for that individual.
Core Tip: Management of pain associated with chronic pancreatitis (CP) is difficult because of the intricate pathophysiology of this pain and the lack of universal gui
- Citation: Nag DS, Swain BP, Anand R, Barman TK, Vatsala. Pain management in chronic pancreatitis. World J Clin Cases 2024; 12(12): 2016-2022
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2016.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2016
Severe abdominal pain is the most debilitating symptom that is associated with chronic pancreatitis (CP)[1]. The patients typically describe a dull-aching pain around the epigastrium, which frequently radiates to the back and flanks. As the disease progresses, the pain becomes severe and excruciating[2]. This intractable pain, if not managed adequately, may drastically reduce the quality of life of patients by interfering with their physical, psychological, and social domains. Since there is no definitive cure for CP, its pain management is primarily aimed at providing patients with symptomatic relief and palliative care. Hence, adequate pain relief is fundamental to the pain management of CP. Despite our improved knowledge of chronic pain management, clinicians still face challenges in treating painful CP because of the complex nature of the disease process and the paucity of universal treatment guidelines. In the current editorial, we have delved into the pathophysiology of CP-associated pain and reviewed the recommended treatment modalities.
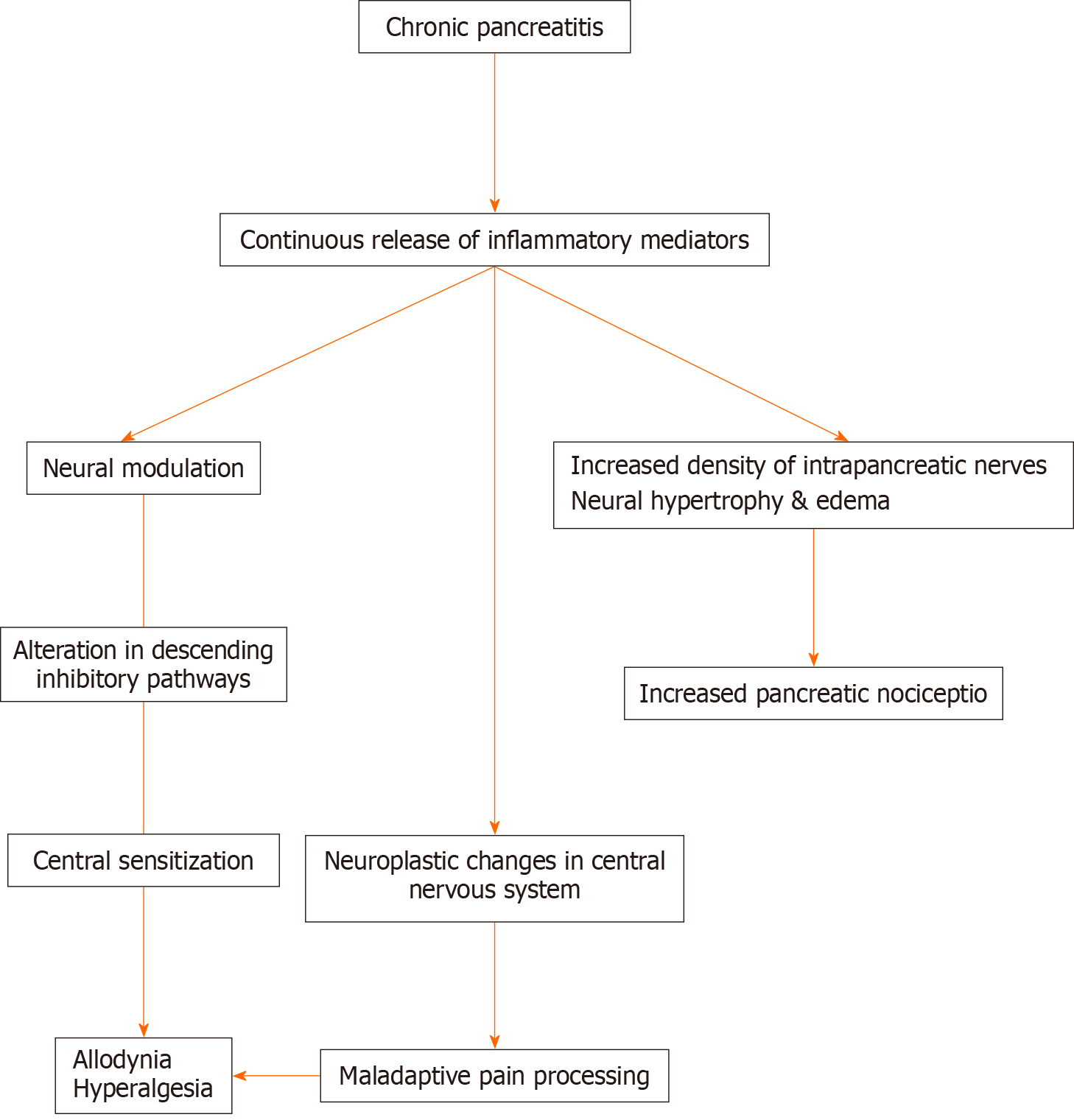
Pain in CP is multifactorial and poorly understood. The pathophysiology of pain was believed earlier to be primarily due to the nociceptive inputs that arise from the inflammatory changes in the pancreas. However, recent evidence suggests that the pain is more neuropathic[3,4]. In the background of continuous bombardment of nociceptive inputs from the inflamed pancreas, there is neural modulation or sensitization of the peripheral and central nervous system. Neural sensitization is clinically exhibited by hyperalgesia and allodynia observed commonly in CP[5,6]. Additionally, electroencephalographic and imaging studies have shown neural remodeling and functional changes in the central nervous system[7,8]. Histopathologically, it is exhibited by neural hypertrophy, edema, and increased density of intrapancreatic nerves. These changes result in the development of neuroplasticity and a maladaptive response to pain[9]. There are two distinct types of clinical manifestations of pain in CP. The “A-type pain” or intermittent pain is characterized by discrete episodes of pain with pain-free periods in between. The “B-type pain” is described as persistent background pain with episodes of acute exacerbation[10]. Studies have shown that the intermittent type of pain has a more predicted response to treatment than the latter one (“B-type pain”)[11]. The mechanism of pain is summarized in Figure 1.
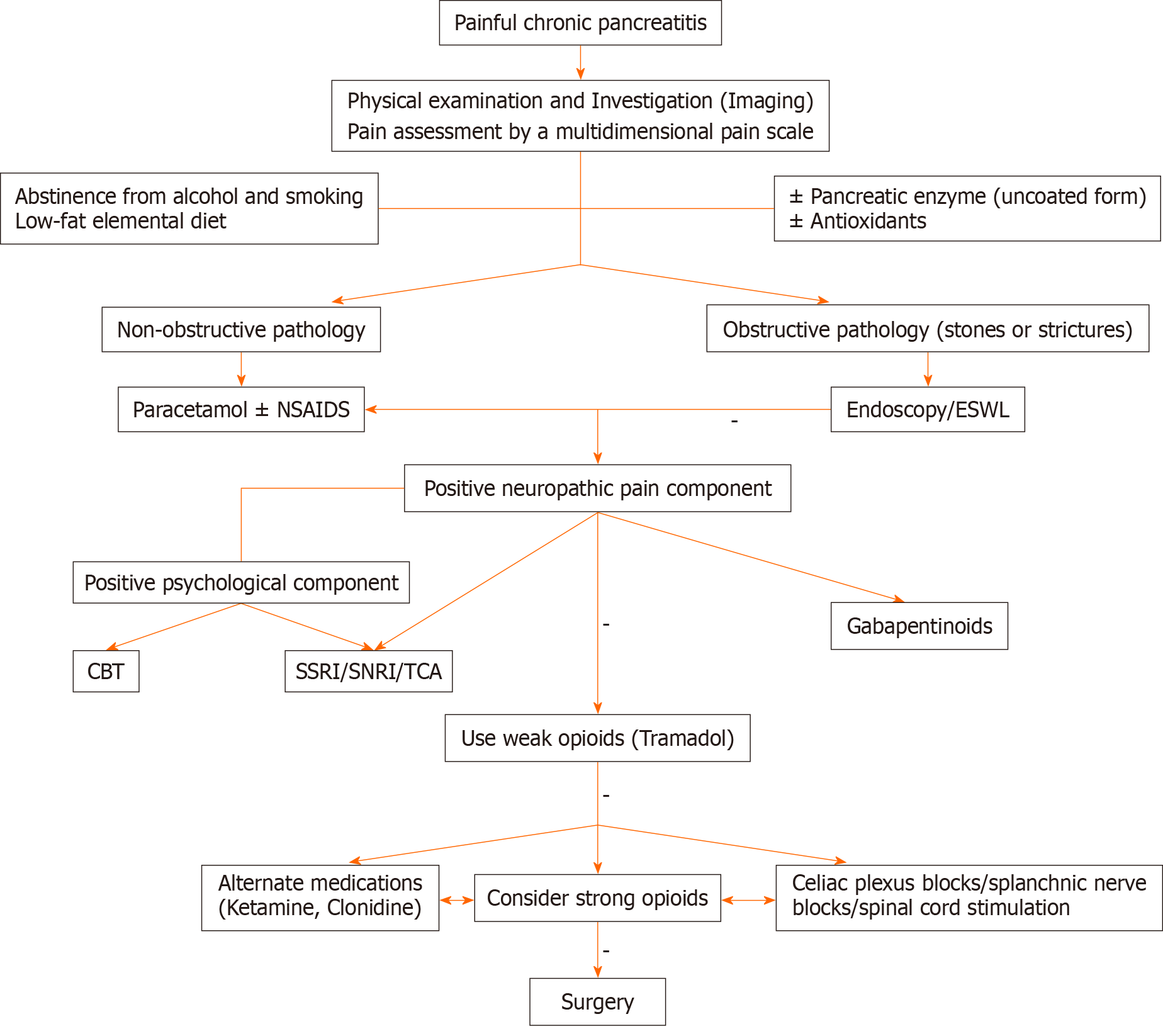
Management of pain in CP requires a structured approach that focuses on the stage, type, and primary pathophysiology of the disease process. A consensus guideline has recently suggested a stepwise approach to managing pain in CP[12]. Even so, one approach may not fit all patients considering that every patient is different. Thus, an individualized treatment plan is the best means to provide optimum benefit to the patient.
Pain management in CP can be divided into the following sections: Pain assessment, lifestyle modification, dietary changes, pharmacotherapy, interventional pain management, endoscopic treatment, and surgical interventions.
The first step of pain management is the accurate assessment of the severity of pain. Multiple pain assessment tools are available, but very few have been validated to be employed in the pain management of CP. Simple pain rating scales such as the numeric rating scale and visual analog scale only measure the intensity of pain and neglect other aspects of pain[13]. CP-associated pain is complex, with a significant psychosocial undertone; hence, it must be assessed through multidimensional pain scales. The Izbicki pain scale is specifically developed to address this aspect of pancreatic pain, but it is not appropriately validated to be applied in the pain management of CP[14].
The brief pain inventory pain assessment scale is a self-administered questionnaire-based tool validated to be used in CP-related pain management[15]. It quantifies the severity of pain and its impact on daily function including general activity, mood, behavior, and sleep[16]. The McGill pain questionnaire is another self-reporting measure of pain that can be useful in the pain management of CP. It provides a holistic view of pain severity by measuring the sensory, cognitive, and emotional aspects of pain[17]. Quantitative sensory testing helps assess and characterize pain mechanisms in patients with CP[18]. It can be employed in treatment-resistant cases of CP to assess pain sensitivity and to check the response of medications to pain[19].
Patients with CP are strongly advised to abstain from alcohol and smoking. Studies have demonstrated that refraining from alcohol intake significantly reduces the frequency of recurrences in pancreatitis and painful episodes[20]. Smoking is frequently associated with alcoholism, and it can be an independent risk factor for pain exacerbation in CP[21].
A low-fat elemental diet has been extensively studied in CP for pain control, considering that it reduces pancreatic secretion and reduces pain by decreasing ductal pressure[22,23]. It is suggested to be more effective in the early stage of the disease when the exocrine function of the pancreas is preserved[23]. The early institution of the nasojejunal tube is also recommended. Besides improving the nutritional status of the patient, nasojejunal feeding also reduces pain[24]. The benefit is achieved probably by a reduction in pancreatic secretion or may be due to bypassing of the stomach. The latter explanation is more plausible since delayed gastric emptying is common in CP cases[25].
Pancreatic enzymes have been shown to ameliorate pain in CP by negative feedback inhibition of pancreatic secretion[26]. It works by degrading the cholecystokinin-releasing factor that releases cholecystokinin responsible for the stimulation of pancreatic secretion[27]. The preparation of pancreatic enzyme must be in the uncoated form (nonacid protected form) to be effective, since the acid resistance form (coated form) may not get released in the duodenum. Nevertheless, a systemic review and meta-analysis was not able to come up with significant evidence of pain relief in CP by using pancreatic enzymes[28].
Antioxidants are advocated with the rationale that there is micronutrient deficiency in CP that results in oxidative stress and free radical injury[29]. A combination of antioxidants (β-carotene, vitamin C, vitamin E, selenium, and methionine) with other pain-relieving medication (Pregabalin) has been shown to avert painful episodes and recurrences[30,31].
The World Health Organization (WHO) analgesic ladder has been an enduring guide for the management of cancer pain for more than two decades, and it is still applicable in planning treatment for pain in CP[32]. The WHO ladder reco
Opioids are invariably added to the pain management regimen as pain severity increases in CP. Despite this, opioids are the most prescribed medications to manage pain, and their role is controversial in nonmalignant chronic pain scenarios such as those of CP-related pain[35]. The controversy is further aggravated by the widespread prevalence of opioid abuse. The recommendation is that opioids should never be the first-line therapy[36]. Before initiating opioid therapy, clinicians must be aware of the long-term side effects including misuse, addiction, opioid-induced hyperalgesia, and bowel dysfunction[37]. The patient who is on opioid therapy, especially strong opioids such as morphine, must be monitored closely to look for the development of such adverse effects. Tramadol, a weak opioid, is suggested to be more effective than morphine in controlling pain in CP with an equianalgesic dose[38]. It does not have any serious adverse effects or dependency potential in therapeutic doses, unlike strong opioids. Tramadol has weak activity on the μ-opioid receptor with an additional inhibitory effect on noradrenaline and serotonin reuptake[39]. It modulates the descending inhibitory pain pathway and can play a significant role in managing central sensitization associated with CP[40]. A maximum adult dose of 400 mg/day can be advocated safely in patients with CP. Transdermal preparation of opioids is also used, but it is usually reserved for patients who cannot tolerate oral preparations[41].
Considering that the neural mechanism of pain in CP is now well established, the drugs interfering with neural transmission are expected to be efficacious. Anticonvulsants (pregabalin and gabapentin), tricyclic antidepressants (amitriptyline), and selective serotonin reuptake inhibitors or selective norepinephrine reuptake inhibitor (duloxetine) are the centrally acting drugs commonly used to treat neuropathic pain and can be beneficial in CP[42]. Pregabalin has been extensively researched in patients with CP. It reduces synaptic release of neurotransmitters (glutamate, noradrenaline, and substance-P) by binding to alpha2-delta subunits of voltage-gated Ca2+ channel and thereby reducing neuronal excitability. Pregabalin must be started at a low dose to prevent its neurological adverse effects and slowly escalate until clinical benefit is appreciated[43]. The maximum recommended dose of pregabalin is 600 mg. Likewise, gabapentin, amitriptyline, and duloxetine can be tried as monotherapy or preferably in combination with other analgesics.
Other novel medications such as ketamine, an N-methyl-D-aspartate antagonist, can be effective by enhancing descending inhibition of pain in CP[43]. The S-enantiomer of ketamine is particularly more effective with fewer psychosomatic side effects and is currently being used in an ongoing trial that involves CP patients[44]. Somatostatin-analog inhibits pancreatic secretions and can lessen pain by reducing ductal pressure. However, current data are limited to suggest its use. Certain experimental drugs such as clonidine and benzodiazepines may be tried in the patient’s refractory to conventional medications[12].
Recent evidence suggested that patients of CP may benefit from sympathetic blocks such as celiac plexus and splanchnic nerve blocks[45,46]. These minimally invasive interventions can reduce analgesic requirements and may be considered as parts of a multimodal analgesic strategy. In one study, pulsed radiofrequency ablation of celiac plexus provided excellent pain relief in two cases of CP[47]. Spinal cord stimulation has shown significant pain relief in multiple studies[48,49]. It may be used in cases of CP refractory to analgesic medications.
The emotional and psychological impact of pain in CP is often a neglected aspect. Recent data support the use of beha
Endoscopic retrograde cholangiopancreatography (ERCP) is one of the most common modalities utilized in the treatment of painful CP. Endoscopic therapy is particularly useful in patients with obstructive pathology in the main pancreatic duct. The rationale behind it is that it releases the outflow obstruction and decompresses the pancreas, thereby reducing the pain[52]. Often extracorporeal shockwave lithotripsy is carried out to reduce pain in CP, especially in cases of large pancreatic stones localized in the head of the pancreas[53].
Surgical management was once the last resort employed when all other modalities failed to provide pain relief in CP. Nevertheless, evidence for the benefits of early surgical interventions is now emerging[54,55]. The surgical approach for pain management in CP depends on the morphological changes in the pancreas, duration of the disease, and response to other treatment modalities. Three modalities of surgery are commonly employed: Decompression surgery, resection, and a combined procedure depending on the pathology in the pancreas. The optimal timing of surgery is controversial. However, surgery should not be delayed beyond 2–3 years of onset of CP and should be done before the patient develops central sensitization[56].
The current evidence suggests that CP-associated pain is less of a nociceptive and more of a neuropathic type with sig
In conclusion, our current understanding of the etiopathogenesis of pain in CP opens multiple pain-relieving options for clinicians. However, to provide the best possible treatment modalities for the successful management of pain in CP, a multidisciplinary approach that involves gastroenterologists, surgeons, and pain physicians must be developed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Indian Society of Anaesthesiology, No. S2863.
Specialty type: Medicine, research and experimental
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dai YC, China S-Editor: Che XX L-Editor: A P-Editor: Zhao S
| 1. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 2. | Goulden MR. The pain of chronic pancreatitis: a persistent clinical challenge. Br J Pain. 2013;7:8-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Dimcevski G, Sami SA, Funch-Jensen P, Le Pera D, Valeriani M, Arendt-Nielsen L, Drewes AM. Pain in chronic pancreatitis: the role of reorganization in the central nervous system. Gastroenterology. 2007;132:1546-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Ceyhan GO, Michalski CW, Demir IE, Müller MW, Friess H. Pancreatic pain. Best Pract Res Clin Gastroenterol. 2008;22:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Atsawarungruangkit A, Pongprasobchai S. Current understanding of the neuropathophysiology of pain in chronic pancreatitis. World J Gastrointest Pathophysiol. 2015;6:193-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Buscher HC, Wilder-Smith OH, van Goor H. Chronic pancreatitis patients show hyperalgesia of central origin: a pilot study. Eur J Pain. 2006;10:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Olesen SS, Hansen TM, Graversen C, Steimle K, Wilder-Smith OH, Drewes AM. Slowed EEG rhythmicity in patients with chronic pancreatitis: evidence of abnormal cerebral pain processing? Eur J Gastroenterol Hepatol. 2011;23:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Olesen SS, Frøkjær JB, Lelic D, Valeriani M, Drewes AM. Pain-associated adaptive cortical reorganisation in chronic pancreatitis. Pancreatology. 2010;10:742-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Mullady DK, Yadav D, Amann ST, O'Connell MR, Barmada MM, Elta GH, Scheiman JM, Wamsteker EJ, Chey WD, Korneffel ML, Weinman BM, Slivka A, Sherman S, Hawes RH, Brand RE, Burton FR, Lewis MD, Gardner TB, Gelrud A, DiSario J, Baillie J, Banks PA, Whitcomb DC, Anderson MA; NAPS2 Consortium. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Drewes AM, Bouwense SAW, Campbell CM, Ceyhan GO, Delhaye M, Demir IE, Garg PK, van Goor H, Halloran C, Isaji S, Neoptolemos JP, Olesen SS, Palermo T, Pasricha PJ, Sheel A, Shimosegawa T, Szigethy E, Whitcomb DC, Yadav D; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17:720-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 196] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 13. | Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15 Suppl 1:S17-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 762] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 14. | Bloechle C, Izbicki JR, Knoefel WT, Kuechler T, Broelsch CE. Quality of life in chronic pancreatitis--results after duodenum-preserving resection of the head of the pancreas. Pancreas. 1995;11:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 1059] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 16. | Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129-138. [PubMed] |
| 17. | Seicean A, Grigorescu M, Tanţău M, Dumitraşcu DL, Pop D, Mocan T. Pain in chronic pancreatitis: assessment and relief through treatment. Rom J Gastroenterol. 2004;13:9-15. [PubMed] |
| 18. | Phillips AE, Faghih M, Kuhlmann L, Larsen IM, Drewes AM, Singh VK, Yadav D, Olesen SS; Pancreatic Quantitative Sensory Testing (P-QST) Consortium. A clinically feasible method for the assessment and characterization of pain in patients with chronic pancreatitis. Pancreatology. 2020;20:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Yadav D, Palermo TM, Phillips AE, Bellin MD, Conwell DL. Painful chronic pancreatitis - new approaches for evaluation and management. Curr Opin Gastroenterol. 2021;37:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | de las Heras G, de la Peña J, López Arias MJ, Gonzalez-Bernal AC, Martín-Ramos L, Pons-Romero F. Drinking habits and pain in chronic pancreatitis. J Clin Gastroenterol. 1995;20:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Han S, Kheder J, Bocelli L, Fahed J, Wachholtz A, Seward G, Wassef W. Smoking Cessation in a Chronic Pancreatitis Population. Pancreas. 2016;45:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kataoka K, Sakagami J, Hirota M, Masamune A, Shimosegawa T. Effects of oral ingestion of the elemental diet in patients with painful chronic pancreatitis in the real-life setting in Japan. Pancreas. 2014;43:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Ikeura T, Takaoka M, Uchida K, Miyoshi H, Okazaki K. Beneficial Effect of Low-Fat Elemental Diet Therapy on Pain in Chronic Pancreatitis. Int J Chronic Dis. 2014;2014:862091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Skipworth JR, Raptis DA, Wijesuriya S, Puthucheary Z, Olde Damink SW, Imber C, Malagò M, Shankar A. The use of nasojejunal nutrition in patients with chronic pancreatitis. JOP. 2011;12:574-580. [PubMed] |
| 25. | Chowdhury RS, Forsmark CE, Davis RH, Toskes PP, Verne GN. Prevalence of gastroparesis in patients with small duct chronic pancreatitis. Pancreas. 2003;26:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Singh VK, Drewes AM. Medical Management of Pain in Chronic Pancreatitis. Dig Dis Sci. 2017;62:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Slaff J, Jacobson D, Tillman CR, Curington C, Toskes P. Protease-specific suppression of pancreatic exocrine secretion. Gastroenterology. 1984;87:44-52. [PubMed] |
| 28. | Yaghoobi M, McNabb-Baltar J, Bijarchi R, Cotton PB. Pancreatic Enzyme Supplements Are Not Effective for Relieving Abdominal Pain in Patients with Chronic Pancreatitis: Meta-Analysis and Systematic Review of Randomized Controlled Trials. Can J Gastroenterol Hepatol. 2016;2016:8541839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Zhou D, Wang W, Cheng X, Wei J, Zheng S. Antioxidant therapy for patients with chronic pancreatitis: A systematic review and meta-analysis. Clin Nutr. 2015;34:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Talukdar R, Murthy HV, Reddy DN. Role of methionine containing antioxidant combination in the management of pain in chronic pancreatitis: a systematic review and meta-analysis. Pancreatology. 2015;15:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Talukdar R, Lakhtakia S, Nageshwar Reddy D, Rao GV, Pradeep R, Banerjee R, Gupta R, Ramchandani M, Tandan M, Murthy HV. Ameliorating effect of antioxidants and pregabalin combination in pain recurrence after ductal clearance in chronic pancreatitis: Results of a randomized, double blind, placebo-controlled trial. J Gastroenterol Hepatol. 2016;31:1654-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Ventafridda V, Saita L, Ripamonti C, De Conno F. WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 1985;7:93-96. [PubMed] |
| 33. | Vantini I, Piubello W, Scuro LA, Benini P, Talamini G, Benini L, Micciolo R, Cavallini G. Duodenal ulcer in chronic relapsing pancreatitis. Digestion. 1982;24:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Sato T, Kameyama J, Sasaki I, Imamura M, Matsuno S. Gastric acid secretion and serum gastrin levels in chronic pancreatitis. Gastroenterol Jpn. 1981;16:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16:405-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 420] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 36. | Houry D, Baldwin G. Announcing the CDC guideline for prescribing opioids for chronic pain. J Safety Res. 2016;57:83-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105-S120. [PubMed] |
| 38. | Wilder-Smith CH, Hill L, Osler W, O'Keefe S. Effect of tramadol and morphine on pain and gastrointestinal motor function in patients with chronic pancreatitis. Dig Dis Sci. 1999;44:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Subedi M, Bajaj S, Kumar MS, Yc M. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother. 2019;111:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 40. | Barakat A. Revisiting Tramadol: A Multi-Modal Agent for Pain Management. CNS Drugs. 2019;33:481-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Shah I, Sheth SG, Kothari DJ. Pain management in chronic pancreatitis incorporating safe opioid practices: Challenge accepted. World J Gastroenterol. 2021;27:3142-3147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 42. | Fornasari D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017;6:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 43. | Olesen SS, Bouwense SA, Wilder-Smith OH, van Goor H, Drewes AM. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology. 2011;141:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 44. | Juel J, Olesen SS, Olesen AE, Poulsen JL, Dahan A, Wilder-Smith O, Madzak A, Frøkjær JB, Drewes AM. Study protocol for a randomised, double-blinded, placebo-controlled, clinical trial of S-ketamine for pain treatment in patients with chronic pancreatitis (RESET trial). BMJ Open. 2015;5:e007087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Cornman-Homonoff J, Holzwanger DJ, Lee KS, Madoff DC, Li D. Celiac Plexus Block and Neurolysis in the Management of Chronic Upper Abdominal Pain. Semin Intervent Radiol. 2017;34:376-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Ahmed A, Arora D. Fluoroscopy-guided Neurolytic Splanchnic Nerve Block for Intractable Pain from Upper Abdominal Malignancies in Patients with Distorted Celiac Axis Anatomy: An Effective Alternative to Celiac Plexus Neurolysis - A Retrospective Study. Indian J Palliat Care. 2017;23:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Brennan L, Fitzgerald J, McCrory C. The use of pulsed radiofrequency treatment for chronic benign pancreatitis pain. Pain Pract. 2009;9:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Kim JK, Hong SH, Kim MH, Lee JK. Spinal Cord Stimulation for Intractable Visceral Pain due to Chronic Pancreatitis. J Korean Neurosurg Soc. 2009;46:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Kapural L, Cywinski JB, Sparks DA. Spinal cord stimulation for visceral pain from chronic pancreatitis. Neuromodulation. 2011;14:423-6; discussion 426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Palermo TM, Law EF, Topazian MD, Slack K, Dear BF, Ko YJ, Vege SS, Fogel E, Trikudanathan G, Andersen DK, Conwell DL, Yadav D; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC). Internet Cognitive-Behavioral Therapy for Painful Chronic Pancreatitis: A Pilot Feasibility Randomized Controlled Trial. Clin Transl Gastroenterol. 2021;12:e00373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Li S, Yin D, Guo XC. Influence of standardized nursing intervention combined with mindfulness stress reduction training on the curative effect in patients with acute pancreatitis. World J Clin Cases. 2023;11:8276-8283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Gabbrielli A, Pandolfi M, Mutignani M, Spada C, Perri V, Petruzziello L, Costamagna G. Efficacy of main pancreatic-duct endoscopic drainage in patients with chronic pancreatitis, continuous pain, and dilated duct. Gastrointest Endosc. 2005;61:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Guda NM, Partington S, Freeman ML. Extracorporeal shock wave lithotripsy in the management of chronic calcific pancreatitis: a meta-analysis. JOP. 2005;6:6-12. [PubMed] |
| 54. | Parekh D, Natarajan S. Surgical Management of Chronic Pancreatitis. Indian J Surg. 2015;77:453-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Yang CJ, Bliss LA, Schapira EF, Freedman SD, Ng SC, Windsor JA, Tseng JF. Systematic review of early surgery for chronic pancreatitis: impact on pain, pancreatic function, and re-intervention. J Gastrointest Surg. 2014;18:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 56. | Bouwense SAW, Kempeneers MA, van Santvoort HC, Boermeester MA, van Goor H, Besselink MG. Surgery in Chronic Pancreatitis: Indication, Timing and Procedures. Visc Med. 2019;35:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |