Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.9
Peer-review started: October 29, 2023
First decision: November 28, 2023
Revised: December 8, 2023
Accepted: December 14, 2023
Article in press: December 14, 2023
Published online: January 6, 2024
Processing time: 64 Days and 16.6 Hours
The knowledge of the pathogenesis of type 1 diabetes mellitus (T1DM) continues to rapidly evolve. The natural course of the disease can be described in four clinical stages based on the autoimmune markers and glycemic status. Not all individuals of T1DM progress in that specific sequence. We hereby present a case of T1DM with a classical third phase (honeymoon phase) and discuss the intri
Core Tip: The insulin requirement reduces drastically in the Honeymoon phase or the partial remission phase in type 1 diabetes mellitus (T1DM). Novel pharmacological interventions and immunomodulating therapies in T1DM are being tried specifically in this phase as it may serve as the potential window of opportunity for possible “cure”. It may therefore be clinically relevant to timely identify this phase in these patients.
- Citation: Mittal M, Porchezhian P, Kapoor N. Honeymoon phase in type 1 diabetes mellitus: A window of opportunity for diabetes reversal? World J Clin Cases 2024; 12(1): 9-14
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/9.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.9
Type 1 diabetes mellitus (T1DM), previously known as Insulin Dependent Diabetes, results from the autoimmune destruction of beta cells of pancreatic islets. With genetic susceptibility conferring the risk of diabetes mellitus since birth, the occurrence of autoantibodies against beta cell components like insulin, Islet-specific Glucose-6-phosphatase catalytic subunit-related protein, Zinc transporter 8, Glutamic Acid Decarboxylase (GAD), Islet Antigen-2 (IA-2) contribute to the loss of immunologic tolerance to beta cells and cause their progressive depletion. Interesting to note is that the mere presence of antibodies doesn’t lead to diabetes, emphasizing the role of other factors like environment as well as stressed beta cell dysfunction in their functional decline.
The natural course of T1DM can be described in four clinical stages based on the autoimmune markers and glycemic status. Stage 1 is characterized by autoantibody positivity which predisposes the individual to autoimmune destruction of pancreatic beta cells thereby leading to insulin deficiency and metabolic dysfunction which constitutes Stage 2. Stage 3 denotes overt hyperglycemia which is classically defined as the T1DM onset. Stage 4 indicates the post-diagnosis period. These stages although assist in understanding the disease, it is to be borne in mind that not all individuals of T1DM progress in that specific sequence. Similarly, T1DM is also described in four phases namely pre-clinical, overt, partial remission, and chronic phase, of which the third phase popularly called the honeymoon phase has instilled extreme interest. This phase is complex in its pathogenesis as well as intervening in this stage seems to be an extremely feasible option for the concept of T1DM reversal. Here we discuss the various criteria for diagnosing the honeymoon phase and present case details of a patient in this phase and the various factors affecting it.
An 11-year-old boy, second born to non-consanguineously married parents, had osmotic symptoms, generalized weakness for 4 wk, and hyperglycemia (> 500 mg/dL). At presentation, he had persisting polyuria with no features of hypothyroidism/adrenal insufficiency/celiac disease. On examination, he was not dehydrated, with height above 97th centile for age and weight between 50-75th centile. The child had a pubertal status of P2 G2 as per Tanner staging. Blood investigations revealed a raised anion gap with mildly positive urine ketones, and no acidosis with normal serum electrolytes. Kidney function, liver function tests, and thyroid profile were within normal limits and HbA1c was > 14.5%. He was initially managed with intravenous fluids, insulin infusion with potassium supplementation as needed and later shifted to subcutaneous basal-bolus insulin regimen. On evaluation, serum C-peptide was 0.34 ng/mL, serum insulin was 1.106 mIU/L, anti-TPO (53.5 IU/mL) and anti-GAD 65 antibodies (208 IU/mL) were positive with other antibodies including IA2 and anti-insulin antibodies being negative. He was discharged on the basal-bolus regimen with a total daily insulin requirement of 48 units/d (1.1 units/kg/d). The patient was followed up closely on an outpatient basis and his insulin requirements gradually decreased when at 8 wk the insulin requirement was only 2-4 units/d (0.1 unit/kg/d) with HbA1C of 7.6%. This minimal insulin requirement persisted for nearly a year with HbA1c values maintaining near 6.5%, suggesting a phase of partial remission.
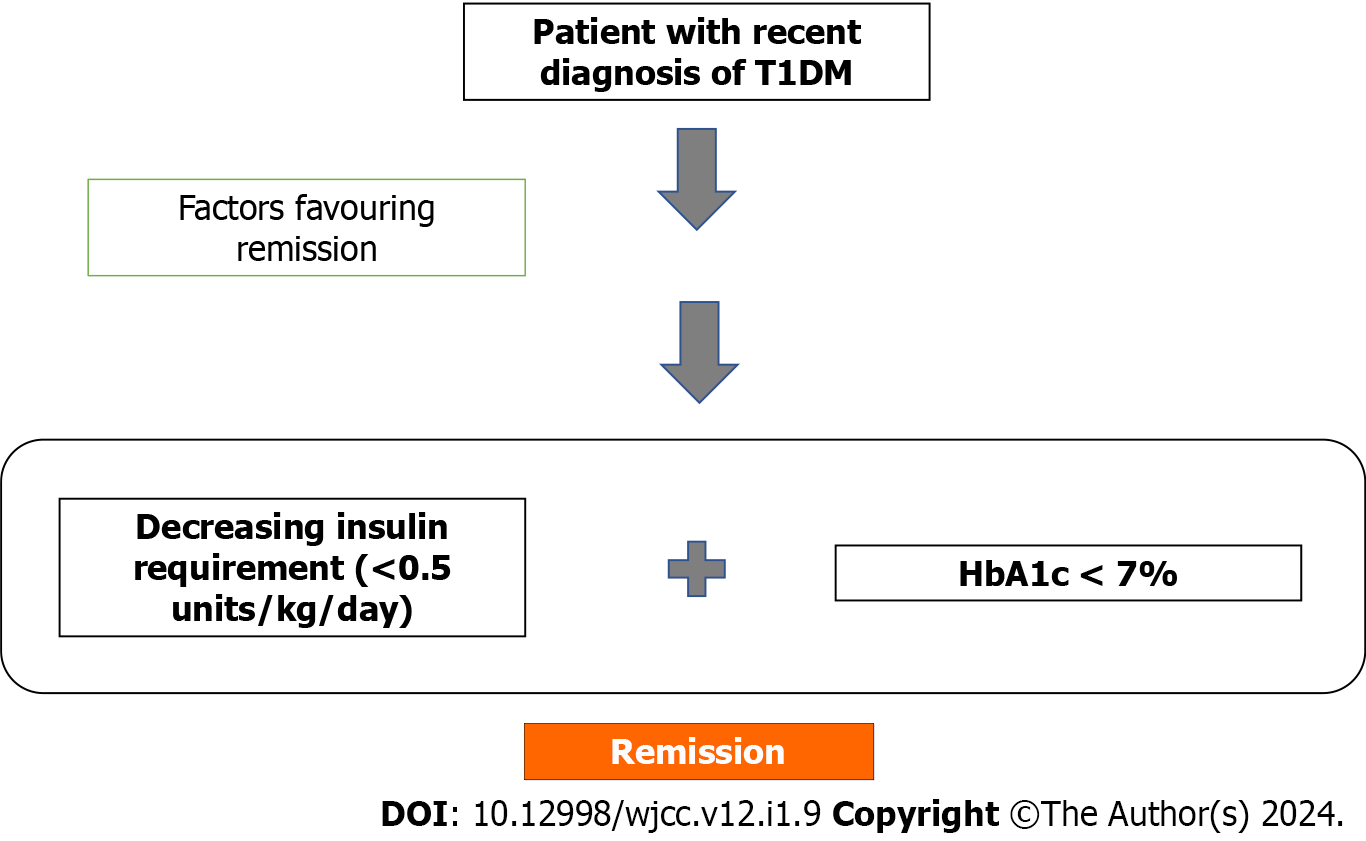
The phase of partial remission (also known as Honeymoon phase) has revived interest owing to the possibility of pharmacological intervention and immunomodulation. This phase is clinically characterized by decreasing (partial) or rarely absent (complete remission) insulin requirements and near-normal glycemic control in patients of type 1 DM. The definitions of this phase have evolved much since Jackson first described it in the 1940s[1]. As in T1DM, the insulin requirement was around 0.8-1.2 units/kg body weight/24 h, authors initially labeled this phase as insulin requirement of < 0.5 units/kg body weight/24 h with few even suggesting a lower dose of 0.3 units/kg body weight/24 h[2,3]. Insulin requirement has been used as a surrogate measure of stimulated C-peptide. The less commonly used definition of decrease in insulin dose of at least 33% from dose at diagnosis has also been employed in a few studies[4]. Achievement of near-normal HbA1C which reflects glycemic control has also been added as a criterion[5]. So, combining both, this phase has classically been defined where insulin requirement is < 0.5 units/kg body weight/ day and HbA1c < 7%[6]. A newer formula proposed by Mortensen et al[7], “Insulin Dose-Adjusted HbA1c” (IDAA1c) is defined as HbA1C+ 4 × (insulin dose in units/kg/24 h) whose value when less than or equal to 9 would indicate partial remission. This definition has been validated well in larger cohorts and correlates with functional beta cell mass and stimulated C-peptide, hence avoiding the need for measurement of stimulated C-peptide[8]. In our patient the IDAA1c value was 8. Another recent retrospective study has claimed a definition of Glycemic Target Adjusted HbA1c which is calculated as HbA1c (%) - [3 × % of normoglycemic values (70-180)] where a value of < 4.5% correlated well with IDAA1c and had a sensitivity and specificity of 72% and 92% in predicting partial remission[9]. Complete remission is defined as insulin independence with HbA1c < 6% on oral or no hypoglycemic agents. The different definitions for partial remission are summarized in Table 1.
| Definitions of partial remission phase | |
| Muhammad et al[3] | Insulin requirement < 0.5 units/kg body weight/24 h |
| Bonfanti et al[2] | Insulin requirement < 0.3 units/kg body weight/24 h |
| Jefferson et al[4] | Fall in insulin dose of at least 33% from dose at diagnosis |
| Chase et al[5] | Achieving near normal HbA1C |
| Araszkiewicz et al[20] | Insulin requirement < 0.3 units/kg body weight/24 h + C-peptide > 0.5 ng/mL + normal 24-h glycemic profile |
| Mortensen et al[7] | Insulin Dose- Adjusted HbA1C < 9 |
| ISPAD[6] | Insulin requirement < 0.5 units/kg body weight/24 h and HbA1C < 7% |
| Nielens et al[9] | Glycemic Target Adjusted HbA1C (GTAA1c) < 4.5% |
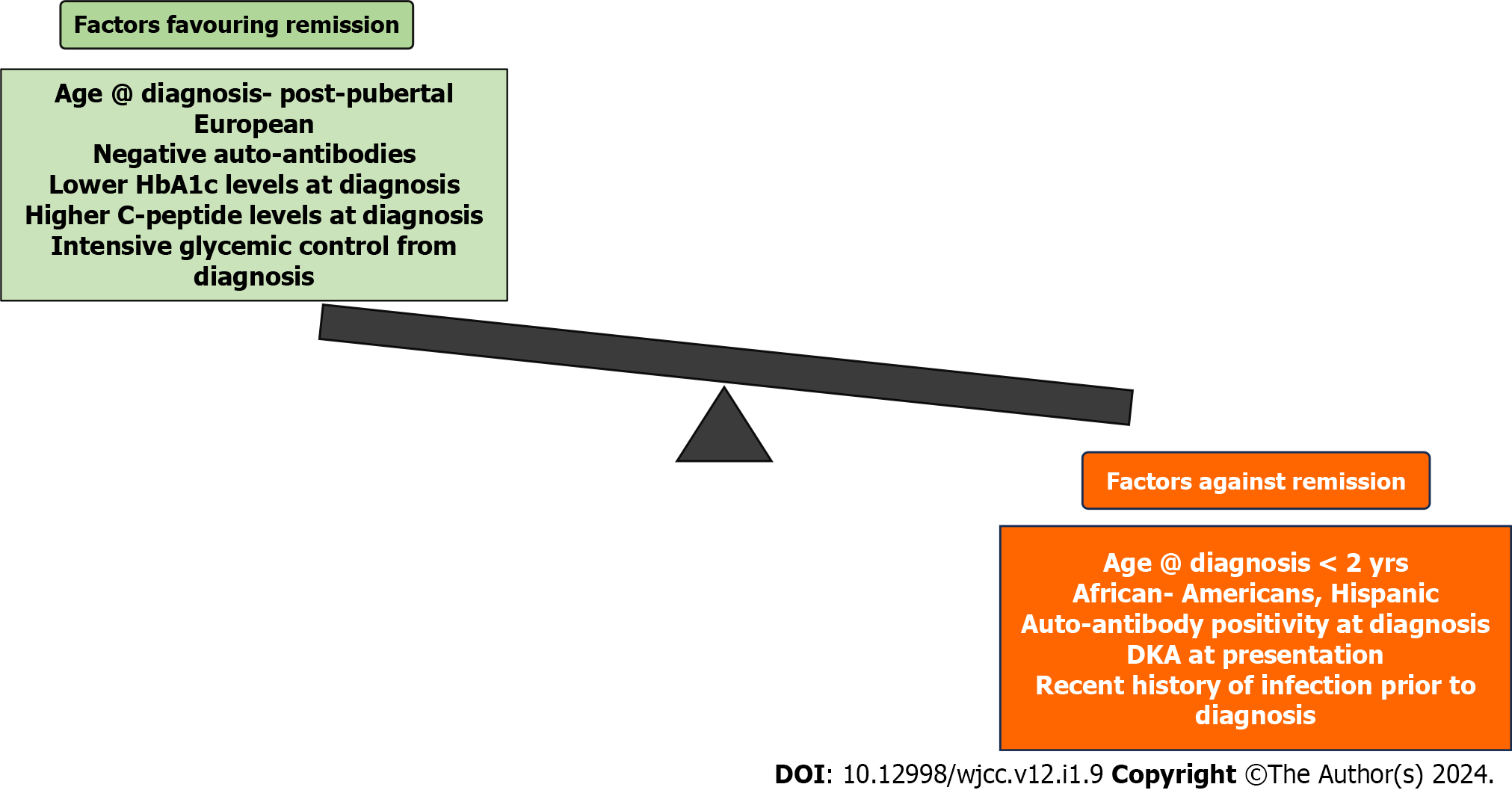
Factors like gender, age at diagnosis of diabetes, body mass index (BMI), duration of diabetes, degree of metabolic compensation, C-peptide levels, HbA1c, antibody positivity, and glycemic control achieved with treatment determine the attainment of partial remission (Figure 1). Boys attain partial remission more frequently than girls whereas in few studies, gender had no association with partial remission occurrence[8,10,11].
In our case, the child was 11 years at diagnosis, male gender, BMI 14.89 kg/m2, 3 mo duration of diabetes, history of mild diabetic ketoacidosis (DKA), C-peptide level 0.34 ng/mL, HbA1c > 14.5% at diagnosis, GAD antibody positive, and very good glycemic control was achieved with multiple subcutaneous insulin injections in 3 mo.
Studies have shown that partial remission was more frequent when the age at diagnosis was above 15 years i.e. post-pubertal, while remission is never attained in patients diagnosed under the age of 2 years[2,3,11]. Few studies have also shown the highest rates of remission occur in children aged 5-9 years[12]. The difference in frequencies among different age groups could be explained by the difference in treatment approach varying with age as a study by Chobot et al[10] showed that there was no association of occurrence of remission with age at the onset. In the guideline followed in their study, treatment was uniform in all age groups.
The study by Chobot et al[10] showed that patients who attained remission had a higher BMI compared to those who failed to attain remission. The possible explanation hypothesized is that higher BMI accelerates insulin resistance and worsening glycemic control which in turn aggravates beta cell apoptosis, thus patients present early and gain therapy. This study also revealed that individuals with another concomitant autoimmune disease had more frequent remission which is explained by the possibility of earlier diagnosis.
Ethnic differences seem to have a role in remission occurrence with studies showing lower remission rates among African-Americans and Hispanic children compared to European children. Environmental factors, genetics, epigenetic modifications, and possible resource inequalities could explain the ethnic variations in remission[3].
The absence of HLA DR3 or HLA DR4 and HLA DQB1*0201 have a lower likelihood of remission as shown by a few studies as these genetic polymorphisms were associated with higher C-peptide levels and beta cell concentration. Lower HbA1C levels at diagnosis showed increased chances of remission in a few studies[3,10]. Similarly, higher C-peptide levels at diagnosis favored higher rates of remission in a few studies[12] while others showed no such association[10,12].
The degree of decompensation in terms of occurrence of DKA at the onset of disease has a role in determining remission. It is believed that when patients present with DKA at onset, it corresponds to lesser remaining functional beta cells, thereby in such cases, the remission chance is also negligible[3,11,12]. Likewise, remitters have higher blood pH at diagnosis than non-remitters[10]. Presence of lesser classical symptoms at diagnosis also predicts partial remission also explainable by the above said concept. Similarly, a recent history of infection before diagnosis also predicts lesser chances of remission as infection increases the insulin requirement.
Antibody negativity at diagnosis predicts a higher chance of remission[8]. Initiating intensive glycemic control from the time of diagnosis either by insulin infusion or insulin pump favors remission as explained by “Beta cell rest theory”. According to this theory, providing exogenous insulin decreases endogenous production and provides beta cell rest which allows decreased antigen exposure and halts further autoimmune destruction. Decreased glucotoxicity on beta cells due to intensive control is an added benefit.
Interestingly, ketosis-prone diabetes (KPD) in a black, middle aged, overweight, or mildly obese, black adult male would point to an entity like Flatbush diabetes[13]. The clinical course of KPD is more like that of type 2 diabetes (controlled on diet with/without oral medications after initial intensive insulin therapy) rather than of patients with type 1 diabetes and such patients may be completely off insulin in the interim. Our patient was a 11-year non-obese male child of South Asian origin with GAD antibody positivity.
The percentage of T1DM patients attaining remission ranges from 18% to 72% whereas complete remission is extremely rare occurring in less than 3%[11]. The partial remission phase occurs around 3 mo from initiation of therapy in most studies although it may range from 3 to 12 mo[3,10,11]. Studies quote that 35%-70% of remission occurs within 3 mo of diagnosis and the rate of remission progressively declines with increasing duration of disease[8]. The duration of remission ranges from 1 month to 13 years with a mean of 7 mo[10]. Similar to factors described in the diabetes risk[14], the duration of partial remission is also affected by multiple factors. Remission is longer in those with one antibody positivity, older age of onset of diabetes, higher blood pH at diagnosis, and in boys[15,10,11]. In addition to the factors described above, others like inflammatory markers, diet, and nicotinamide are some factors which may also have a role in predicting the occurrence of remission[16]. Flowchart to detect partial remission phase in type 1 diabetes mellitus similar to a simplified model suggested for retinopathy detection in diabetic patients by Johora et al[17] (Figure 2).
The potential explanations for this third phase of the disease have been multiple. Immunological tolerance to beta cell antigens, enhanced insulin sensitivity, beta cell regeneration or recovery, and satisfactory metabolic control by insulin therapy reversing the effects of glucose toxicity have been the most plausible theories. The insulin resistance in peripheral tissues exists immediately post ketoacidosis resolution and then improves with insulin administration and this enhances insulin sensitivity which correlates with the partial remission phase. With the accepted fact of the autoimmune process with near-total destruction of beta cells leading to disease manifestation, it is also to be considered that the concurrent beta-cell turnover with net regeneration and recovery of residual cells play a significant part in remission. It has also been emphasized that hyperglycemia directly attenuates pancreatic cells’ secretory response as well as insulin-mediated glucose transport, leading to a vicious cycle of hyperglycemia and glucotoxicity which when appropriately halted by satisfactory metabolic control may transiently restore the beta cell function[18].
Patients with a history of partial remission in the past have better metabolic control later in the disease course in terms of lower HbA1C and better lipid profiles as well as reduced risk of chronic microvascular complications[10,11]. It is also seen that the hypoglycemic events are lower compared to those with no remission, because the residual b-cell assists in the maintenance of appropriate alpha cells’ glucagon secretion to avoid hypoglycemia[19].
Although various therapeutic options have been tried in different stages of diabetes with the agenda of primary or secondary prevention, the overall curiosity of the phase of partial remission rests on the predicted prospect of attempting a reversal of diabetes if intervened well in time. With autoimmunity as the main etiology in the pathogenesis of T1DM, drugs like prednisone, cyclosporine, and azathioprine which are non-specific in immunosuppression have been used. Although proving some efficacy, the concerns of long-term drug use, toxicity, and lifelong immunosuppression make their use impractical. For innate immunity, agents like etanercept and canakinumab targeting Tumor Necrosis Factor-alpha and interleukin-1-β respectively have been tried, of which the latter has failed to show any benefit. Furthermore, anti-IL-6 receptor monoclonal antibodies and anti-IL-12/23 monoclonal antibodies are also being evaluated. The role of adaptive immunity has been exploited for the use of anti-CD-20 monoclonal antibody rituximab and anti-CD3 antibody teplizumab in new-onset T1DM. T-cell modulating agents have also been studied in the quest for type 1 diabetes reversal.
With multiple existing criteria posing a challenge in diagnosis, rightly picking up the Honeymoon phase or the partial remission phase may serve as the potential window of opportunity for using novel pharmacological interventions and immunomodulating therapies to attain the elusive “Diabetes Reversal” in T1DM. Targeted immunotherapies hold the promise of a possible “cure” in this disease with complex autoimmune-mediated pathogenesis and can thus influence long-term outcomes. Identifying this phase of partial remission is thus a key for using immunomodulatory therapies in T1DM.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abu Yousuf M, Bangladesh S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Jackson RL, Body JD, Smith TE. Stabilization of the diabetic child. Am J Dis Child. 1940;59:332-337. [DOI] [Full Text] |
| 2. | Bonfanti R, Bognetti E, Meschi F, Brunelli A, Riva MC, Pastore MR, Calori G, Chiumello G. Residual beta-cell function and spontaneous clinical remission in type 1 diabetes mellitus: the role of puberty. Acta Diabetol. 1998;35:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Muhammad BJ, Swift PG, Raymond NT, Botha JL. Partial remission phase of diabetes in children younger than age 10 years. Arch Dis Child. 1999;80:367-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Jefferson IG, Smith MA, Baum JD. Insulin dependent diabetes in under 5 year olds. Arch Dis Child. 1985;60:1144-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Chase HP, MacKenzie TA, Burdick J, Fiallo-Scharer R, Walravens P, Klingensmith G, Rewers M. Redefining the clinical remission period in children with type 1 diabetes. Pediatr Diabetes. 2004;5:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Couper JJ, Haller MJ, Greenbaum CJ, Ziegler AG, Wherrett DK, Knip M, Craig ME. ISPAD Clinical Practice Consensus Guidelines 2018: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes. 2018;19 Suppl 27:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Mortensen HB, Hougaard P, Swift P, Hansen L, Holl RW, Hoey H, Bjoerndalen H, de Beaufort C, Chiarelli F, Danne T, Schoenle EJ, Aman J; Hvidoere Study Group on Childhood Diabetes. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32:1384-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Nagl K, Hermann JM, Plamper M, Schröder C, Dost A, Kordonouri O, Rami-Merhar B, Holl RW. Factors contributing to partial remission in type 1 diabetes: analysis based on the insulin dose-adjusted HbA1c in 3657 children and adolescents from Germany and Austria. Pediatr Diabetes. 2017;18:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Nielens N, Pollé O, Robert A, Lysy PA. Integration of Routine Parameters of Glycemic Variability in a Simple Screening Method for Partial Remission in Children with Type 1 Diabetes. J Diabetes Res. 2018;2018:5936360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Chobot A, Stompór J, Szyda K, Sokołowska M, Deja G, Polańska J, Jarosz-Chobot P. Remission phase in children diagnosed with type 1 diabetes in years 2012 to 2013 in Silesia, Poland: An observational study. Pediatr Diabetes. 2019;20:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Abdul-Rasoul M, Habib H, Al-Khouly M. 'The honeymoon phase' in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Pecheur A, Barrea T, Vandooren V, Beauloye V, Robert A, Lysy PA. Characteristics and determinants of partial remission in children with type 1 diabetes using the insulin-dose-adjusted A1C definition. J Diabetes Res. 2014;2014:851378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Lebovitz HE, Banerji MA. Ketosis-Prone Diabetes (Flatbush Diabetes): an Emerging Worldwide Clinically Important Entity. Curr Diab Rep. 2018;18:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Alam Miah MB, Yousuf MA. Analysis the significant risk factors on type 2 diabetes perspective of Bangladesh. Diabetes Metab Syndr. 2018;12:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 15. | Dost A, Herbst A, Kintzel K, Haberland H, Roth CL, Gortner L, Holl RW. Shorter remission period in young versus older children with diabetes mellitus type 1. Exp Clin Endocrinol Diabetes. 2007;115:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Sokołowska M, Chobot A, Jarosz-Chobot P. The honeymoon phase - what we know today about the factors that can modulate the remission period in type 1 diabetes. Pediatr Endocrinol Diabetes Metab. 2016;22:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Johora FT, Md. Mahbub-Or-Rashid, Yousuf MA, Saha TR, Ahmed B. Diabetic Retinopathy Detection Using PCA-SIFT and Weighted Decision Tree. In: Uddin, M., Bansal, J. (eds) Proceedings of International Joint Conference on Computational Intelligence. Algorithms for Intelligent Systems. Springer, Singapore. 2020. [DOI] [Full Text] |
| 18. | Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985;28:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 152] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 537] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 20. | Araszkiewicz A, Bandurska-Stankiewicz E, Budzyński A. 2019 Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clin Diabetol. 2019;8:1-95. |