Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.95
Peer-review started: October 13, 2023
First decision: November 28, 2023
Revised: December 12, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: January 6, 2024
Processing time: 81 Days and 1.9 Hours
Endoscopic submucosal dissection (ESD) and transanal endoscopic submucosal dissection (TES) are widely employed surgical techniques. However, the comparative efficacy and safety of both remain inconclusive.
To comprehensively analyze and discern differences in surgical outcomes between ESD and TES.
We conducted a systematic search of the electronic databases PubMed, Embase, Cochrane Central Register of Controlled Trials, Scopus, and CINAHL from inception till August 2023. We analyzed outcomes including recurrence rate, en bloc resection, R0 resection rate, perforation rate, procedure length, and hospital stay length applying a random-effects inverse-variance model. We assessed publication bias by conducting an Egger’s regression test and sensitivity analyses.
We pooled data from 11 studies involving 1013 participants. We found similar recurrence rates, with a pooled odds ratio of 0.545 (95%CI: 0.176-1.687). En bloc resection, R0 resection, and perforation rate values were also similar for both ESD and TES. The pooled analysis for procedure length indicated a mean difference of -4.19 min (95%CI: -22.73 to 14.35), and the hospital stay was on average shorter for ESDs by about 0.789 days (95%CI: -1.671 to 0.093).
Both ESD and TES displayed similar efficacy and safety profiles across multiple outcomes. Our findings show that individualized patient and surgeon preferences, alongside specific clinical contexts, can be considered when selecting between these two techniques.
Core Tip: This meta-analysis compares Endoscopic Submucosal Dissection (ESD) and Transanal Endoscopic Surgery (TES) for rectal tumours, focusing on recurrence rates, resection efficacies, and procedural outcomes. Our findings reveal no significant differences in recurrence or resection rates between ESD and TES, highlighting their comparable efficacies. However, the methods differ in anaesthesia requirements, impacting patient experience and recovery. With substantial heterogeneity in study designs and patient populations, our analysis underscores the need for standardized multicentric studies. This comprehensive comparison provides crucial insights for clinicians in selecting the most appropriate surgical technique based on tumour characteristics, patient profiles, and institutional resources.
- Citation: Huang LW, Zhong Y. Endoscopic submucosal dissection vs transanal endoscopic surgery for rectal tumors: A systematic review and meta-analysis. World J Clin Cases 2024; 12(1): 95-106
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/95.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.95
The realm of colorectal surgery has witnessed transformative advancements over the past few decades, especially in the domain of minimally invasive procedures[1]. These innovations reflect the fusion of technology with the surgical craft and mark the relentless pursuit to achieve optimal outcomes with minimal surgical intrusion[2]. In this context, the most suitable approach to resect rectal tumors needs to be identified. Two leading techniques have emerged at the forefront: Endoscopic submucosal dissection (ESD) and transanal endoscopic surgery (TES)[3]. However, their relative merits, applications, and outcomes demand a meticulous evaluation. With this systematic review and meta-analysis we compare the two techniques, shedding light on their efficacies and potential roles in modern colorectal surgical practice.
Rectal tumors, whether benign or malignant, pose considerable management challenges due to their anatomical location and the complications and morbidity associated with their surgical resection[4]. Traditional surgical techniques, while effective, often require extensive tissue dissection, resulting in prolonged recovery, potential for morbidity, and significant bowel function alterations[5]. By contrast, minimally invasive techniques offer patients a combination of effective tumor resection and quality of life preservation[6].
The ESD approach originated in East Asia and has become a promising technique for the removal of early neoplasms of the gastrointestinal tract[7]. By allowing en bloc resection of lesions irrespective of their size, ESDs aim to achieve clear histological margins to enhance the accuracy of histopathological assessments and reduce recurrence rates[8]. Moreover, the endoluminal procedure significantly reduces the potential for abdominal or pelvic surgical complications[9]. How
The TES, evolved from the earlier transanal endoscopic microsurgery, presents a more familiar technique for colorectal surgeons[11]. By offering a direct, magnified view of the rectal lumen and leveraging well-understood principles of surgical dissection, TES seems to combine the advantages of endoscopic and open surgical techniques. The potential for achieving R0 resection, coupled with the promise of reduced post-operative morbidity, positions TES as a compelling alternative to traditional and other endoscopic techniques[12]. However, like all surgical techniques, TES is not devoid of challenges. The technical demands of the procedure, potential complications, and the need for specialized equipment have been cited as limiting factors[11]. Moreover, while TES has demonstrated promise in several studies, its comparative efficacy vis-à-vis ESD, especially in terms of oncological outcomes, remains a point of contention[3].
Thus, comparing the ESD and TES techniques for rectal tumor resections is important. With this systematic review and meta-analysis, we seek to synthesize the collective wisdom of the global surgical community, to derive evidence-based conclusions that may potentially guide clinical decision-making. By analyzing outcomes, complications, learning curves, and patient-centric parameters, we hope to present a comprehensive analysis that stands up to the rigorous standards of evidence-based medicine.
As the realm of colorectal surgery continues to evolve, driven by technology and a deeper understanding of disease processes, the surgical community needs to continually evaluate, adapt, and optimize techniques. It is with this goal of continual refinement and progress that we performed this review on two leading techniques for rectal tumor resection.
We adhered stringently to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines[13] to conduct this systematic review and meta-analysis. The protocol was registered at PROSPERO, CRD42023463730.
We compared outcomes of patients with rectal tumors after either ESD or TES.
Population: Our review encompassed studies with participants diagnosed as presenting rectal tumors who had undergone either ESD or TES for management. We imposed no limitations on age, gender, ethnicity, or geographic location.
Intervention group: The population in this group comprised patients with rectal tumors who underwent ESD.
Comparison group: The population in this group comprised patients with rectal tumors who underwent TES.
Outcomes: We analyzed outcomes such as local recurrence, en bloc resection rate, R0 resection rate, procedure length, hospital stay length, or complication rates.
Study design: We included randomized controlled trials (RCTs), observational studies, and cohort studies published in English between the inception of searchable databases and August 2023. To mitigate publication bias, we considered both published articles and grey literature.
We conducted a systematic search on the PubMed, Embase, Cochrane Central Register of Controlled Trials, Scopus, and CINAHL databases. Our manual search included the reference lists of relevant studies and reviews. We contacted authors to obtain additional data or clarification of study details as necessary. We combined terms associated with "endoscopic submucosal resection," "transanal endoscopic surgery," and the specific outcomes mentioned above, using both Medical Subject Headings and relevant keywords. We set restrictions on language (only English) and publication dates (till August 2023). The Supplementary material details our exhaustive search strategy.
Data management: We managed the retrieved studies using the EndNote X9 citation management software. We eliminated duplicates, and screened the remaining articles for inclusion.
Selection process: Two independent reviewers performed the screening of titles and abstracts of the retrieved studies. After that, they evaluated the full texts of potentially eligible studies for inclusion. Disagreements were resolved through discussions.
Data collection process: Two reviewers independently extracted data using a standardized form. The extracted data encompassed study characteristics (authors, publication year, study design, setting), participant characteristics (number of participants, age, gender, severity of condition), details of intervention, and procedure outcomes. We also collected information on funding sources and potential conflicts of interest.
To evaluate the risk of bias, we used the Newcastle Ottawa Scale[14] for observational studies. We considered studies with scores between 0 and 3 as having a high risk of bias, those with scores between 4 and 6 as having a moderate risk, and those with scores between 7 and 9 as having a low risk of bias. Two reviewers independently performed the assessments and resolved disagreements through discussion.
We performed a meta-analysis with data from studies that were sufficiently homogeneous concerning design, parti
We assessed heterogeneity using the I2 statistic[15]. We used funnel plots and Egger’s regression tests to evaluate publication bias, and we checked for selective reporting within studies by comparing reported outcomes with those listed in study protocols or trial registries. We performed sensitivity analyses to check the robustness of the effect estimates.
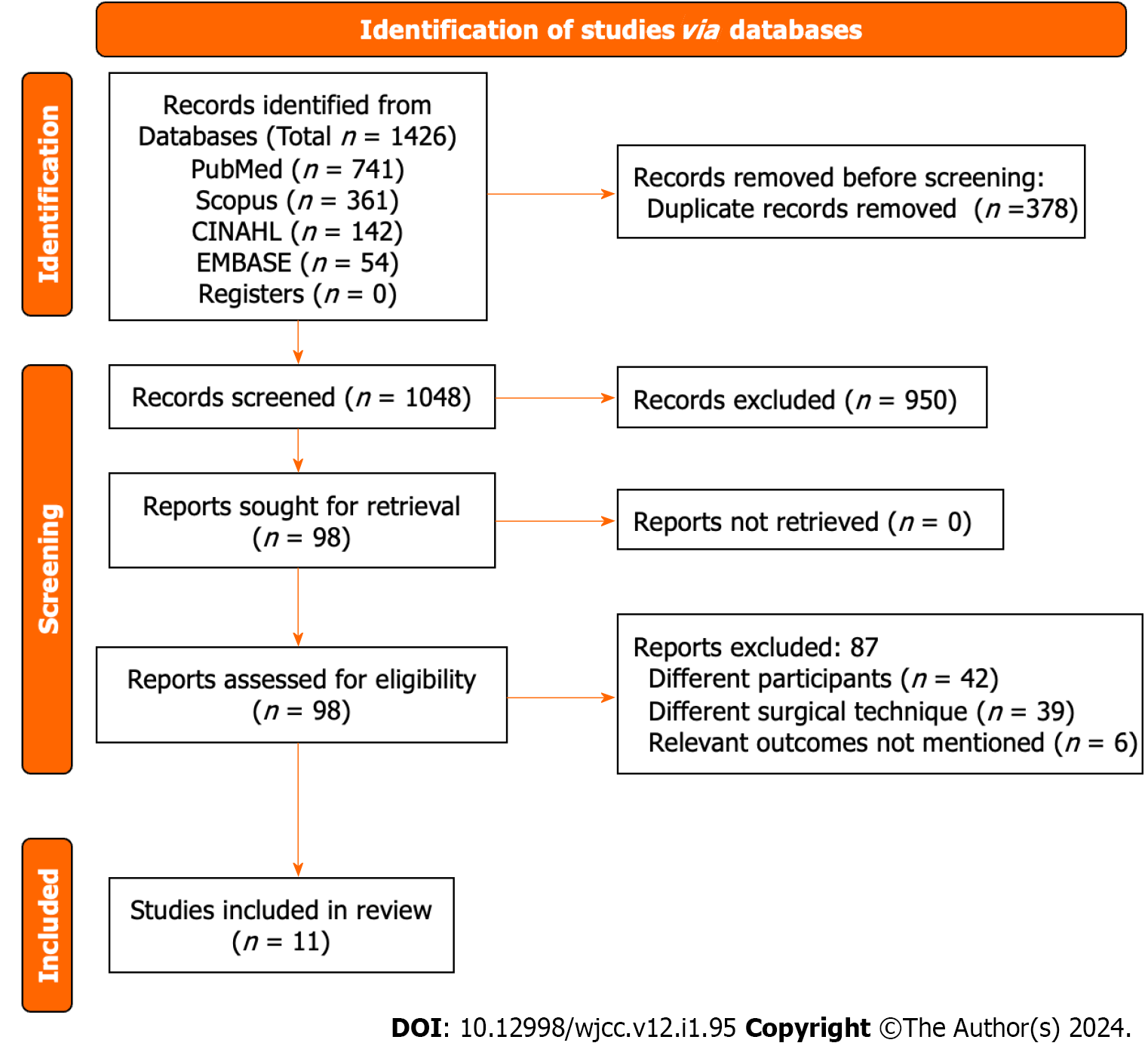
After the primary screening, we identified 1426 citations across the databases. Following removal of duplicates, we retrieved 98 full text articles. After the secondary screening, we included 11 studies that satisfied the eligibility criteria (Figure 1)[16-26].
This study included data from 11 retrospective studies spanning various countries including Italy, China, Korea, Brazil, the United States, and India, and covering a period between 2014 and 2023. The sample sizes for the ESD groups ranged from 11 to 226, and for the TES group from 13 to 103. Most studies focused on patients with early-stage rectal tumors, rectal neuroendocrine tumors, or specific rectal lesions such as adenomas and high-grade dysplasias. Surgeon experience varied across studies, with some specifying the extensive experience of the endoscopists and surgeons and others failing to do so. The mean age of participants across studies was generally similar between the ESD and TES groups. We identified high risk of bias in some studies, moderate in others, and low in two studies (Table 1).
| Ref. | Country | Study design | Sample size in ESD group | Sample size in TES group | Study participants details | Surgeons for ESD | Surgeons for TES | Mean age (yr) | Risk of bias |
| Bisogni et al[22], 2020 | Italy | Retrospective | 13 | 36 | Patients with superficial rectal lesions such as adenomas with negative lifting sign, T1 carcinoma with indication for endoscopic resection and subepithelial colorectal tumour | Three over ten years’ experience endoscopists | Three experienced surgeons performed all the TES procedures | ESD = 74.92; TES = 69.17 | High |
| Jin et al[23], 2023 | China | Retrospective | 14 | 49 | Patients with rectal neuroendocrine tumours ≤ 2 cm | Experienced endoscopist team, who had completed over 5000 cases for colonoscopy training and completed more than 300 cases for ESD | Experienced surgeon who had completed over 300 cases for TES training | ESD = 52.9; TES = 51.1 | Moderate |
| Jung et al[19], 2018 | Korea | Retrospective | 40 | 16 | Patients with epithelial rectal tumour | One experienced endoscopist | One experienced surgeon | ESD = 67.4; TES = 68.4 | Moderate |
| Kawaguti et al[21], 2014 | Brazil | Retrospective | 11 | 13 | Patients with early-stage rectal cancer | NR | NR | ESD = 62.3; TES =61.5 | High |
| Kim et al[24], 2023 | United States | Retrospective | 101 | 103 | Patients with early-stage rectal cancer | NR | NR | ESD = 63; TES = 62 | Moderate |
| Kimura et al[25], 2021 | Brazil | Retrospective | 71 | 27 | Consecutive patients undergoing either ESD or TES | Endoscopist who performed all the ESD procedures had previous training in this technique and had performed more than 20 cases/yr | Two colorectal surgeons performed TEM and both had previous training in this method, had performed more than 20 cases per year | ESD = 65.5; TES = 64.9 | Moderate |
| Mao et al[18], 2017 | China | Retrospective | 31 | 26 | Patients with early-stage rectal tumour | Senior colorectal surgeons and assistants with expertise in endoscopic operations | Experienced colorectal surgeons | ESD = 52.1; TES =54.8 | High |
| Mittal et al[16], 2018 | India | Retrospective | 31 | 19 | Patients with rectal polyps | NR | NR | NR | High |
| Park et al[20], 2012 | Korea | Retrospective | 30 | 33 | Patients with nonpolypoid high-grade dysplasia or cancer invading the submucosa | One experienced endoscopist | Experienced surgeons | ESD = 58.6; TES = 59.5 | Low |
| Park et al[26], 2021 | Korea | Retrospective | 226 | 59 | Patients with rectal neuroendocrine tumours | Four experienced endoscopist | One surgeon | ESD = 48.92; TES = 50.85 | Low |
| Tajika et al[17], 2016 | India | Retrospective | 48 | 28 | Patients with lower rectal tumour | NR | NR | NR | High |
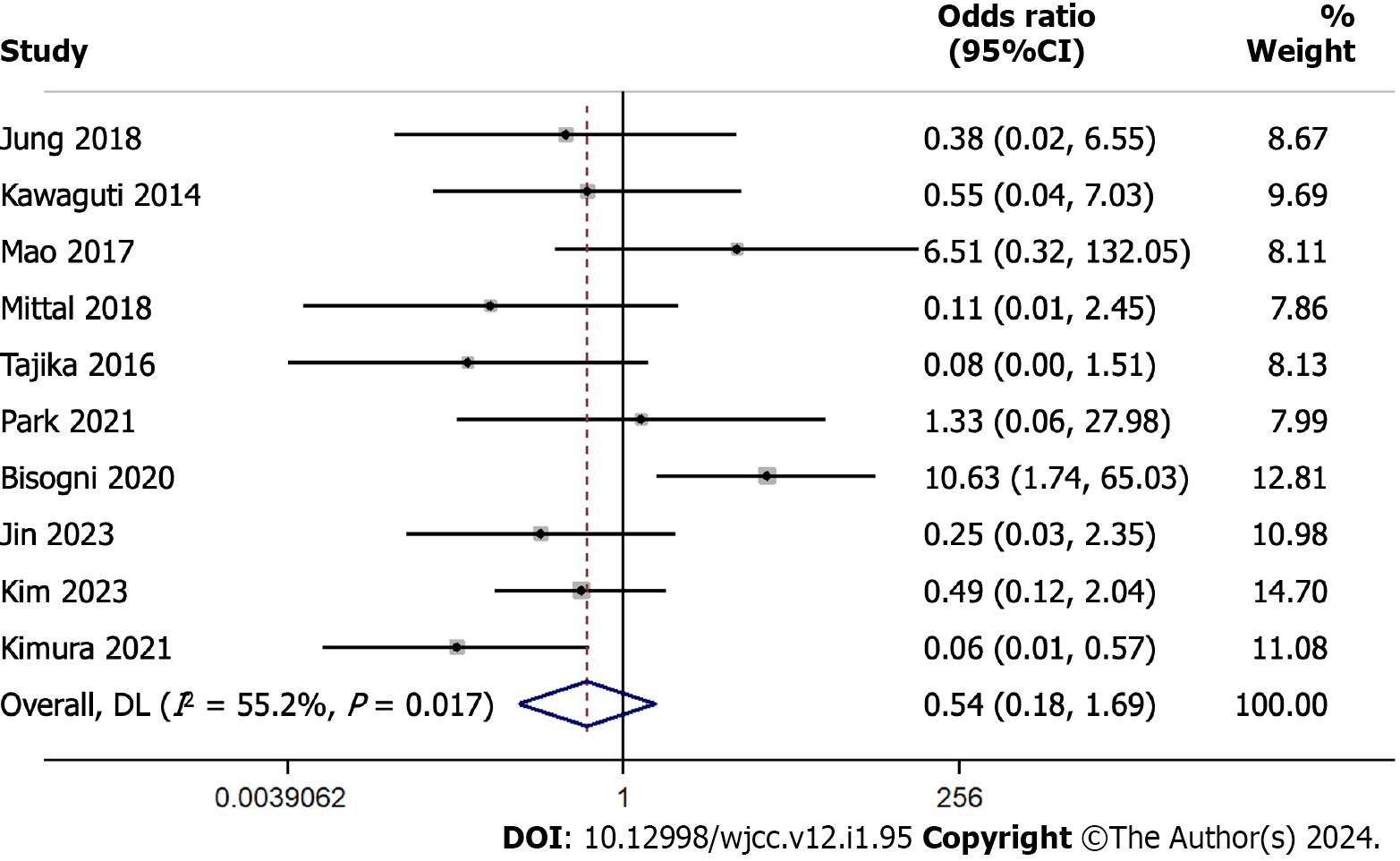
We conducted a comprehensive meta-analysis to discern the difference in recurrence rates between ESD and TES. This involved a pool of 10 studies with 1013 participants. The pooled odds ratio was 0.545 with a 95%CI ranging from 0.176 to 1.687 (Figure 2). This suggests similar recurrence rates for both techniques, as substantiated by a Z-value of -1.053 (P = 0.292). The Cochran’s Q test indicated significant heterogeneity with a value of 20.10 (df = 9, P = 0.017). The I² statistic was 55.2%.
The Egger’s regression test showed that the slope (regression coefficient for the standard effect) was 0.5943 (95%CI: -4.2978 to 5.4863, P = 0.786). The intercept (bias) was -0.9814 (95%CI: -5.1888 to 3.2261, P = 0.605). The P values associated with both the slope and the intercept indicate the absence of statistically significant publication bias in this meta-analysis, as both values exceed the conventional significance threshold (P > 0.05) as shown in the funnel plot (Supple
The sensitivity analysis, systematically omitting one study at a time, revealed a stable combined effect estimate across exclusions ranging from 0.352 to 0.793. This consistency underscores the robustness of our meta-analysis findings, suggesting that no individual study influenced the overall result disproportionately (Supplementary Figure 2).
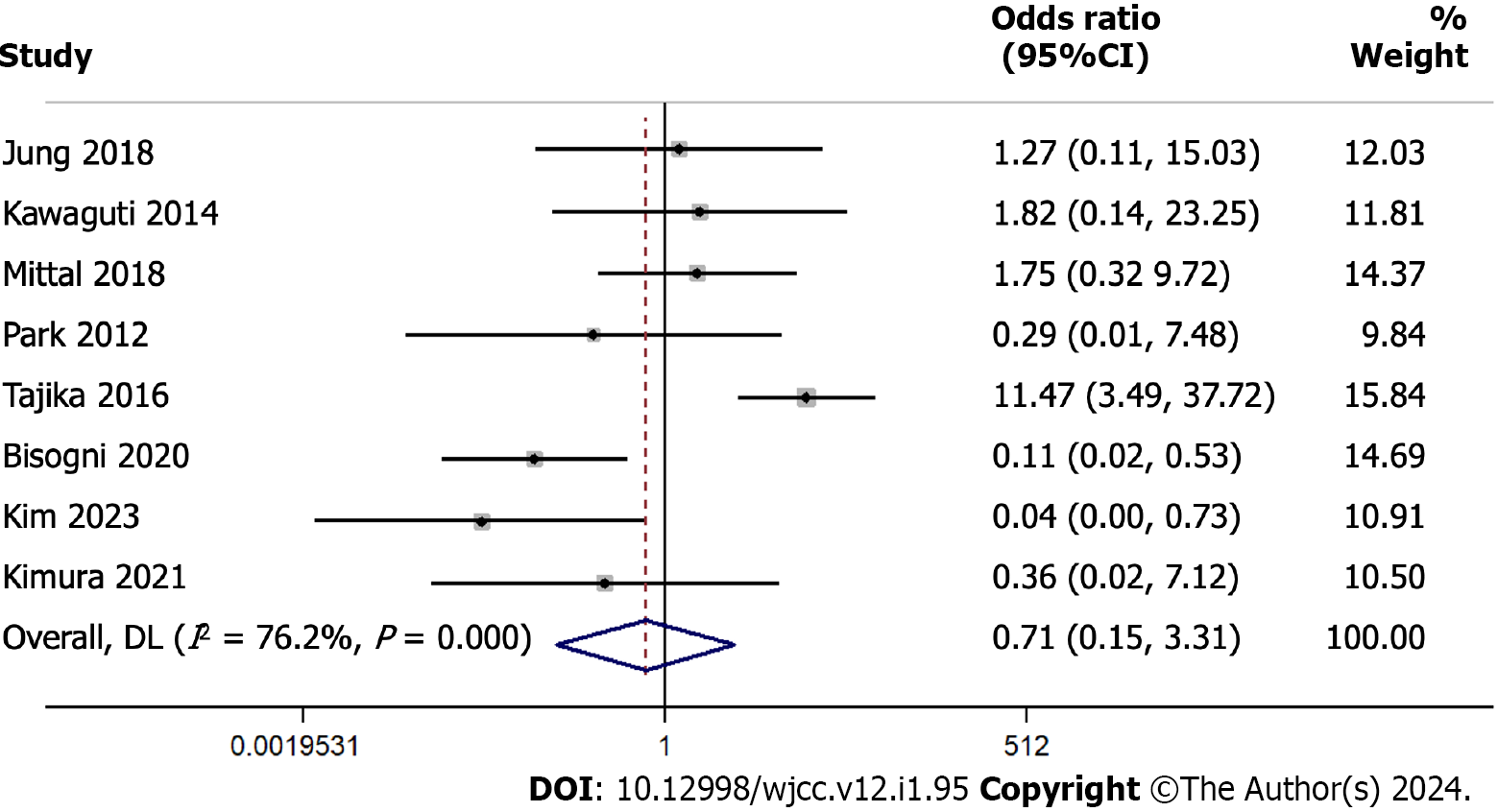
From a collection of 8 studies with 620 participants, we analyzed the difference in en bloc resection rates between ESD and TES. The pooled odds ratio stood at 0.713 with a 95%CI from 0.154 to 3.306 (Figure 3). The test of the overall effect yielded a Z-value of -0.432 (P = 0.665), indicating similar en bloc resection rates for the two techniques.
Cochran’s Q test demonstrated substantial heterogeneity with a value of 29.44 (df = 7, P < 0.001). The I² statistic, a measure of the proportion of total variance attributed to between-study differences, was 76.2%, which is relatively high. After individually omitting each study, we found a combined effect estimate ranging from 0.427 to 2.314. This analysis indicates that these results are relatively stable and not overly influenced by any single study (Supplementary Figure 3).
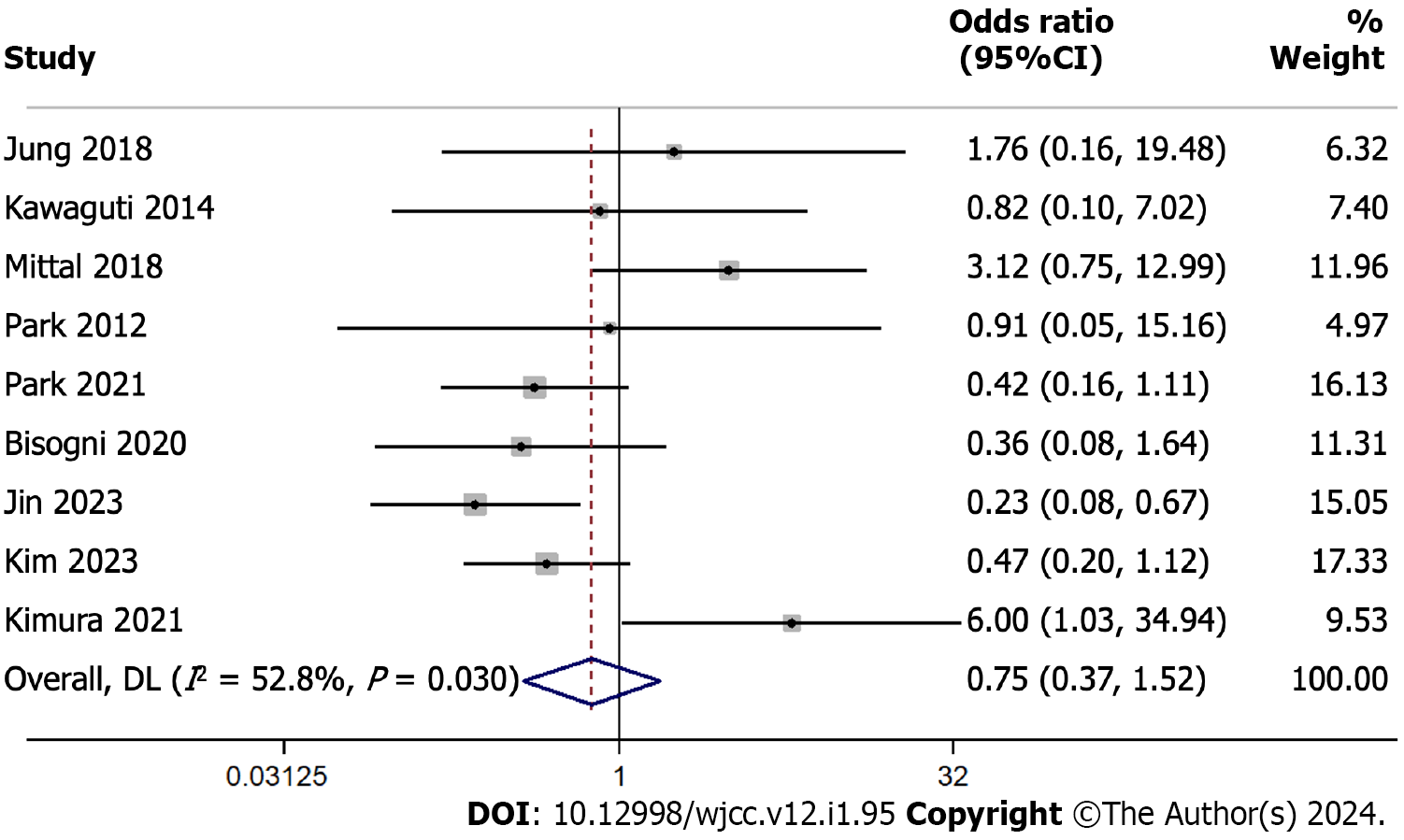
We compared R0 resection rates between ESD and TES across 9 studies encompassing 935 participants. Using a random-effects model, the pooled OR was 0.751 with a 95%CI ranging from 0.370 to 1.524 (Figure 4). This suggests similar R0 resection rates in the two surgical techniques, as evidenced by the z-value of -0.793 (P = 0.428).
We found a moderate level of heterogeneity among the studies with an I² of 52.8%, and the Cochran’s Q test yielded a statistically significant P value of 0.030. After individually omitting each study from the meta-analysis examining R0 resection rates between ESD and TES, the combined effect estimate remained consistent, ranging between odds ratios of 0.514 and 0.751. This suggests the overall finding is robust and not overly influenced by any single study (Supple
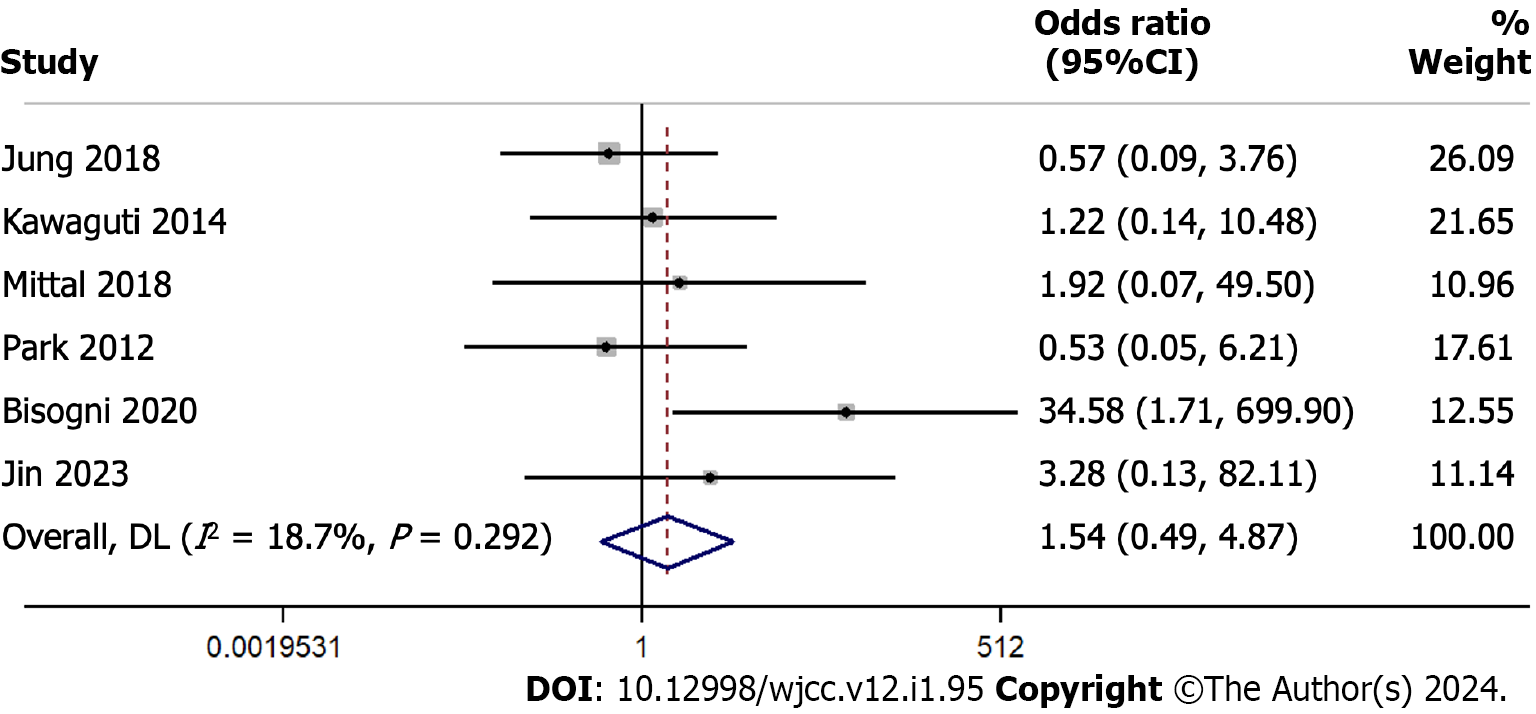
In the analysis evaluating perforation rates across 6 studies with 356 participants, the overall odds ratio was 1.543 (95%CI: 0.489 to 4.870), suggesting similar rates in both groups (Figure 5). The heterogeneity across the studies was relatively low with an I² of 18.7%.
In a sensitivity analysis omitting one study at a time, the estimated overall odds ratio for perforation rates ranged between 0.9509 and 2.0979. The combined effect, after accounting for each omitted study, remained consistent, with an odds ratio of 1.4361 (95%CI: 0.5185 to 3.9774), suggesting that no single study had overwhelmingly influenced the overall results (Supplementary Figure 5).
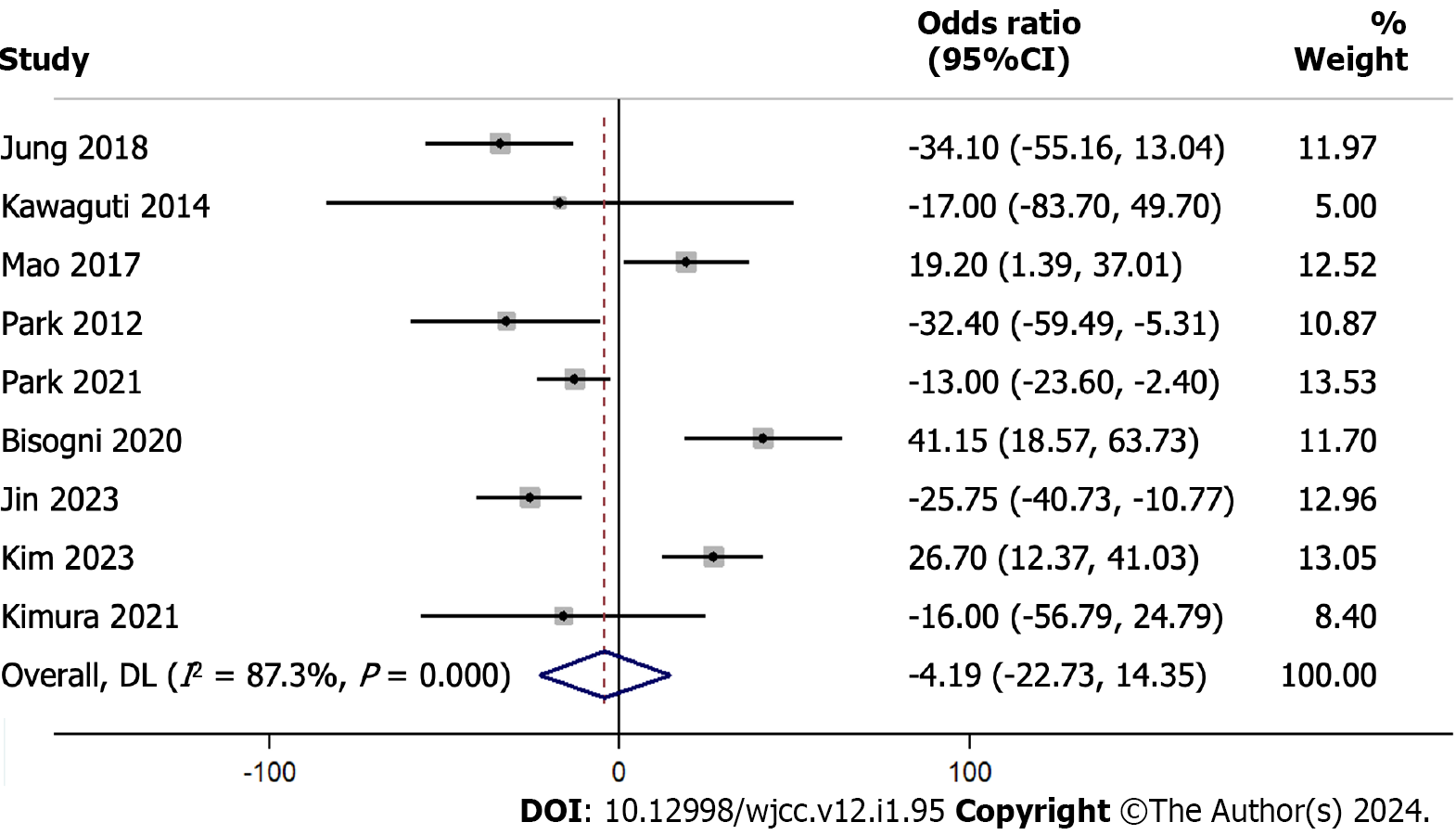
The pooled analysis results from 9 studies with a total of 899 participants evaluating the difference in the procedure length using the WMD indicated a mean difference of -4.19 min (95%CI: -22.73 to 14.35), which was not statistically significant (P = 0.658) (Figure 6). We found evidence for substantial heterogeneity among the studies in an I² value of 87.3%.
After omitting one study at a time and recalculating the values, the sensitivity analysis results suggest that the overall estimate of the difference in procedure length ranges between -9.76 min and 1.17 min (Supplementary Figure 6). Exclusion of Bisogni et al[22] and Kim et al[24] changed the direction of the association from a non-significant to a significant one. This indicates that those studies had a strong influence on the overall effect.
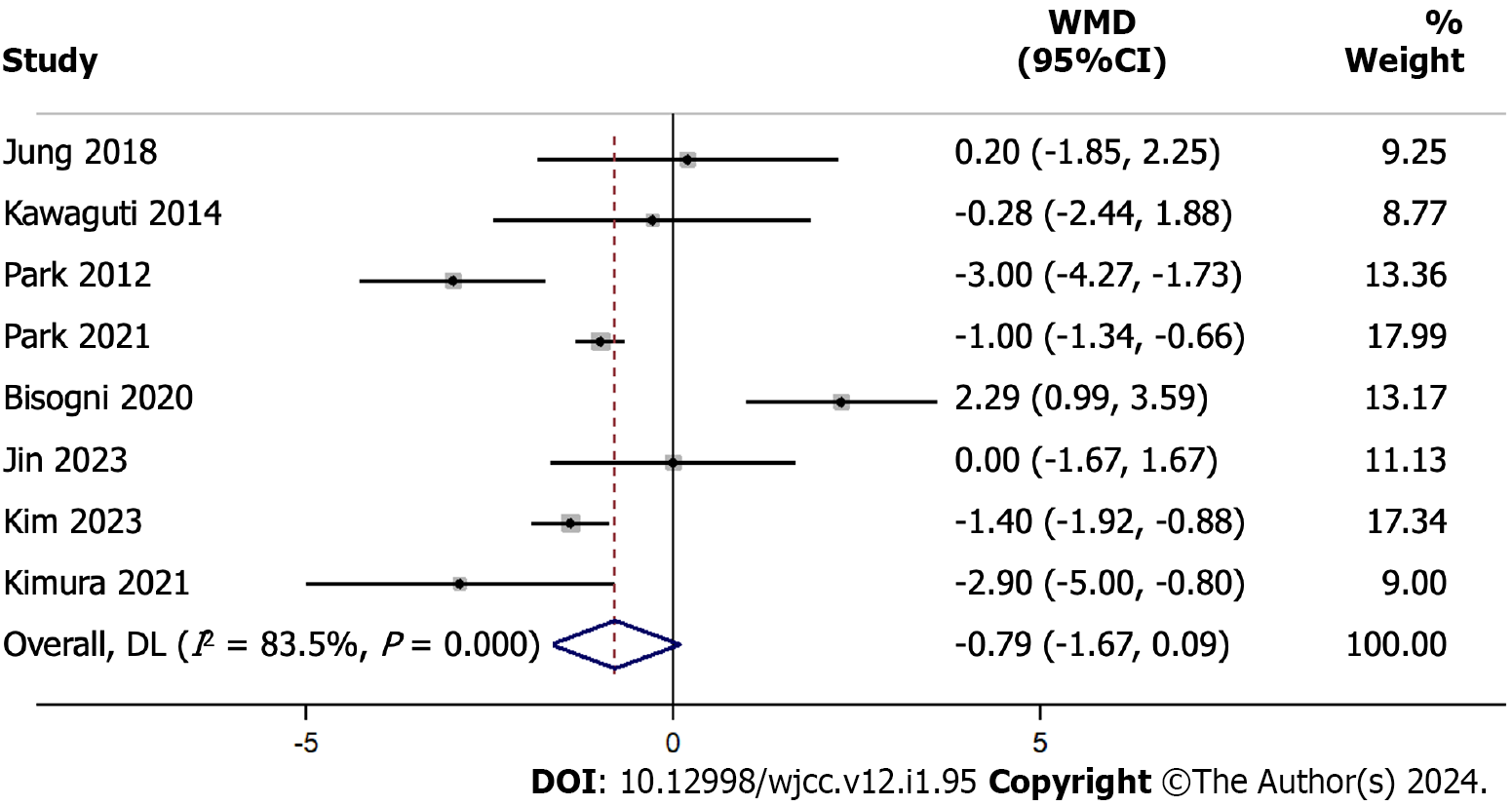
Our hospital stay length results, based on 8 studies with 893 participants, indicate an overall WMD at -0.789 d, with a 95%CI ranging from -1.671 d to 0.093 d (Figure 7). This suggests that, on average, the hospital stay was shorter by about 0.789 d for one method compared to the other, but the difference was not statistically significant (P = 0.079). The heterogeneity among the studies was high, with an I² value of 83.5%.
Our sensitivity analysis demonstrated how the pooled estimate of the weighted mean difference changed after sequentially omitting one study at a time from the analysis (Supplementary Figure 7). The combined estimate of the WMD was -1.0259613, with a 95%CI ranging from -1.286812 to -0.76511061. This indicates an overall reduction in the hospital stay length for ESD compared to that for TES.
In this comprehensive meta-analysis, we sought to compare the surgical ESD and TES techniques in terms of their relative efficacies and outcomes. We assessed a range of outcomes including recurrence rates, en bloc and R0 resection rates, perforation rates, and procedure and hospital stay lengths. We chose these variables to help us evaluate the effectiveness and feasibility of the two approaches.
Our findings on recurrence rate are important, because recurrence remains a post-operative challenge in surgical oncology. The pooled odds ratio of 0.545 with a 95%CI ranging from 0.176 to 1.687 suggests that both techniques have similar recurrence rates, and the associated Z-value and P values confirm this. This finding is crucial for clinicians weighing the long-term implications of either surgical method. However, we also found considerable heterogeneity among the studies involved. An I² statistic of 55.2% underscores a moderate-to-high variance in outcomes, indicating potential differences in study designs, patient populations, or surgical technique implementation. Thus, standardized multicentric studies are needed to generate a clear picture.
ESD offers direct visualization with a minimal invasion of the submucosal layer. The endoscopic technique is particularly useful in instances where tumors are located in difficult-to-access areas or when they are large and display a high risk of malignancy[3]. By contrast, TES is often preferred for localized tumors, especially when invasion beyond the mucosa is suspected[11]. Understanding the context in which each technique excels is critical for making informed surgical decisions.
Resection methods—whether en bloc or R0—affect the prognosis, recurrence, and overall patient morbidity differently[27]. Our pooled analyses in these domains yielded non-significant differences between the two techniques. This apparent equivalency between ESD and TES should be interpreted with caution due to the substantial heterogeneity in the en bloc resection data (I² = 76.2%). Considering the broader context of surgical goals is important; while complete tumor removal is the principal aim, the assurance that a procedure will accomplish this without increasing the recurrence risk is equally essential. ESDs are used primarily for precise en bloc tumor removals, especially in patients with tumors that are large or are spread superficially. By contrast, TES provides a direct and effective approach for tumors in the lower rectum. These technical differences must be weighed in when assessing the equivalent outcomes we are presenting.
Perforation, albeit a rare complication, is associated with substantial morbidity and potential mortality. Our findings suggesting similarly low perforation rates for both groups is reassuring. With an odds ratio of 1.543, both methods appear to offer comparable safety profiles. The relatively low heterogeneity (I² of 18.7%) further strengthens our confidence in these results.
Efficiency in surgical procedures, in terms of duration, impacts hospital resource allocation and can have implications on patient outcomes. Our meta-analysis indicates a non-significant difference in procedure length between ESD and TES. However, the substantial heterogeneity we observed (I² = 87.3%) highlights the need for standardization in procedural techniques, patient selection, and post-operative care protocols. The steps in the ESD and TES procedures reveal clues behind the similar procedure lengths. While ESD demands careful dissection of the submucosal layer, especially in patients with large tumors; TES requires precise localization and access through the transanal approach. Such technical nuances, coupled with surgeon expertise, play a decisive role in procedure length.
An important aspect to consider in comparing ESD and TES is the difference in anaesthesia methods used for these procedures. ESD often requires conscious sedation or general anaesthesia, depending on the size and location of the tumour, as well as patient factors. This type of anaesthesia allows for patient comfort while maintaining a degree of responsiveness. In contrast, TES typically necessitates general anaesthesia due to its more invasive nature and the potential for discomfort in the transanal approach. The choice of anaesthesia can impact patient recovery, procedure duration, and overall patient experience. This distinction is crucial for a comprehensive comparison of ESD and TES, as it not only influences the procedural approach but may also have implications for post-operative recovery and patient satisfaction.
The hospital stay length, which directly impacts healthcare costs and patient convenience, showed a trend towards a shorter stay for ESD. Although this difference did not reach statistical significance, the trend can have substantial implications for high-throughput centers. The explanation for this difference in hospital stay lengths may be due to ESD’s minimal invasiveness. By contrast, TES, may involve more extensive tissue manipulation, potentially necessitating longer post-operative recovery.
Other meta-analyses have compared outcomes of ESD and TES[3,28]. However, the most notable of those meta-analyses, with similar outcome findings to ours incorporated only half the number of studies we scrutinized in here[3,28]. Our expanded population numbers broaden the empirical base and potentially incorporate a wider range of specific populations, surgical techniques, and clinical settings. We believe the large population in our analysis makes our conclusions more generalizable and robust.
In any case, the parallels between our findings and those of previous research highlight the consistency across analyses. However, with our analysis of a large population, we also uncovered heterogeneity among the studies.
In pondering the similarities in the outcomes of these different techniques, we highlight a few factors. First, advances in both the ESD and TES techniques over the years may have converged their efficacy profiles. Surgeons may have optimized the techniques, leading to similar outcomes across both modalities[29]. Second, the standard of care in post-operative settings has evolved to minimize complications and improve recovery, irrespective of the surgical method applied to individual cases[30]. Finally, patient selection, a pivotal aspect of any surgical intervention, may have been refined with accumulating clinical experience, ensuring that the most appropriate individual patient profiles have been applied[31].
Our rigorous approach to assessing publication bias, involving the Egger’s regression test, showed no significant evidence of such bias. This enhances the validity of our findings. Moreover, our systematic sensitivity analyses across all domains underscore the robustness of our conclusions, with no single study unduly influencing the collective results. This is paramount in a meta-analytic approach, ensuring that our findings are not skewed by outlier data or disproportionately influential studies.
When choosing a technique between ESD and TES, several factors need to be considered: Tumor location, size, and depth of invasion are pivotal. For instance, superficial tumors, especially those in challenging locations, may be better suited for ESD, given its fine-tuned submucosal access. By contrast, TES might be the method of choice for tumors that are deeper or situated in the lower rectum. Beyond tumor characteristics, surgeon expertise and familiarity with each procedure, as well as available institutional resources, also have a decisive role.
Patient-specific variables, such as age, comorbidities, and prior surgical history need to be taken into consideration because they can influence post-operative recovery and the risk of complications. The choice between ESD and TES should be individualized after consideration of the entire clinical picture.
While our analysis is robust and comprehensive, it has its limitations. The possibility of selection bias and a lack of RCTs are our primary concerns. The heterogeneity we observed across outcomes warrants attention. Differences in patient populations, surgical expertise, hospital resources, and post-operative care can all contribute to this variance. However, future studies should strive for standardized methodologies and clear reporting of outcomes to minimize this heterogeneity.
Our meta-analysis offers a comprehensive comparison between ESD and TES across multiple critical domains. The lack of significant differences in recurrence, resection rates, perforation rates, and procedure lengths suggests that both surgical techniques have their merits and can be chosen based on individual patient needs, surgeon expertise, and institutional resources. The potential reduction in hospital stays with ESD, though not statistically significant, may offer strategic advantages in specific healthcare settings. We hope that this synthesis will aid in clinical decision-making, and we underscore the need for further research with standardized methodologies.
Rectal tumors, whether benign or malignant, pose considerable management challenges due to their anatomical location and the complications and morbidity associated with their surgical resection. Traditional surgical techniques, while effective, often require extensive tissue dissection, resulting in prolonged recovery, potential for morbidity, and significant bowel function alterations.
This study addresses the comparative efficacy and safety of two widely employed surgical techniques for rectal tumours - endoscopic submucosal dissection (ESD) and transanal endoscopic surgery (TES), which are yet to be conclusively compared.
The primary objective was to analyze and discern the differences in surgical outcomes between ESD and TES. By reviewing various studies, the research aims to provide a clearer understanding of these techniques' efficacies and potential roles in modern surgical practice.
A systematic review and meta-analysis were conducted following the PRISMA 2020 guidelines. The study included randomized controlled trials, observational studies, and cohort studies that compared outcomes such as local recurrence, en bloc resection rate, R0 resection rate, procedure length, hospital stay length, and complication rates in patients with rectal tumours undergoing ESD or TES.
The meta-analysis of 11 studies found no significant differences between ESD and TES in terms of recurrence rates, en bloc and R0 resection rates, and perforation rates. The study noted similar procedure lengths and a non-significant trend towards shorter hospital stays for ESD. Substantial heterogeneity was observed in some outcomes, indicating variations in study designs, patient populations, and surgical techniques.
The study concludes that both ESD and TES have similar efficacy and safety profiles for treating rectal tumours. The choice between these techniques can be based on individual patient needs, tumour characteristics, surgeon expertise, and available resources. However, the presence of heterogeneity in study results and the lack of randomized controlled trials suggest that future standardized studies are needed.
Future research should focus on standardizing methodologies and reporting outcomes to minimize heterogeneity observed in current studies. Additionally, a deeper exploration of patient-specific variables and an emphasis on surgeon expertise and institutional capabilities will be crucial in refining the choice between ESD and TES for individual patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kinami S, Japan S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Chi P, Wang XJ. [Historical evolution and ultimate goal of minimally invasive surgery for colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2022;25:675-681. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Hasan O, Ayaz A, Masood L, Baig AM, Baloch N. Innovations in surgery between the past and future: A narrative review of targeted literature. J Pak Med Assoc. 2022;72 (Suppl 1):S55-S58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Aihara H. Endoscopic submucosal dissection (ESD) versus transanal endoscopic microsurgery (TEM) for treatment of rectal tumors: a comparative systematic review and meta-analysis. Surg Endosc. 2020;34:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (2)] |
| 4. | Fazeli MS, Keramati MR. Rectal cancer: a review. Med J Islam Repub Iran. 2015;29:171. [PubMed] |
| 5. | Scott MJ, Baldini G, Fearon KC, Feldheiser A, Feldman LS, Gan TJ, Ljungqvist O, Lobo DN, Rockall TA, Schricker T, Carli F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand. 2015;59:1212-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 6. | Devane LA, Burke JP, Kelly JJ, Albert MR. Transanal minimally invasive surgery for rectal cancer. Ann Gastroenterol Surg. 2021;5:39-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Ma MX, Bourke MJ. Endoscopic submucosal dissection in the West: Current status and future directions. Dig Endosc. 2018;30:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Lee DJ, Sagar PM, Sadadcharam G, Tan KY. Advances in surgical management for locally recurrent rectal cancer: How far have we come? World J Gastroenterol. 2017;23:4170-4180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 9. | Pak H, Maghsoudi LH, Soltanian A, Gholami F. Surgical complications in colorectal cancer patients. Ann Med Surg (Lond). 2020;55:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Gkionis IG, Flamourakis ME, Tsagkataki ES, Kaloeidi EI, Spiridakis KG, Kostakis GE, Alegkakis AK, Christodoulakis MS. Multidimensional analysis of the learning curve for laparoscopic colorectal surgery in a regional hospital: the implementation of a standardized surgical procedure counterbalances the lack of experience. BMC Surg. 2020;20:308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Leong KJ, Evans J, Davies MM, Scott A, Lidder P. Transanal endoscopic surgery: past, present and future. Br J Hosp Med (Lond). 2016;77:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Martin ST, Heneghan HM, Winter DC. Systematic review of outcomes after intersphincteric resection for low rectal cancer. Br J Surg. 2012;99:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40620] [Article Influence: 10155.0] [Reference Citation Analysis (2)] |
| 14. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1646] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 15. | Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. Cochrane Handbook for Systematic Reviews of Interventions. 2019. Available from: https://training.cochrane.org/handbook. |
| 16. | Mittal R, Manji F, Antillon-Galdamez M, Ogilvie JW. Is endoscopic submucosal dissection for rectal polyps an alternative to trans anal minimally invasive surgery: a retrospective comparative study. Dis Colon Rectum. 2018;61:E53. [DOI] [Full Text] |
| 17. | Ishihara M, Tajika M, Tanaka T, Hirayama Y, Ohnishi S, Hara K, Mizuno N, Hijioka S, Okuno N, Niwa Y. Clinical outcome of salvage ESD for esophageal cancer after chemoradiation therapy. J Gastroenterol Hepatol. 2016;31:7-441. [DOI] [Full Text] |
| 18. | Mao W, Liao X, Shao S, Wu W, Yu Y, Yang G. Comparative evaluation of colonoscopy-assisted transanal minimally invasive surgery via glove port and endoscopic submucosal dissection for early rectal tumor. Int J Surg. 2017;42:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Jung Y, Lee J, Cho JY, Kim YD, Park CG, Kim MW, Kim KJ, Kim SW. Comparison of efficacy and safety between endoscopic submucosal dissection and transanal endoscopic microsurgery for the treatment of rectal tumor. Saudi J Gastroenterol. 2018;24:115-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 20. | Park SU, Min YW, Shin JU, Choi JH, Kim YH, Kim JJ, Cho YB, Kim HC, Yun SH, Lee WY, Chun HK, Chang DK. Endoscopic submucosal dissection or transanal endoscopic microsurgery for nonpolypoid rectal high grade dysplasia and submucosa-invading rectal cancer. Endoscopy. 2012;44:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Kawaguti FS, Nahas CS, Marques CF, Martins BC, Retes FA, Medeiros RS, Hayashi T, Wada Y, de Lima MS, Uemura RS, Nahas SC, Kudo SE, Maluf-Filho F. Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc. 2014;28:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Bisogni D, Manetti R, Talamucci L, Coratti F, Naspetti R, Valeri A, Martellucci J, Cianchi F. Comparison among different techniques for en-bloc resection of rectal lesions: transanal endoscopic surgery vs. endoscopic submucosal dissection vs. full-thickness resection device with Over-The-Scope Clip® System. Minerva Chir. 2020;75:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 23. | Jin R, Bai X, Xu T, Wu X, Wang Q, Li J. Comparison of the efficacy of endoscopic submucosal dissection and transanal endoscopic microsurgery in the treatment of rectal neuroendocrine tumors ≤ 2 cm. Front Endocrinol (Lausanne). 2022;13:1028275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 24. | Kim M, Bareket R, Eleftheriadis NP, Kedia P, Seewald S, Groth S, Nieto J, Kumta NA, Deshmukh AA, Katz J, Suresh S, Zamarripa F, Martínez MG, Liu-Burdowski J, Gaidhane M, Sarkar A, Shahid HM, Tyberg A, Kahaleh M. Endoscopic Submucosal Dissection (ESD) Offers a Safer and More Cost-effective Alternative to Transanal Endoscopic Microsurgery (TEM): An International Collaborative Study. J Clin Gastroenterol. 2023;57:486-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 25. | Kimura CMS, Kawaguti FS, Nahas CSR, Marques CFS, Segatelli V, Martins BC, de Paulo GA, Cecconello I, Ribeiro-Junior U, Nahas SC, Maluf-Filho F. Long-term outcomes of endoscopic submucosal dissection and transanal endoscopic microsurgery for the treatment of rectal tumors. J Gastroenterol Hepatol. 2021;36:1634-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Park SS, Kim BC, Lee DE, Han KS, Kim B, Hong CW, Sohn DK. Comparison of endoscopic submucosal dissection and transanal endoscopic microsurgery for T1 rectal neuroendocrine tumors: a propensity score-matched study. Gastrointest Endosc. 2021;94:408-415.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Ouyang Y, Zhu Y, Chen H, Li G, Hu X, Luo H, Li Z, Han S. Case Report: Long-term survival of a patient with advanced rectal cancer and multiple pelvic recurrences after seven surgeries. Front Oncol. 2023;13:1169616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 28. | Sagae VMT, Ribeiro IB, de Moura DTH, Brunaldi VO, Logiudice FP, Funari MP, Baba ER, Bernardo WM, de Moura EGH. Endoscopic submucosal dissection versus transanal endoscopic surgery for the treatment of early rectal tumor: a systematic review and meta-analysis. Surg Endosc. 2020;34:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial Intelligence in Surgery: Promises and Perils. Ann Surg. 2018;268:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 603] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 30. | O'Leary MP, Choong KC, Thornblade LW, Fakih MG, Fong Y, Kaiser AM. Management Considerations for the Surgical Treatment of Colorectal Cancer During the Global Covid-19 Pandemic. Ann Surg. 2020;272:e98-e105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Bernier L, Balyasnikova S, Tait D, Brown G. Watch-and-Wait as a Therapeutic Strategy in Rectal Cancer. Curr Colorectal Cancer Rep. 2018;14:37-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |