Published online Apr 6, 2024. doi: 10.12998/wjcc.v12.i10.1799
Peer-review started: November 16, 2023
First decision: December 26, 2023
Revised: January 7, 2024
Accepted: March 8, 2024
Article in press: March 8, 2024
Published online: April 6, 2024
Processing time: 137 Days and 22.8 Hours
The precise mechanism by which severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) impacts the central nervous system remains unclear, with manifestations spanning from mild symptoms (e.g., olfactory and gustatory deficits, hallucinations, and headache) to severe complications (e.g., stroke, seizures, encephalitis, and neurally demyelinating lesions). The occurrence of single-pass subdural effusion, as described below, is extremely rare.
A 56-year-old male patient presented with left-sided limb weakness and slurred speech as predominant clinical symptoms. Through comprehensive imaging and diagnostic assessments, he was diagnosed with cerebral infarction complicated by hemorrhagic transformation affecting the right frontal, temporal, and parietal regions. In addition, an intracranial infection with SARS-CoV-2 was identified during the rehabilitation process; consequently, an idiopathic subdural effusion developed. Remarkably, the subdural effusion underwent absorption within 6 d, with no recurrence observed during the 3-month follow-up.
Subdural effusion is a potentially rare intracranial complication associated with SARS-CoV-2 infection.
Core Tip: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may transmit via the retrograde axonal pathway, bloodstream, or direct penetration through the blood-brain barrier, exerting its effects on angiotensin-converting enzyme-2 receptors. This intricate interaction can cause neurological complications, including subdural effusion, which is very rare. Clinical vigilance is advised for cranial imaging in individuals with SARS-CoV-2 infection to enhance diagnostic precision. Considering its unique characteristics, subdural effusion, a seldom reported complication, warrants attention.
- Citation: Xue ZY, Xiao ZL, Cheng M, Xiang T, Wu XL, Ai QL, Wu YL, Yang T. Subdural effusion associated with COVID-19 encephalopathy: A case report. World J Clin Cases 2024; 12(10): 1799-1803
- URL: https://www.wjgnet.com/2307-8960/full/v12/i10/1799.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i10.1799
Subdural effusion or subdural hydrocele commonly stems from craniocerebral trauma, postcranial surgery, or cerebral atrophy. Typically presenting unilaterally or bilaterally in the frontotemporal region, symptoms include headache, dizziness, cognitive alterations, and mood changes. Its slow absorption may progress to chronic subdural hematoma, with treatment options ranging from medications to surgical interventions such as bone flap craniotomy, subdural effusion capsule wall stripping, abdominal shunt, and external drainage[1-4]. This report presents a unique case of subdural effusion that formed after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection but was remarkably resolved within 6 d, showcasing a rare and expeditious resolution.
A 56-year-old male presented with a sudden onset of left-sided limb weakness for over 20 d.
On November 25, 2021, the patient experienced a sudden onset of left-sided limb weakness accompanied by slurred speech. After a comprehensive examination, he was diagnosed with cerebral infarction with hemorrhagic transformation affecting the right frontal, temporal, and parietal lobes. He was then treated for his cranial hemorrhage symptoms. Throughout the hospitalization period, he exhibited fever, dyspnea, and generalized muscular aches and pains. The patient’s temperature fluctuated at 38.0 °C, accompanied by headache, hallucinations, and incoherent speech.
He had primary hypertension for 4 years, with a maximum blood pressure of 188/106 mmHg. The patient was on long-term oral medication with sacubitril/valsartan sodium tablets (100 mg) once a day.
The patient denied any personal or family history of related diseases.
The patient was unresponsive, a shallow left nasolabial groove, slurred speech, difficulty swallowing fluids (leading to choking), slight neck resistance, a positive Kernig’s sign, and muscle strength graded at 0, 2, and 5 in the left-upper, left-lower, and right limbs, respectively, were observed. Reduced muscle tone in the left upper limb, decreased pharyngeal reflexes, and a positive Babinski’s sign on the left side were also observed. The remaining systematic examination did not reveal any positive signs.
The patient’s laboratory examination results were as follows: Platelet count, 462 × 109/L (reference range: 100-300/L); sodium level, 125 mmol/L (reference range: 135-145 mmol/L); positive throat swab test result for coronavirus disease 2019 (COVID-19); C-reactive protein level, 44.18 mg/L (reference range: 0-10 mg/L); and blood gas analysis, 60 mmHg (reference range: 83-108 mmHg). Furthermore, inflammatory factors were elevated, with interleukin (IL)-2, IL-6, IL-10, and tumor necrosis factor alpha (TNF-α) at 878.54, 62.83, 136.15, and 111.14 U/mL (reference range: 160-625, 0-7, 0-9.5, and 0-8.5 U/mL), respectively. Lumbar puncture revealed a cerebrospinal fluid pressure of 150 mmH2O (reference range: 80-180 mmH2O). The cerebrospinal fluid analysis for SARS-CoV-2 showed specific gene Ct values: N gene Ct value of 35.5429 and ORF1ab gene Ct value of 30.3402 (reference range: > 40 for both). Routine biochemistry of cerebrospinal fluid revealed no abnormalities. Cultures and a full set of viral screenings did not detect pathogens such as bacteria, fungi, parasites, Mycobacterium tuberculosis complex, Mycoplasma, or Chlamydia. Moreover, cerebrospinal fluid and serum specimens were negative for autoimmune encephalitis antibodies, paraneoplastic syndrome autoantibody profile semiquantitative test, and ganglioside antibody profile. Coagulation function and procalcitonin levels also showed no abnormalities. Electroencephalogram results showed bilateral symmetrical diffuse slow waves.
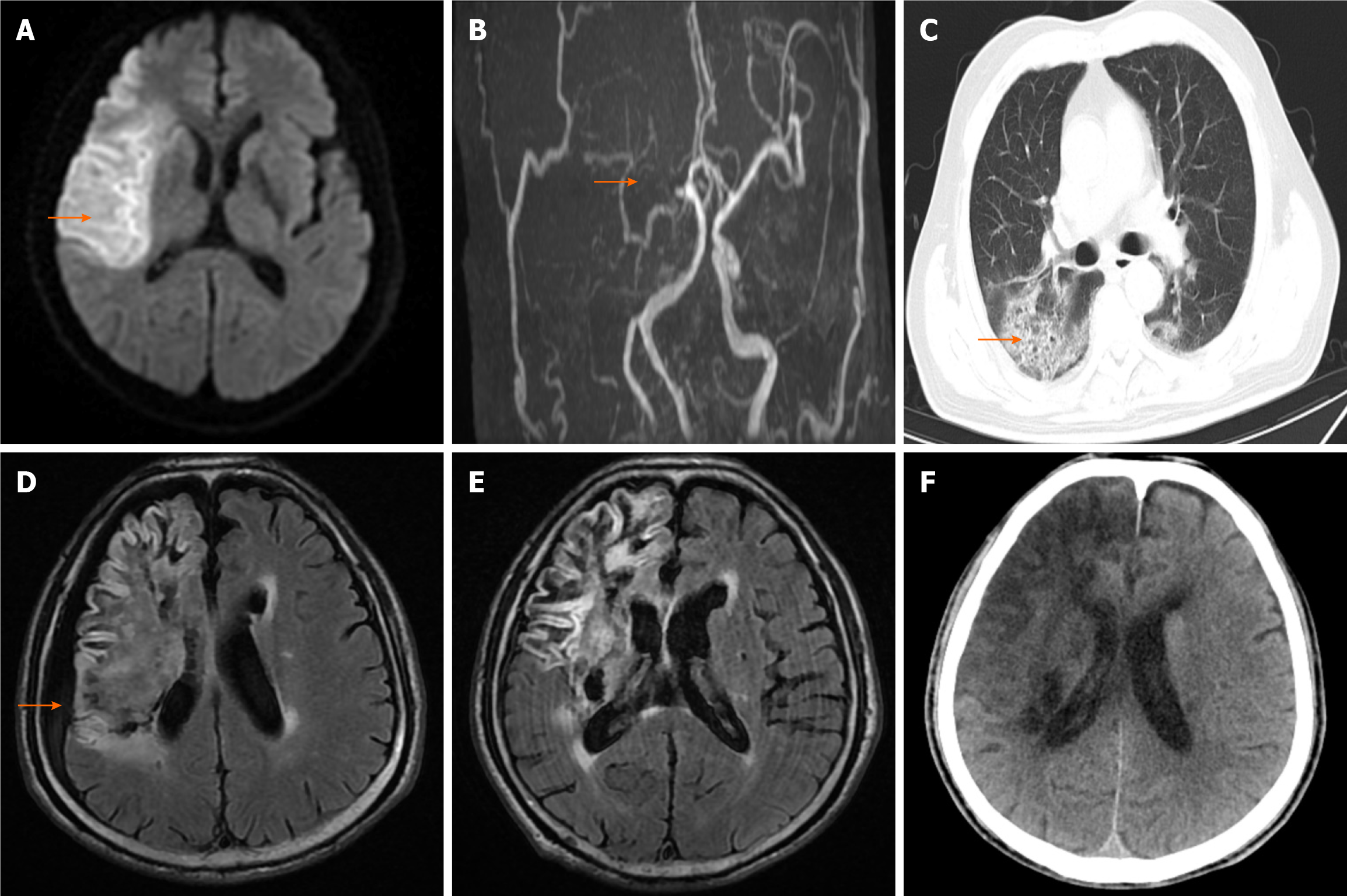
Cranial magnetic resonance imaging (MRI) revealed a typical infarction in the right frontal, temporal, and parietal lobes, along with brain atrophy indications. On magnetic resonance angiography, the right internal carotid artery and middle cerebral artery were largely undetected, and the walls of the left internal carotid artery, left middle cerebral artery M1 segment, and bilateral posterior cerebral arteries exhibited abnormalities (Figure 1A and B). On December 28, 2021, a repeat cranial computed tomography (CT) scan revealed infarction with hemorrhagic transformation in the right frontal, temporal, parietal, and basal ganglia areas, and chest CT showed scattered patchy shadows in the lungs (Figure 1C). On March 15, 2022, cranial MRI revealed new subdural effusion in the right frontal, temporal, and parietal areas (Figure 1D), with subsequent absorption of the subdural effusions on March 21 (Figure 1E).
The patient was diagnosed with the following: (1) Cerebral infarction with hemorrhagic transformation on the right frontal, temporal, and parietal lobes (atherosclerotic large artery type); (2) Cerebral atrophy; (3) Primary hypertension, grade 3 (extremely high-risk group); (4) Pneumonia associated with COVID-19; (5) COVID-19 encephalitis; (6) Subdural effusion; and (7) Hyponatremia.
The patient received the following: Aspirin enteric-coated tablet (100 mg) orally once a day, rosuvastatin calcium tablet (10 mg) orally at night, butylphthalide soft capsule (0.2 g) orally thrice a day, sacubitril/valsartan sodium tablet (100 mg) orally once a day, and early comprehensive rehabilitation training. He was also prescribed nemavirit (300 mg)/ritonavir (100 mg) tablet orally every 12 h for 5 d, methylprednisolone sodium succinate injection (40 mg) intravenously once a day, and acetylglutamide injection (0.6 g) intravenously once a day.
At 3 months after discharge, we noted that the patient’s left-sided limb weakness had improved, and he had clear speech and mild headache that did not disrupt his sleep or daily activities (Figure 1F). The patient’s condition has remained stable without any noteworthy concerns.
Subdural effusions encompass regressive, stable, progressive, and evolving types, with craniocerebral trauma being the predominant cause. The stable and evolving types are more common among older adults, often progressing slowly and occasionally evolving into chronic subdural hematomas necessitating surgical intervention when conservative approaches fail. Conversely, regressive types are more prevalent in young individuals, showcasing optimal healing outcomes, and progressive types, which are found more commonly in pediatrics, exhibit severe symptoms, neurological deficits, and the highest mortality rate[5]. In the case of our elderly male patient, the subdural effusion spontaneously resolved in only 6 d, aligning with the typical characteristics of regressive subdural effusion.
We analyzed the potential causes of subdural effusion. First, SARS-CoV-2 has a neuroinvasive and neurophilic nature, as seen in the associations of subdural effusion with other viruses, such as enterovirus 71, herpes simplex virus type 1, and Epstein-Barr virus[6]. However, currently, only one case of subdural effusion associated with SARS-CoV-2 has been reported[7]. Involvement in the central nervous system (CNS) by the virus primarily occurs through direct invasion, inflammatory response activation, and autoimmune response induction. Excessive immune activation leads to the intracranial infiltration of inflammatory cells and upregulation of proinflammatory factors such as IL-1α, IL-6, and TNF-α, resulting in a “cytokine inflammatory storm”. The virus enters the CNS through the interstitial space between endothelial cells and affects the angiotensin-converting enzyme-2 receptor, causing brain tissue damage and cerebrospinal fluid extravasation[8-12]. Second, blood-brain barrier permeability may be altered. The complex mechanism of cerebral infarction with hemorrhagic transformation involves the release of oxygen-free radicals, inflammatory factors, and cytokines and the degradation of collagen and laminin by brain cell extracellular matrix metalloproteins. This event affects the structural integrity of endothelial cells, basement membranes, and the perivascular pedicle of astrocytes. The patient’s right cerebral hemispheric parenchyma displayed edema, and MRI revealed fragmented protein deposition in the lesion, further implying blood-brain barrier disruption. Third, brain atrophy could be a potential cause. Cranial CT and MRI revealed ventricular enlargement, cerebral pool enlargement, cerebral sulcus and fissure deepening, and brain tissue volume reduction. Brain atrophy coupled with compensatory dilation of the subdural space was considered to be a factor leading to subdural effusion.
In summary, the mechanisms by which SARS-CoV-2 affects the CNS are insufficiently understood. This virus may use various routes of transmission, ranging from retrograde axonal pathways to bloodstream transmission and direct transmission through the blood-brain barrier. Clinical manifestations may range from mild symptoms (e.g., olfactory and taste deficits) to severe ones (e.g., stroke, seizures, encephalitis, and neurally demyelinating lesions)[13-15]. Subdural effusion has been proposed as a potentially rare feature of SARS-CoV-2 infection. Hence, early evaluation of brain ima
Subdural effusion is a potentially rare intracranial complication associated with SARS-CoV-2 infection.
We thank Dr. Ming Cheng for his editing assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Kawahara H, Japan S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Zhao S
| 1. | Yu J, Tang J, Chen M, Ren Q, He J, Tang M, Zhang X, Liu Z, Ding H. Traumatic subdural hygroma and chronic subdural hematoma: A systematic review and meta-analysis. J Clin Neurosci. 2023;107:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Xie D, Xie J, Wan Y, Qi X, Yang S. The Comparison Between Surgical Procedure and Conservative Treatment in the Management of Traumatic Subdural Effusion. Turk Neurosurg. 2016;26:725-731. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Zhou JY, Zhang X, Gao HB, Cao Z, Sun W. Endoscopic-assisted surgery for skull defects with subdural effusion. Wideochir Inne Tech Maloinwazyjne. 2021;16:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Hagan MJ, Volpe JK. Subdural Hygroma: A Rare Complication of a Common Brain Malformation. R I Med J (2013). 2021;104:40-43. [PubMed] |
| 5. | Fan G, Ding J, Wang H, Wang Y, Liu Y, Wang C, Li Z. Risk factors for the development of chronic subdural hematoma in patients with subdural hygroma. Br J Neurosurg. 2021;35:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Lee KY. Enterovirus 71 infection and neurological complications. Korean J Pediatr. 2016;59:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Martio AE, Carregosa ALDS, Karam OR, Padua WL, Mesquita Filho PM. Spontaneous subdural effusion in a hospitalized Covid-19 patient: Case report. Brain Hemorrhages. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 8. | Ladopoulos T, Zand R, Shahjouei S, Chang JJ, Motte J, Charles James J, Katsanos AH, Kerro A, Farahmand G, Vaghefi Far A, Rahimian N, Ebrahimzadeh SA, Abedi V, Papathanasiou M, Labedi A, Schneider R, Lukas C, Tsiodras S, Tsivgoulis G, Krogias C. COVID-19: Neuroimaging Features of a Pandemic. J Neuroimaging. 2021;31:228-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Wu H, Zhu H, Yuan C, Yao C, Luo W, Shen X, Wang J, Shao J, Xiang Y. Clinical and Immune Features of Hospitalized Pediatric Patients With Coronavirus Disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3:e2010895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 10. | Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, Winkler CW, Sun J, Dickey JM, Ylaya K, Ko SH, Platt AP, Burbelo PD, Quezado M, Pittaluga S, Purcell M, Munster VJ, Belinky F, Ramos-Benitez MJ, Boritz EA, Lach IA, Herr DL, Rabin J, Saharia KK, Madathil RJ, Tabatabai A, Soherwardi S, McCurdy MT; NIH COVID-19 Autopsy Consortium, Peterson KE, Cohen JI, de Wit E, Vannella KM, Hewitt SM, Kleiner DE, Chertow DS. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 527] [Article Influence: 175.7] [Reference Citation Analysis (0)] |
| 11. | Payus AO, Jeffree MS, Ohn MH, Tan HJ, Ibrahim A, Chia YK, Raymond AA. Immune-mediated neurological syndrome in SARS-CoV-2 infection: a review of literature on autoimmune encephalitis in COVID-19. Neurol Sci. 2022;43:1533-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Yachou Y, El Idrissi A, Belapasov V, Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41:2657-2669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 13. | Erickson MA, Rhea EM, Knopp RC, Banks WA. Interactions of SARS-CoV-2 with the Blood-Brain Barrier. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 14. | Mahalakshmi AM, Ray B, Tuladhar S, Bhat A, Paneyala S, Patteswari D, Sakharkar MK, Hamdan H, Ojcius DM, Bolla SR, Essa MM, Chidambaram SB, Qoronfleh MW. Does COVID-19 contribute to development of neurological disease? Immun Inflamm Dis. 2021;9:48-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 15. | Iroegbu JD, Ifenatuoha CW, Ijomone OM. Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2. Neurol Sci. 2020;41:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |