Published online Apr 6, 2024. doi: 10.12998/wjcc.v12.i10.1844
Peer-review started: January 4, 2024
First decision: January 17, 2024
Revised: January 31, 2024
Accepted: March 15, 2024
Article in press: March 15, 2024
Published online: April 6, 2024
Processing time: 88 Days and 16.6 Hours
Suprasellar germinomas are rare intracranial tumors frequently associated with permanent endocrine disorders. We present the clinical picture, treatment, and complications of suprasellar germinoma at pediatric age which, besides being life-threatening, has lifelong endocrinological consequences.
A 12-year-old female patient was presented having had intensive headaches for three weeks and visual disturbances for six months. An ophthalmological exa
This complex case highlights the importance of timely diagnosis, a multidisciplinary approach, and close follow-up in children with suprasellar germinomas.
Core Tip: Suprasellar germinomas are rare tumors that may be associated with hypopituitarism at diagnosis and after therapy. We report the presentation, diagnosis, treatment, and complications of a suprasellar germinoma in a 12-year-old patient. The tumor caused visual impairment, headaches, and hypopituitarism. The patient was treated surgically, followed by adjuvant chemotherapy and radiotherapy. Post-treatment panhypopituitarism required hormonal replacement therapy. The case under
- Citation: Roganovic J, Saric L, Segulja S, Dordevic A, Radosevic M. Panhypopituitarism caused by a suprasellar germinoma: A case report. World J Clin Cases 2024; 12(10): 1844-1850
- URL: https://www.wjgnet.com/2307-8960/full/v12/i10/1844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i10.1844
Germinomas are germ cell neoplasms that primarily affect the gonads but can appear extragonadally, along midline structures of the body[1]. Intracranial germinomas are rare brain tumors predominantly affecting pediatric and young adult patients. They often occur in the pineal or the sellar/suprasellar regions, and less frequently in the basal ganglia/thalamus[2]. Patients may present with a wide spectrum of non-specific endocrinological, visual, and cognitive distur
Germinomas of the suprasellar region cause the clinical picture of diabetes insipidus, visual disturbances, and hypopituitarism, due to the lack of stimulating hormones from the pituitary gland[10,11]. The aim of this case report is to present a patient with a rare brain tumor – a suprasellar germinoma, associated with a rare endocrine condition – hypopituitarism, that persists even after the underlying cause has been cured.
A twelve-year-old female patient presented to the Emergency Department with gradual vision loss for 6 months and frontoparietal headache in the morning for 3 wk.
The patient reported gradual vision loss over the previous 6 months and a 3-wk long frontoparietal headache in the morning. She had no nausea, vomiting, double vision, or other symptoms.
The patient was being followed-up regularly by an ophthalmologist due to strabismus surgery performed at the ages of 8 and 11.
Parents denied any family history of malignant tumors.
The patient was in a good general condition. Her vital signs included blood pressure 105/69 mmHg, pulse rate 82 beats/min, oxygen saturation 100%, and axillar temperature of 36.7 ºC. The girl had age-appropriate growth [height 157 cm (75th percentile), body weight (BW) 49 kg (75th percentile), and body mass index (BMI) 19.8 (75th percentile)]. Sexual maturation was normal with Tanner stage 2 breast development and Tanner stage 1 pubic hair development. Neurological exami
Abnormal laboratory findings included lower levels of thyroxine (7.46 pmol/L, reference range 10-26 pmol/L), cortisol (53.52 nmol/L, range 171-536 nmol/L for a blood sample taken at 8 in the morning), follicle stimulating hormone (FSH) (< 0.1 IU/L, range 3-10 IU/L), luteinizing hormone (LH) (< 0.1 IU/L, range 2-8 IU/L), estradiol (< 18 pg/mL, range 30-400 pg/mL), progesterone (< 0.05 ng/mL, range 0.1-0.3 ng/mL for prepubescent girls), testosterone (< 0.087 nmol/L, range 0.29-1.67 nmol/L), and insulin-like growth factor 1 (IGF-1) (6.7 nmol/L, range 12.5-70.9 nmol/L). Thyroid stimulating hormone (TSH) (1.53 mIU/L, range 0.58-4.1 mIU/L) and adrenocorticotropic hormone (ACTH) (2.20 pmol/L, range 2-11 pmol/L) were within normal limits. Prolactin level (1063 mIU/L, range 40-530 mIU/L) was elevated. The diagnosis of hypopituitarism was established on the basis of diminished levels of anterior pituitary gland hormones (gonadotropins FSH and LH) and target hormones, thyroxine and cortisol. Beta-human chorionic gonadotropin (β-hCG) and alpha fetoprotein (AFP) tumor markers were negative. Lactate dehydrogenase (318 U/L, range 152-284 U/L) and uric acid concentrations (436 µmol/L, range 125-293 µmol/L) were slightly elevated.
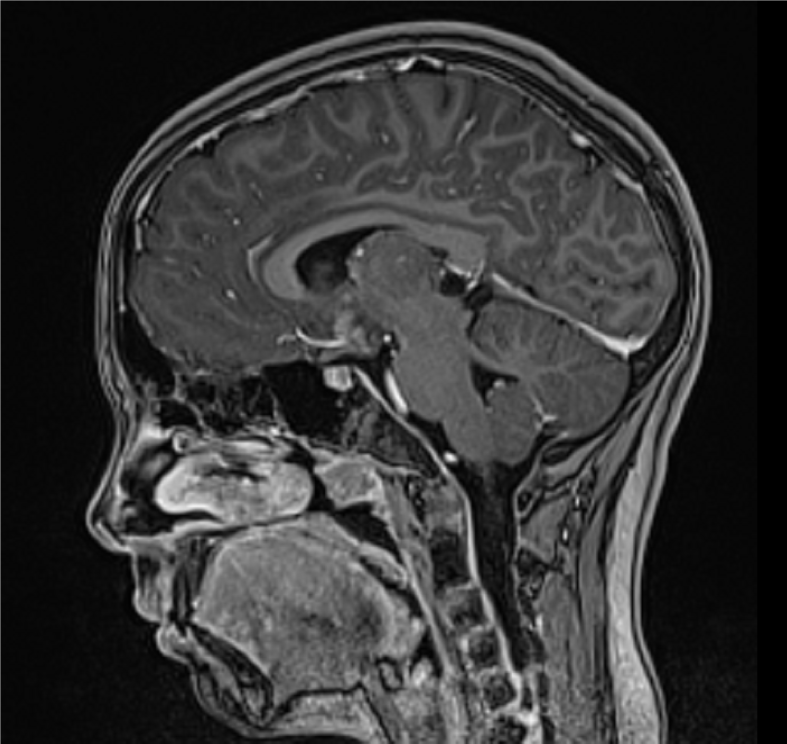
Emergency magnetic resonance imaging (MRI) of the brain showed a suprasellar mass measuring 22 mm × 18 mm. The tumor was compressing the anterior commissure and the tuber cinereum, extending to the bottom of the third ventricle. The lesion was isointense to the brain on non-contrast T1 weighted image (WI) and showed homogenous contrast enhancement. The axial T2WI MRI scan showed the lesion isointense to the brain. The infundibulum and optic chiasm were incorporated into the mass, and a cyst 13 mm × 10 mm was found in the pineal region. No calcifications, signs of ischemia, or hemorrhage were observed (Figure 1).
The final diagnosis of suprasellar germinoma was established by imaging studies and immunohistopathology.
Replacement therapy with hydrocortisone and levothyroxine was started, but no additional hormone levels in basal conditions and dynamic stimulation tests were performed as the patient’s clinical condition dictated emergency intervention.
The patient was transferred to another hospital for neurosurgery. Maximal tumor reduction was performed, and brain MRI at 48 h after surgery showed minimal residual tumor. The postoperative course was uneventful. MRI of the spine and cytology of the cerebrospinal fluid (CSF) confirmed non-metastatic disease. A diagnosis of suprasellar germinoma was established by pathohistological examination. Immunohistochemistry revealed positivity for CD117, OCT-3/4, focal cytokeratin, and placental alkaline phosphatase, and negative staining for CD30, β-hCG and AFP, contributing to the accurate identification of the tumor type.
Postoperative treatment was continued according to the SIOP CNS GCT II protocol for children, adolescents, and young adults with intracranial germ cell tumors. Pretreatment investigations included repeated endocrine status including TSH (0.66 mIU/L, range 0.58-4.1 mIU/L) and ACTH (< 0.22 pmol/L, range 1.6-13.9 pmol/L) and tumor markers, routine blood tests, renal function, and hearing, ophthalmological and neurocognitive assessments.
A central venous catheter was implanted. Adjuvant chemotherapy consisted of two courses of carboplatin/etoposide, alternating with two courses of ifosfamide/etoposide. The courses were given at 21-d intervals; only the second course was delayed due to hematological toxicity. MRI of the brain after four courses of chemotherapy revealed no residual tumor (Figure 2). In the later course the patient was treated with radiotherapy. She received whole ventricular irradiation and tumor bed irradiation in a total dose of 24 Gy (15 fractions, dose per fraction 1.6 Gy).
Due to persistent hypopituitarism, hormonal replacement therapy with recombinant human growth hormone (GH) (somatropin, 1 × 2.25 mg subcutaneously daily), hydrocortisone (10 + 5 + 5 mg per os daily), levothyroxine (1 × 125 mcg per os daily, and estrogens (17 β-oestradiol, 1 × 1 mg per os daily) was conducted. A water deprivation test provided a diagnosis of central diabetes insipidus, requiring therapy with desmopressin (2 × 60 mcg sublingually daily). Progestogens (medroxyprogesterone acetate, 1 × 5 mg per os daily) were added after two years.
The patient was followed-up regularly by an oncologist and endocrinologist. Eight months of treatment with GH led to a height increase of 7 centimeters (from 162 to 169 centimeters). After the end of antitumor treatment, significant weight gain was observed, from BW of 49 kg and BMI of 19.2 to BW 95 kg (94% increase) and BMI of 32.3 (68% increase) over a three-year period. The girl had her first menstrual period at the age of 15. She had marked psychological changes in the form of low self-esteem, depression, anxiety, and maladjustment to her peer group, which required psychological and psychiatric support.
Three and a half years after the end of treatment, the patient is in remission, with regular oncological follow-up (MRI) and hormonal replacement therapy.
Hypopituitarism is defined as partial or complete deficiency of a single or multiple pituitary hormones[12]. The clinical presentation varies depending on the underlying disorder, and the number and severity of specific pituitary hormone deficiencies. The presence of signs and symptoms suggestive of hypopituitarism warrants prompt further investigation, including a thorough clinical history and physical examination, baseline biochemical testing, measurement of hormone levels in basal conditions and after appropriate stimulation, and imaging of the brain with pituitary focus[12,13].
Hypopituitarism is divided into primary (caused by disorders of the pituitary gland), and secondary (caused by disorders of the hypothalamus). It can be congenital (associated with structural pituitary and hypothalamic abnormalities) and acquired. Acquired hypopituitarism can result from any damage to the pituitary gland, including tumor, trauma, infection, autoimmune disease, chemotherapy, and irradiation[13,14]. The beginning is insidious, GH is usually the first to be lacking, followed by gonadotropins, and finally TSH and ACTH. Vasopressin deficiency is rare in primary pituitary disorders, but the frequency is higher in lesions of the pituitary stalk and hypothalamus. Lack of all hormones (panhypopituitarism) leads to hypofunction of all target glands. In children, the lack of GH leads to slow height growth, and the lack of gonadotropins leads to a delay in pubertal development. TSH deficiency causes hypothyroidism, with the typical clinical picture of a puffy face, a hoarse voice, bradycardia, and cold intolerance. ACTH deficiency leads to hypoadrenalism, with fatigue, hypotension, reduced tolerance to stress, and infections[13,15].
Treatment is based on the removal of the primary pathology, along with hormone replacement of hypofunctioning target glands. In the case of pituitary apoplexy and hypothalamic compression with progressive deterioration of con
The predominant cause of primary hypopituitarism is pituitary tumors, including tumors of germ cell origin[14]. Germinomas are rare intracranial tumors, with an estimated frequency between 0.4%-3.4% in Western countries[10]. Most of these tumors develop along the midline, mainly from the pineal gland, and are usually manifested by diabetes insipidus, visual impairment, and failure of the hypothalamic-pituitary axis[2]. Histopathological and immunohistochemical diagnostics are necessary for optimal treatment[5]. Preoperative staging includes craniospinal MRI, CSF cytology, and measurement of biological tumor markers in the serum and CSF. The surgical approach is determined by the MRI findings, and the extent of resection by staging and intraoperative histopathological evaluation of frozen sections[2]. Treatment should be individualized, and usually consist of a combination of surgery, platinum-based chemotherapy, and focal radiotherapy (whole ventricular and tumor bed radiotherapy[2,17]. Germinomas are very responsive to chemotherapy and radiotherapy, with excellent therapeutic outcomes[8]. The greatest challenge is to minimize the adverse effects of irradiation treatment, and to improve the quality of life of patients who develop neurological, neurocognitive, and endocrine impairments[19].
Our patient had a typical presentation with vision loss and headaches related to the underlying disorder. Her hypopituitarism, with reduced levels of thyroxine, cortisol, IGF-1, and gonadotropins, was clinically silent. Emergency MRI of the brain demonstrated the suprasellar tumor, which was diagnosed as a germinoma with immunohistopathology after surgery. Adjuvant chemotherapy and radiotherapy were performed, and complete remission was achieved. Due to post-treatment panhypopituitarism, hormonal replacement therapy with somatropin, hydrocortisone, levothyroxine, estrogens, progestogens and desmopressin was prescribed.
There are several other reports of suprasellar germinomas with hypopituitarism at a younger age. Jevalikar et al[20] reported a 10-year-old boy with central diabetes insipidus and multiple autoimmune disorders, who developed progressive panhypopituitarism. Serial brain MRI showed changes suggestive of germinoma, and the patient was successfully treated with radiotherapy.
Celik et al[21] reported on lasting remission from multimodal treatment in an 18-year male presenting with blurred vision, headache and delayed sexual development. Pal et al[22] reported a 12-year-old girl with a sellar/suprasellar germinoma, masquerading as secondary granulomatous hypophysitis, presenting with panhypopituitarism and central diabetes insipidus. da Nóbrega et al[23] described a 14-year-old boy with a suprasellar germinoma, initially presenting with diabetes insipidus, in whom panhypopituitarism developed after chemotherapy and radiotherapy. Partenope et al[24] examined endocrine manifestations in 55 children with intracranial germ cell tumors at diagnosis and during follow-up. Endocrine disorders were present in 50.9% of patients at diagnosis. The most common was diabetes insipidus (85.7%), followed by central adrenal insufficiency (57.1%), central hypothyroidism (50%), GH deficiency (28.5%), hypogonadotrophic hypogonadism (10.7%), and precocious puberty (10.7%). If not diagnosed previously, endocrinopathies arose 15.15 months (1.3-404.2) after the end of treatment in an additional 16.4% patients[24].
All reported cases emphasize the significance of careful clinical and radiological follow-up in patients with endocrine and neurological manifestations. Integrated multidisciplinary involvement, including pediatric oncology, endocrinology, neurosurgery, pathology, radiation oncology and psychology, can optimize the complex care of children with suprasellar tumors and hypopituitarism.
Suprasellar germinomas are rare malignant brain tumors that may be associated with hypopituitarism at diagnosis and after treatment. Although the prognosis is good, they still present a diagnostic and therapeutic challenge. Our case highlights the importance of timely diagnosis, multimodality treatment with a multidisciplinary approach, and careful lifelong care of affected patients.
We wish to acknowledge Dr. Dragan Javoran of the Department of Radiology at Clinical Hospital Centre Rijeka for high-quality images and image interpretations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Liu TF, China; Yahya FS, Iraq S-Editor: Zhang H L-Editor: A P-Editor: Zhao S
| 1. | Weil BR, Billmire DF. Management of Germ Cell Tumors in Pediatric Patients. Surg Oncol Clin N Am. 2021;30:325-338. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Kremenevski N, Buchfelder M, Hore N. Intracranial Germinomas: Diagnosis, Pathogenesis, Clinical Presentation, and Management. Curr Oncol Rep. 2023;25:765-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Nakamura H, Takami H, Yanagisawa T, Kumabe T, Fujimaki T, Arakawa Y, Karasawa K, Terashima K, Yokoo H, Fukuoka K, Sonoda Y, Sakurada K, Mineharu Y, Soejima T, Fujii M, Shinojima N, Hara J, Yamasaki K, Fujimura J, Yamasaki F, Takahashi M, Suzuki T, Sato I, Nishikawa R, Sugiyama K. The Japan Society for Neuro-Oncology guideline on the diagnosis and treatment of central nervous system germ cell tumors. Neuro Oncol. 2022;24:503-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Sethi RV, Marino R, Niemierko A, Tarbell NJ, Yock TI, MacDonald SM. Delayed diagnosis in children with intracranial germ cell tumors. J Pediatr. 2013;163:1448-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4523] [Cited by in RCA: 6353] [Article Influence: 1588.3] [Reference Citation Analysis (1)] |
| 6. | Zhang Y, Zhong C, Ke X, Liu J, Ye Z, Lu L, Deng K, Zhu H, Yao Y. Advances in genetic abnormalities, epigenetic reprogramming, and immune landscape of intracranial germ cell tumors. Acta Neuropathol Commun. 2023;11:188. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Aridgides P, Janssens GO, Braunstein S, Campbell S, Poppe M, Murphy E, MacDonald S, Ladra M, Alapetite C, Haas-Kogan D. Gliomas, germ cell tumors, and craniopharyngioma. Pediatr Blood Cancer. 2021;68 Suppl 2:e28401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Bartels U, Onar-Thomas A, Patel SK, Shaw D, Fangusaro J, Dhall G, Souweidane M, Bhatia A, Embry L, Trask CL, Murphy ES, MacDonald S, Wu S, Boyett JM, Leary S, Fouladi M, Gajjar A, Khatua S. Phase II trial of response-based radiation therapy for patients with localized germinoma: a Children's Oncology Group study. Neuro Oncol. 2022;24:974-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Calaminus G, Kortmann R, Worch J, Nicholson JC, Alapetite C, Garrè ML, Patte C, Ricardi U, Saran F, Frappaz D. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 10. | Srinivasan N, Pakala A, Mukkamalla C, Oswal A. Pineal germinoma. South Med J. 2010;103:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Xue K, Han G, Wang Z, Zhang X. Primary suprasellar germinoma: A series of 15 cases. J Clin Neurosci. 2023;111:71-77. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Yeliosof O, Gangat M. Diagnosis and management of hypopituitarism. Curr Opin Pediatr. 2019;31:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Hoffman RP. Panhypopituitarism. 2022 Apr 21 [cited 2 January 2024]. Available from: https://emedicine.medscape.com/article/923789. |
| 14. | Kim SY. Diagnosis and Treatment of Hypopituitarism. Endocrinol Metab (Seoul). 2015;30:443-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Stieg MR, Renner U, Stalla GK, Kopczak A. Advances in understanding hypopituitarism. F1000Res. 2017;6:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Mayol Del Valle M, De Jesus O. Pituitary Apoplexy. 2023 Aug 23. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 17. | Mesquita Filho PM, Santos FP, Köhler LR, Manfroi G, De Carli F, Augusto de Araujo M, Schwingel D. Suprasellar Germinomas: 2 Case Reports and Literature Review. World Neurosurg. 2018;117:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Scherz A, Feller K, Berezowska S, Genitsch V, Zweifel M. Successful Treatment of Pituitary Germinoma with Etoposide, Cisplatin, Vincristine, Methotrexate and Bleomycin Chemotherapy Without Radiotherapy. Anticancer Res. 2017;37:3111-3115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Osorio DS, Allen JC. Management of CNS germinoma. CNS Oncol. 2015;4:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Jevalikar G, Wong SC, Zacharin M. Rapidly evolving hypopituitarism in a boy with multiple autoimmune disorders. J Paediatr Child Health. 2013;49:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Celik O, Ozyurt S, Saglican Y. Suprasellar germinoma with hypopituitarism in an 18-year old man: A case report and review of literature. Clin Neurol Neurosurg. 2020;196:106026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Pal R, Rai A, Vaiphei K, Gangadhar P, Gupta P, Mukherjee KK, Singh P, Ray N, Bhansali A, Dutta P. Intracranial Germinoma Masquerading as Secondary Granulomatous Hypophysitis: A Case Report and Review of Literature. Neuroendocrinology. 2020;110:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | da Nóbrega VA, da Nóbrega VA, Soares RD. Childhood-onset panhypopituitarism and differential diagnosis of polyuria. Medicina (Ribeirão Preto). 2021;54:e-178625. |
| 24. | Partenope C, Pozzobon G, Weber G, Arya VB, Carceller F, Albanese A. Endocrine manifestations of paediatric intracranial germ cell tumours: from diagnosis to long-term follow-up. Endocrine. 2022;77:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |