Published online Apr 6, 2024. doi: 10.12998/wjcc.v12.i10.1733
Peer-review started: January 5, 2024
First decision: January 30, 2024
Revised: February 25, 2024
Accepted: March 8, 2024
Article in press: March 8, 2024
Published online: April 6, 2024
Processing time: 88 Days and 0.4 Hours
Diabetic patients with cataracts encounter specific difficulties during cataract surgery due to alterations in microcirculation, blood supply, metabolism, and the microenvironment. Traditional phacoemulsification may not fully tackle these issues, especially in instances with substantial preoperative astigmatism. The utilization of femtosecond laser-assisted phacoemulsification, in conjunction with Toric intraocular lens (IOL) implantation, offers a potentially more efficient strategy. This research seeks to evaluate the efficacy and possible complications of this approach in diabetic cataract patients.
To investigate the clinical efficacy and complications of femtosecond laser-assisted phacoemulsification combined with Toric IOL implantation in diabetic cataract patients, comparing it with traditional phacoemulsification methods.
This retrospective study enrolled 120 patients with diabetes cataract from May 2019 to May 2021. The patients were divided into two groups: the control group underwent traditional phacoemulsification and Toric IOL implantation, while the treatment group received Len Sx femtosecond laser-assisted treatment. Outcome measures included naked eye vision, astigmatism, high-level ocular phase di
There were no significant preoperative differences in astigmatism or naked eyesight between the two groups. However, postoperative improvements were observed in both groups, with the treatment group showing greater enhan
This study suggests that femtosecond laser-assisted phacoemulsification combined with Toric IOL implantation appears to be more effective in enhancing postoperative vision in diabetic cataract patients compared to traditional methods offering valuable insights for clinical practice.
Core Tip: Retrospective studies have shown that femtosecond laser-assisted phacoemulsification produces favorable clinical results in the management of cataracts related to diabetes. This method significantly improves vision after surgery and provides important information for the clinical treatment of cataracts.
- Citation: Tang YF, Duan ZH. Clinical efficacy of femtosecond laser-assisted phacoemulsification in diabetic cataract patients. World J Clin Cases 2024; 12(10): 1733-1741
- URL: https://www.wjgnet.com/2307-8960/full/v12/i10/1733.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i10.1733
The microcirculation, blood supply, metabolism and microenvironment in diabetic patients differ from those in normal individuals, resulting in abnormal blood supply and terminal circulation. This leads to lens epithelial cell disorder, lens fiber cell opacity, and cataract development[1,2]. Cataract, affecting the eye lens, can be influenced by factors such as trauma, aging, immune dysfunction, poisoning, metabolic abnormalities, nutritional disorders, radiation, and genetics[3]. It is characterized by the opacity due to lens protein degeneration and metabolic dysfunction. Age-related cataract specifically refers to cataracts that develop as individuals age, with progressive vision impairment as the main clinical manifestation[4].
Cataract surgery has now advanced into the realm of refractive surgery. In many countries, approximately 23.0% to 47.0% of cataract patients have preoperative astigmatism greater than 1.0 diopter (D)[5]. In China, this percentage is 25.4% for cataract patients with preoperative astigmatism greater than 1.5D. When astigmatism exceeds 0.75D, it can lead to symptoms such as blurred vision, double images, halos, glare, and others[6]. Currently, the Toric intraocular lens (IOL) is being used to correct cataract and corneal astigmatism. However, he design of this lens, which accounts for as
In this study, we selected 120 patients with diabetic cataract who underwent surgery at our hospital from May 2019 to May 2021 as the retrospective research subjects. All of the patients had monocular cataracts. They were divided into a control group and a treatment group of 60 patients each using the random residue grouping method. Prior to the start of the study, the patients and their families were informed about the study and signed an informed consent form, adhering to principles of voluntariness, confidentiality, benefit, and harmlessness. The study also received approval from the medical ethics committee of our hospital. The general data, such as gender and age, of the two patient groups did not have any impact on the test results, as shown in Table 1.
| Group | Control group (n = 60) | Therapy group (n = 60) | χ2/t/z | P value |
| Gender (men and women) | 28/32 | 27/33 | 0.034 | 0.855 |
| Average age (yr) | 79.78 ± 4.32 | 80.62 ± 2.66 | -1.171 | 0.245 |
| Eye axis (mm) | 23.2 (20.1-26.7) | 22.9 (21.2-25.6) | 0.019 | 0.992 |
| Intraocular pressure (mmHg) | 14.78 ± 3.32 | 13.62 ± 3.66 | 1.660 | 0.100 |
The inclusion criteria for this study were as follows: (1) All patients met the “diabetic macular edema management in Asian population: Expert panel consensus guidelines” for the diagnosis of diabetic cataract, and had a clear history of type 2 diabetes, combined with cataracts, and required surgical treatment[9]; (2) The study included patients with progressive decline in vision, blurred vision, or even light perception, but no symptoms such as eye pain, photophobia, and tearing. The axial length of the eye ranged from 24 mm to 30 mm; and (3) The affected eye had a dilated pupil with a diameter of at least 6mm, and regular corneal astigmatism of 0.75D and above. The exclusion criteria were as follows: (1) Patients with corneal disease, nuclear hardness above grade 4, glaucoma, eyelid adhesion, small eyelid fissure, nystagmus, lens dislocation, those who had undergone corneal or internal eye surgery, and those who could not coo
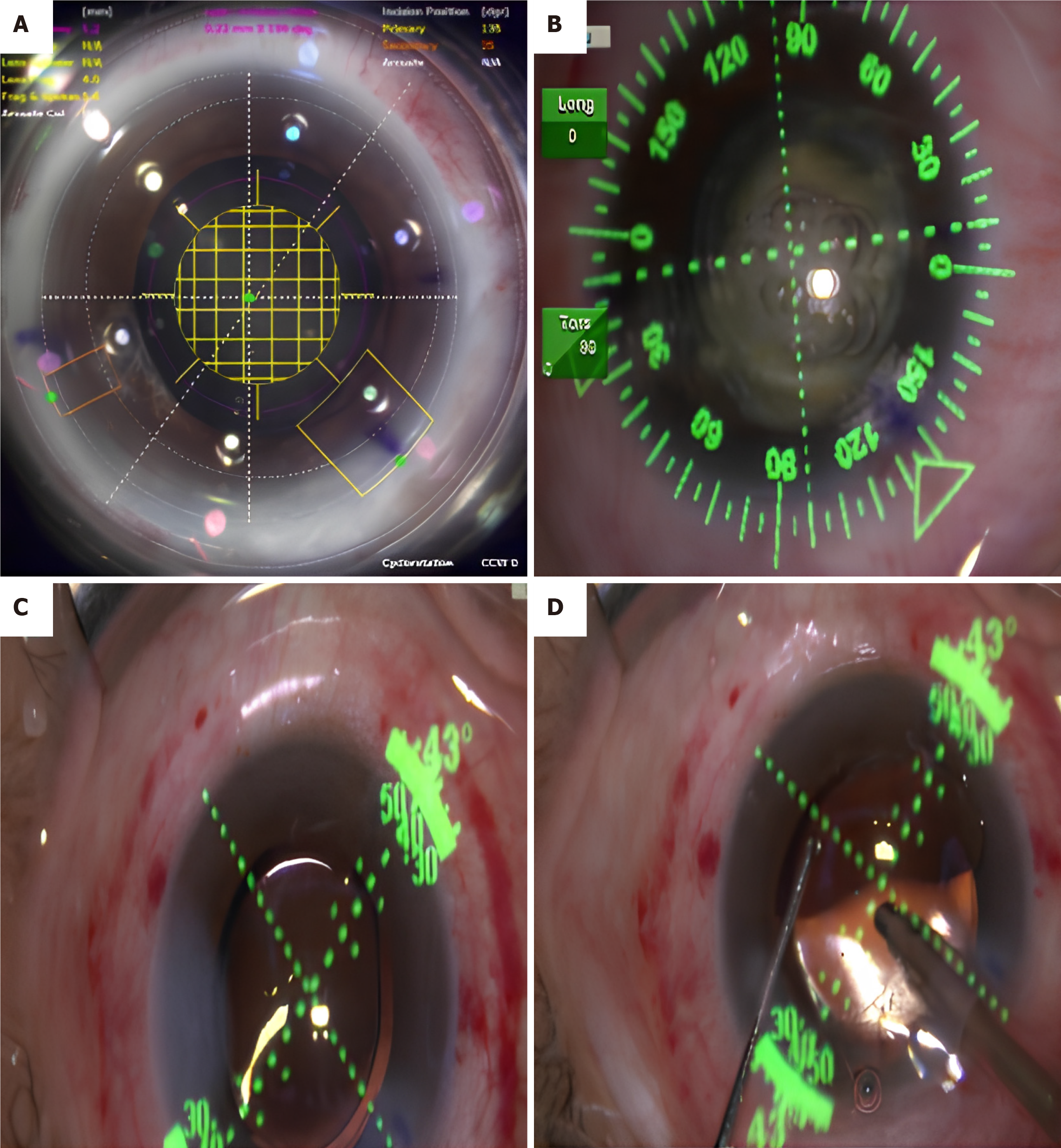
The treatment group utilized the Len-Sx femtosecond laser for adjuvant treatment. During the procedure, the patient is seated and the corneal parameters are positioned at 0 and 180° under the slit lamp. The axial direction for Toric IOL implantation is determined by the corneal locator mark and the axial direction obtained from the toric calculator (www.acrysoft-calculator.com). The femtosecond group of patients undergoes several steps with the femtosecond laser system (United States Alcon's LenSx femtosecond laser system), including capsulorhexis, nucleus splitting and incision making. The size of the anterior capsular opening is set to 5.2 mm. The main incision position is consistent with the position set in the preoperative Toric calculation program, with a width of 2.4 mm. Phacoemulsification is performed using the American Alcon Centurion cataract intelligent phacoemulsification instrument. Toric IOL is then implanted, and the viscoelastic agent is removed after the lens is completely extracted. The IOL axis is adjusted to the predetermined angle of the mark, and the center of the IOL is gently pressed to ensure attachment to the capsular bag and water tightness (Figure 1).
The control group underwent phacoemulsification combined with Toric IOL implantation. Conventional disinfection drapes were used, and a 5% povidone-iodine stock solution was used to rinse the second conjunctival sac after the upper eyelid opener. The main side of the cornea incision, capsulorhexis, and water separation along the mark were performed. The Centurion phacoemulsification instrument was used, and the phacoemulsification nucleus chopping method was employed for nuclear processing and lens nucleus, aspiration. Cortex aspiration was done using IA. Toric IOL was implanted, and the back of the IOL was first aspirated with Viscoelastic agent. The axis of the IOL was adjusted to align with the marking line, and imbibition cortex was applied to the IOL. The viscoelastic agent in front of the IOL was then sucked out, and the mouth was closed with appropriate water. The third conjunctival sac was flushed with a 5% povidone-iodine stock solution, and tobramycin and dexamethasone ophthalmic ointment was applied to the con
Logarithm of the minimum angle of resolution visual acuity was assessed using various tools including uncorrected visual acuity, intraocular pressure, IOL-Master, and Od-sCAIII visual quality analyzer. Among these, OPD-SCAN III was used to detect 5 indicators related to the corneal area: the sum of all the advanced phase differences of 0-8 orders (TO-TAL), IOL tilt prism (tilt), the sum of all the advanced phase differences of 3-8 orders (high), high-order coma (S3 + S5 + S7), and high-order spherical aberration (S4 + S6 + S8). The online calculation program (www.acrysoftoriccalculator.com) can be used to calculate the prism power and positioning axis of Toric IOLs. The surgical induced astigmatism entered into the computer program is calculated based on the postoperative data collected from the surgeon before the operation, with an input of 0.3D. Follow-up with the patients will be conducted for 6 months after the surgery.
All statistical data in this study were entered into the excel software by the first author and the corresponding author, respectively. The included data was tested using the Shapiro Wilk method to determine if it conformed to a normal distribution. Measurement data that conformed to the normal distribution were described using the mean ± SD. Between-groups comparisons were performed using independent sample or paired sample t-tests. Count data were described using integers or percentages (%), and χ2 tests were used for comparisons between or within groups. Data that did not conform to the normal distribution were described using M (QR) and analyzed using the Mann-Whitney test with a significance level of α = 0.05.
In the 60 therapy group, the mean age was 80.62 ± 2.66 years with 81.82% of the participants being male. The eye axis (mm) was measured at 22.9 (21.2 to 25.6) and the intraocular pressure (mmHg) was 13.62 ± 3.66. In the control group of 60 participants, the mean age was 79.78 ± 4.32 years with 87.5% of the participants being male. The eye axis (mm) was measured at 23.2 (20.1-26.7) and the intraocular pressure (mmHg) was 14.78 ± 3.32. There were no significant differences in gender, age, eye axis, and intraocular pressure between the two groups (P > 0.05) (Table 1).
Before surgery, the control group had a measured naked eye visual acuity of 1.15 ± 0.10, while the treatment group had a visual acuity of 1.16 ± 0.12. There was no statistical significance between the two groups (P > 0.05). However, one day after surgery, the naked eye visual acuity of the control group decreased to 0.28 ± 0.02, whereas the treatment group had a visual acuity of 0.46 ± 0.04. The difference between the two groups was significant (P < 0.05). After one month of treatment, the naked eye visual acuity of the control group improved to 0.35 ± 0.02, and the treatment group had a visual acuity of 0.61 ± 0.11. Once again, the difference between the two groups was significant (P < 0.05). At the six-month mark after surgery, the naked eye visual acuity of the control group was 0.48 ± 0.12, while the treatment group had a visual acuity of 0.65 ± 0.10. Once more, the difference between the two groups was significant (P < 0.05). These results indicate a gradual improvement in naked eye visual acuity for both groups after surgery (Table 2).
| Group | Control group (60) | Therapy group (60) | t | P value |
| Before surgery | 1.15 ± 0.10 | 1.16 ± 0.12 | -0.453 | 0.652 |
| 1 d after surgery | 0.28 ± 0.02 | 0.46 ± 0.04 | -28.460 | 0.000 |
| 1 month after surgery | 0.35 ± 0.02 | 0.61 ± 0.11 | -16.444 | 0.000 |
| 6 months after surge | 0.48 ± 0.12 | 0.65 ± 0.10 | -7.696 | 0.000 |
Before surgery, the naked eye astigmatism of the two groups was measured. In the control group, it was 2.62 ± 0.36, while in the treatment group, it was 2.56 ± 0.54. There was no statistically significant difference between the two groups (P > 0.05). One day after surgery, the naked eye astigmatism was 0.28 ± 0.02 in the control group and 2.40 ± 0.32 in the treatment group. The difference between the groups was not statistically significant (P > 0.05). After one month of treatment, the naked eye astigmatism measured 2.43 ± 0.63 in the control group and 2.33 ± 0.64 in the treatment group, with no statistically difference (P > 0.05). Half a year after surgery, the naked eye astigmatism was measured. It was 2.14 ± 0.23 in the control group and 1.18 ± 0.24 in the treatment group. The difference between the groups was found to be significant (P < 0.05). These results indicate an improvement in naked eye astigmatism in both groups six months after the operation (Table 3).
| Group | Before surgery | Therapy group (60) | t | P value |
| Before surgery | 2.62 ± 0.36 | 2.64 ± 0.30 | 0.302 | 0.763 |
| 1 d after surgery | 2.56 ± 0.54 | 2.40 ± 0.32 | 1.802 | 0.075 |
| 1 month after surgery | 2.43 ± 0.63 | 2.33 ± 0.64 | 0.787 | 0.433 |
| 6 months after surgery | 2.14 ± 0.23 | 1.18 ± 0.24 | 20.421 | 0.000 |
The initial total value of naked eyes in the control group and was 2.52 ± 0.63 and in the treatment group was 2.08 ± 0.32. There was a significant difference observed between the two groups (P < 0.05). The tilt values of naked eyes were 0.62 ± 0.16 in the control group and 0.94 ± 0.10 in the treatment group, showing a significant difference (P < 0.05). Similarly, the naked eye high values were 0.46 ± 0.10 in the control group and 0.60 ± 0.12 in the treatment group, with a significant difference (P < 0.05). The combined values of S3 + S5 + S7 in the naked eye were 0.31 ± 0.06 in the control group and 0.53 ± 0.08 in the treatment group, with a significant difference (P < 0.05). Similarly, the combined values of S4 + S6 + S8 in the naked eye were 0.24 ± 0.03 in the control group and 0.35 ± 0.11 in the treatment group, with a significant difference (P < 0.05) (Table 4).
| Group | Control group (60) | Therapy group (60) | t | P value |
| TOTAL (μm) | 2.52 ± 0.63 | 2.08 ± 0.32 | 4.403 | 0.000 |
| Tilt (μm) | 0.62 ± 0.16 | 0.94 ± 0.10 | -11.993 | 0.000 |
| High (μm) | 0.46 ± 0.10 | 0.60 ± 0.12 | -6.338 | 0.000 |
| S3 + S5 + S7 (μm) | 0.31 ± 0.06 | 0.53 ± 0.08 | -15.556 | 0.000 |
| S4 + S6 + S8 (μm) | 0.24 ± 0.03 | 0.35 ± 0.11 | -6.822 | 0.000 |
The corneal epithelium and endothelium of diabetic patients are easily damaged and have a slow recovery rate. Additionally, due to abnormal metabolism of microvascular and peripheral nerves, diabetic cataract surgery is more prone to complications such as miosis, iris hemorrhage, and corneal incision edema[10,11]. Diabetic cataract patients are also more sensitive to surgical stimulation. IOL implantation, which is a common method for treating cataracts with corneal astigmatism, has been widely used in refractive cataract surgery. Diabetes, being a metabolic disease that affects the entire body, impairs microvascular function and damages body tissues[12]. Traditional phacoemulsification , with its excessive release of ultrasound energy, can be particularly irritating to the eyes of diabetic patients[13]. However, with the continuous development of society and technology, femtosecond laser technology has gained popularity in eye surgery, especially in cataract surgery[14]. The femtosecond laser, operating in a pulsed form using near-infrared light, offers precise targeting and positioning accuracy, making it more accurate than conventional surgery. This technology is now being used in clinical practice[6]. During femtosecond laser surgery, a single pulse of light creates micro-plasma bubbles, which contain CO2 and H2O, These bubbles gradually approach and fuse with each other, resulting in a gradual decrease in tissue adhesion between the bubbles[15].
To achieve the purpose of precise tissue separation, femtosecond laser-assisted surgery can be employed. This technique reduces intraoperative ultrasound energy, lowers the risk of postoperative corneal edema, and promotes early recovery of corneal transparency and refractive stability[16]. However, miosis, which is the constriction of the pupil, is a common complication of femtosecond laser anterior lens capsulotomy. To prevent this, preoperative administration non-steroidal anti-inflammatory drugs can be used to reduce the energy and the number of negative pressure suctions during laser operations[17].
Our study demonstrated a significant improvement in both astigmatism of the ametropia and naked eye vision after six months of surgery. The mean astigmatism was 1.18 ± 0.24. Similar results were reported by Rückl et al[18], who observed a significant reduction in dioptometric astigmatism after femtosecond laser-assisted arcuate keratotomy (FSAK) procedure involves a pair of curved incisions with an arc diameter of 7.5 mm within the corneal stroma. Another study by Day et al[19] investigated intra-corneal FSAK in 196 eyes and found a 39% decrease in corneal astigmatism from 1.21D before surgery to 0.74D after surgery. Furthermore, our study revealed a gradual and statistically significant improvement in astigmatism and naked eye vision six months after operation. The comparison of various corneal phase difference tests between the two groups of patients, including TOTAL, tilt, high, S3 + S5 + S7, and S4 + S6 + S8, also showed significant differences and statistical significance. The comparative study of diabetic cataract shows that femtosecond laser-assisted phacoemulsification has a better clinical effect, effectively improving postoperative vision in patients. Toric IOL can also be used in patients with traumatic cataract and binocular cataract. There is no statistical difference between photopic, photopic glare, scotopic vision, and scotopic glare when compared to ordinary IOLes. The use of toric IOL does not increase discomfort in cataract patients and does not affect proper vision. The results of this study demonstrate that the toric IOL is relatively stable, with only a slight position shift. The shift angle of more than 90% of patients is less than 10°, which has minimal impact on postoperative vision. The corneal phase difference test reveals significant differences in TOTAL, tilt, high, S3 + S5 + S7, S4 + S6 + S8, and other high-order corneal phase difference tests between the two patient groups. This may be attributed to the negative pressure generated during femtosecond laser operation, such as the making of suction ring or incision, which increases corneal high-order coma. However, further confirmation of this finding requires larger clinical datasets[20]. After cataract surgery, the intraocular high-order phase difference of the IOL in the affected eye is mostly produced by the IOL in addition to the influence of the retina of the affected eye. The displacement and deflection of the lens will cause the phase difference to change[15].
Macular cystoid edema (CME) is a frequently observed condition following femtosecond laser-assisted surgery, particularly in patients with diabetes[21]. This condition is known to have a negative impact on vision, especially during the 7-60 d follow-up period[22,23]. Even minimal and non-central preoperative CME in patients with diabetes can worsen after cataract surgery[24]. Therefore, based on the current findings, it can be concluded that femtosecond laser-assisted cataract surgery may lead to a significant decline in short-term vision when compared to conventional phacoemulsification surgery.
Although this study is somewhat innovative, it also has its limitations. The clinical impact of Len Sx femtosecond laser as an adjuvant treatment for diabetic cataract is notably significant, but its specific mechanism has not been thoroughly investigated. The cases collected for this study were solely from one hospital, which may not adequately represent the general population. The criteria for participant selection were subjective, potentially leading to biased outcomes. In conclusion, the comparative study on diabetic cataracts using femtosecond laser-assisted phacoemulsification de
Diabetic cataract is a common complication among diabetic patients, characterized by altered ocular physiology. Traditional cataract surgery methods have limitations in addressing the unique challenges posed by the diabetic eye. The integration of femtosecond laser-assisted phacoemulsification with Toric intraocular lens (IOL) implantation presents a novel approach in the treatment of diabetic cataracts.
This study was motivated by the need to improve surgical outcomes in diabetic cataract patients. The specific focus was on evaluating whether the advanced technique of femtosecond laser-assisted phacoemulsification combined with Toric IOL implantation could offer better results compared to traditional methods, particularly in terms of postoperative vision and complication rates.
The primary objective was to assess the clinical efficacy and potential complications of femtosecond laser-assisted phacoemulsification combined with Toric IOL implantation in diabetic cataract patients. The study aimed to compare this method with traditional phacoemulsification techniques.
A retrospective study design was employed, involving 120 diabetic cataract patients from May 2019 to May 2021. They were randomly divided into a control group (traditional phacoemulsification with Toric IOL) and a treatment group (Len Sx femtosecond laser-assisted surgery). Key metrics for evaluation included naked eye vision, astigmatism levels, high-level ocular phase difference detection, clinical efficacy, and analysis of complications.
The study found no significant preoperative differences between the two groups in terms of astigmatism and naked eye vision. However, postoperatively, the treatment group showed more significant improvements in both naked eye vision and astigmatism at the six-month follow-up. High-level corneal phase difference tests also indicated better outcomes for the treatment group.
Femtosecond laser-assisted phacoemulsification combined with Toric IOL implantation is more effective in improving postoperative visual outcomes in diabetic cataract patients than traditional phacoemulsification. This method could represent a significant advancement in the surgical treatment of diabetic cataracts.
This study opens up new perspectives for the treatment of diabetic cataracts. Future research should focus on further refining femtosecond laser-assisted techniques, exploring long-term outcomes, and broadening the scope to include diverse patient populations. Additionally, further studies could delve into the underlying mechanisms of improved outcomes with this method.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arnold SV, United States S-Editor: Gong ZM L-Editor: A P-Editor: Zhao S
| 1. | Kelkar A, Kelkar J, Mehta H, Amoaku W. Cataract surgery in diabetes mellitus: A systematic review. Indian J Ophthalmol. 2018;66:1401-1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Panozzo G, Staurenghi G, Dalla Mura G, Giannarelli D, Alessio G, Alongi S, Appolloni R, Baldascino A, Boscia F, Caporossi A, Cereda M, D'Ugo E, Fallico M, Avitabile T, Galan A, La Spina C, Lo Giudice G, Mastropasqua L, Palmisano C, Panico C, Parravano MC, Penna R, Pintore P, Vaiano A, Reibaldi M, Rizzo S, Rossi T, Varano M, Virgili G. Prevalence of diabetes and diabetic macular edema in patients undergoing senile cataract surgery in Italy: The DIabetes and CATaract study. Eur J Ophthalmol. 2020;30:315-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Feng Y, Zhu S, Skiadaresi E, McAlinden C, Tu R, Gao R, Stephens JW, Wang Q, Huang J. Phacoemulsification cataract surgery with prophylactic intravitreal bevacizumab for patients with coexisting diabetic retinopathy: A Meta-Analysis. Retina. 2019;39:1720-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Kaliaperumal R, Venkatachalam R, Nagarajan P, Sabapathy SK. Association of Serum Magnesium with Oxidative Stress in the Pathogenesis of Diabetic Cataract. Biol Trace Elem Res. 2021;199:2869-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Žiak P, Halička J, Mojžiš P, Kapitánová K, Michal J, Piñero DP. Presbyopic lens exchange (PRELEX) cataract surgery outcomes with implantation of a rotationally asymmetric refractive multifocal intraocular lens: femtosecond laser-assisted versus manual phacoemulsification. Int Ophthalmol. 2019;39:2875-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kaur M, Titiyal JS, Shaikh F, Rani D. Femtosecond laser-assisted refractive capsulorhexis - Precise capsulotomy with accurate toric intraocular lens alignment. Indian J Ophthalmol. 2020;68:2562-2564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Yoo A, Yun S, Kim JY, Kim MJ, Tchah H. Femtosecond Laser-assisted Arcuate Keratotomy Versus Toric IOL Implantation for Correcting Astigmatism. J Refract Surg. 2015;31:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Espaillat A, Pérez O, Potvin R. Clinical outcomes using standard phacoemulsification and femtosecond laser-assisted surgery with toric intraocular lenses. Clin Ophthalmol. 2016;10:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Chhablani J, Wong K, Tan GS, Sudhalkar A, Laude A, Cheung CMG, Zhao P, Uy H, Lim J, Valero S, Ngah NF, Koh A. Diabetic Macular Edema Management in Asian Population: Expert Panel Consensus Guidelines. Asia Pac J Ophthalmol (Phila). 2020;9:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Higashi Y, Higashi K, Mori A, Sakamoto K, Ishii K, Nakahara T. Anti-cataract Effect of Resveratrol in High-Glucose-Treated Streptozotocin-Induced Diabetic Rats. Biol Pharm Bull. 2018;41:1586-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Rossi T, Panozzo G, Della Mura G, Giannarelli D, Ferrari D, Alessio G, Palmisano C, Telani S, Ripandelli G. Diabetes and diabetic retinopathy in patients undergoing cataract surgery: a prevalence study-DiCat study report #2. Acta Diabetol. 2020;57:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Yao Q, Zhou Y, Yang Y, Cai L, Xu L, Han X, Guo Y, Li PA. Activation of Sirtuin1 by lyceum barbarum polysaccharides in protection against diabetic cataract. J Ethnopharmacol. 2020;261:113165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Diakonis VF, Kounis GA, Yesilirmak N, Warren D, Tsaousis KT, Davis Z, Yoo SH, Donaldson KE. Outcomes of toric intraocular lens implantation after femtosecond laser and traditional cataract surgery. Clin Exp Optom. 2021;104:69-73. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Lai KR, Zhang XB, Yu YH, Yao K. Comparative clinical outcomes of Tecnis toric IOL implantation in femtosecond laser-assisted cataract surgery and conventional phacoemulsification surgery. Int J Ophthalmol. 2020;13:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Oakley CL, Ewe SY, Allen PL, Vote BJ. Visual outcomes with femtosecond laser-assisted cataract surgery vs conventional cataract surgery in toric IOL insertion. Clin Exp Ophthalmol. 2016;44:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Scorsetti D, Viteri E, Mayorga E. Iberoamerican Ophthalmologists IOL Selection for Use on Themselves: Survey Results. Clin Ophthalmol. 2021;15:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Steinwender G, Shajari M, Kohnen T. Refractive Outcomes After Femtosecond Laser-Assisted Cataract Surgery in Eyes With Anterior Chamber Phakic Intraocular Lenses. J Refract Surg. 2018;34:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Rückl T, Dexl AK, Bachernegg A, Reischl V, Riha W, Ruckhofer J, Binder PS, Grabner G. Femtosecond laser-assisted intrastromal arcuate keratotomy to reduce corneal astigmatism. J Cataract Refract Surg. 2013;39:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Day AC, Lau NM, Stevens JD. Nonpenetrating femtosecond laser intrastromal astigmatic keratotomy in eyes having cataract surgery. J Cataract Refract Surg. 2016;42:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Kim JW, Eom Y, Yoon EG, Choi Y, Song JS, Jeong JW, Park SK, Kim HM. Comparison of Nd:YAG Laser Capsulotomy Rates Between Refractive Segmented Multifocal and Multifocal Toric Intraocular Lenses. Am J Ophthalmol. 2021;222:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology. 2007;114:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Daien V, Papinaud L, Domerg C, Lacombe S, Daures JP, Villain M. Incidence and Characteristics of Cystoid Macular Edema after Cataract Surgery. Ophthalmology. 2016;123:663-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Guo S, Patel S, Baumrind B, Johnson K, Levinsohn D, Marcus E, Tannen B, Roy M, Bhagat N, Zarbin M. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2015;60:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Diabetic Retinopathy Clinical Research Network Authors/Writing Committee; Baker CW, Almukhtar T, Bressler NM, Glassman AR, Grover S, Kim SJ, Murtha TJ, Rauser ME, Stockdale C. Macular edema after cataract surgery in eyes without preoperative central-involved diabetic macular edema. JAMA Ophthalmol. 2013;131:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |