Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.32
- This article has been corrected.
- See: World J Clin Cases. Dec 26, 2024; 12(36): 6950-6951
Peer-review started: August 21, 2023
First decision: November 28, 2023
Revised: November 30, 2023
Accepted: December 15, 2023
Article in press: December 15, 2023
Published online: January 6, 2024
Processing time: 134 Days and 2.8 Hours
Prostate cancer (PCa) is a widespread malignancy, predominantly affecting elderly males, and current methods for diagnosis and treatment of this disease continue to fall short. The marker Ki-67 (MKI67) has been previously demon
To explore the diagnostic and prognostic efficacy of antigens identified by MKI67 expression in PCa.
For cohort 1, the efficacy of MKI67 diagnosis was evaluated using data from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) data
In cohort 1, MKI67 expression was correlated with prostate-specific antigen (PSA), Gleason Score, T stage, and N stage. The receiver operating characteristic (ROC) curve showed a strong diagnostic ability, and the Kaplan-Meier method demon
MKI67 expression was positively associated with the Gleason Score, T stage, and N stage and showed a strong diagnostic and prognostic ability in PCa.
Core Tip: Marker Ki-67 (MKI67) has been established to correlate with the proliferation and metastasis of various malignant tumor cells, including those implicated in prostate cancer (PCa). Our objective is to validate the connection between MKI67 and the diagnosis as well as prognosis of PCa, by deploying two distinct patient cohorts from bioinformatics and clinical data. Within the bioinformatics data cohort, comprising 496 PCa tissue samples juxtaposed with 152 normal controls, we ascertained that MKI67 possesses a strong diagnostic ability for PCa along with a moderate prognostic prediction potential. Similarly, through our retrospective analysis of clinical data from 271 PCa patients, we confirmed the potent diagnostic capacity of MKI67 for PCa and its capability to predict prognosis to a certain extent.
- Citation: Song Z, Zhou Q, Zhang JL, Ouyang J, Zhang ZY. Marker Ki-67 is a potential biomarker for the diagnosis and prognosis of prostate cancer based on two cohorts. World J Clin Cases 2024; 12(1): 32-41
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/32.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.32
Prostate cancer (PCa) is the second most common malignancy worldwide[1] and has become the most prevalent diagnosed carcinoma in American males, accounting for 20% of new diagnoses[2,3]. Although PCa has the highest probability of survival, its protopathic mortality rate is approximately 10%[2]. In China, PCa has surpassed bladder and kidney cancers in terms of incidence and mortality, and is currently the most common tumor in adult urology[4]. Clinicians must correctly and appropriately diagnose and treat all types of PCa. Prostate-specific antigen (PSA) testing, prostate biopsy, and pathological diagnoses are frequently used to detect and diagnose PCa. However, given the insidious onset of PCa, early symptoms are not obvious, and the disease progresses slowly. Therefore, there is a persistent need to further improve the detection rate to achieve early diagnosis and early treatment and increase the survival rate of patients with PCa. Regarding treatment, androgen deprivation therapy in conjunction with radiotherapy is preferable to other treatments in patients with high-risk PCa[5]. Radical prostatectomy (RP) is one of the most efficacious treatments[6]. However, biochemical recurrence still occurs in approximately 15%-35% of patients after radical surgery[7]. Approximately 29% of these patients eventually experience the recurrence of clinical lesions[8]. Therefore, it is imperative to discover and validate biomarkers that can assist in disease diagnosis and prognosis, thereby aiding clinicians in improving treatment choices.
It is worth noting that all prognostic parameters commonly used in clinical practice for PCa have major limitations. For example, there exists substantial interobserver variation in the Gleason grade, even among specialized urogenital pathologists[9]. Therefore, there is a need for additional prognostic parameters that are not necessarily statistically independent of established parameters but are more reproducible and reliable than established parameters. Despite the recent advances in molecular pathology, immunohistochemistry (IHC)-based biomarkers offer substantial advantages in terms of universal, rapid, and reliable transfer to clinical routine owing to the widespread use of IHC. Increased cell proliferation is a characteristic of cancer[10]. The marker Ki-67 (MKI67) is employed to indicate the proliferation of tumor cells, including the prostate, and is closely associated with epithelial-mesenchymal transition[11]. Typically, the expression of this functionally unknown DNA-binding protein is determined using IHC[12]. Recently, several researchers have found that the proliferation of MKI67 is related to PCa prognosis[13-15]. Herein, we further evaluated the value of MKI67 expression in the diagnosis and prognosis of PCa by undertaking a comparative analysis of two cohorts, i.e., a bioinformatics database, and clinical data, and attempted to construct a nomogram to guide the assessment of the clinical prognosis of PCa.
The RNA-sequencing data in transcripts per million (TPM) format containing 496 PCa tissues and 152 normal samples from the database ID named “TCGA_GTEx-PRAD” from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) databases were uniformly processed by the Toil process from the University of California Santa Cruz (UCSC) Xena (https://xenabrowser.net/datapages/) when first downloaded[16]. Subsequently, the RNA-sequencing data and clinical information of 499 cases of PCa projects accompanied by 52 counterpart samples from database ID “TCGA-PRAD” were recorded from the TCGA database (https://portal.gdc.cancer.gov/). All data formats were converted from level 3 HTSeq fragments per kilobase per million into TPM for further analysis. All the procedures were performed in accordance with the Declaration of Helsinki (as revised in 2013).
Inclusion and exclusion criteria: Patients with PCa who underwent RP and bilateral pelvic lymph node dissection at the First Affiliated Hospital of Soochow University between January 2018 and January 2019 were analyzed. The inclusion criteria were as follows: (1) Patients with PCa at the first diagnosis; (2) Patients who had not undergone preoperative endocrine therapy, neoadjuvant radiotherapy, or chemotherapy; (3) PCa confirmed by pathology and IHC after PR in all patients; (4) PSA < 0.2 ng/mL at 6-wk postoperative recheck; and (5) Complete clinicopathological features and follow-up data were available. Exclusion criteria were as follows: (1) Combined autoimmune diseases; (2) Combined other tumors; and (3) Patients or family members who refused to participate in the clinical study. The study was approved by the Ethics Committee of the First Affiliated Hospital of Soochow University (No. 119, 2021), and all the patients signed informed consent forms before study participation.
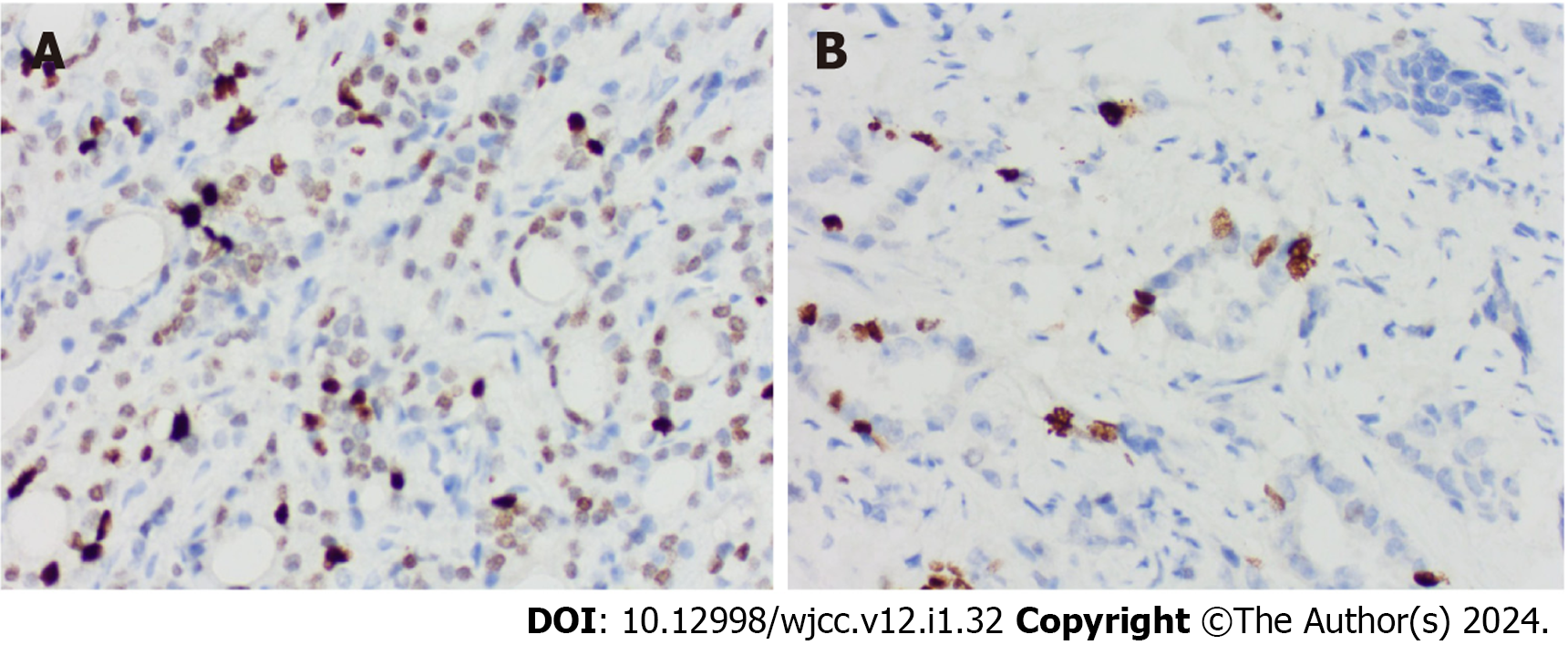
IHC: Prostate tissue specimens were collected and fixed in a 10% formaldehyde solution. The tissue samples were embedded in paraffin and sliced for immunohistochemical analysis. Paraffin slices were subsequently subjected to sequential dewaxing, hydration, washing with phosphate-buffered saline (PBS), and rinsing with citric acid buffer. To inactivate the endogenous peroxidase, a 3% hydrogen peroxide solution was applied and allowed to react for 10 min at room temperature. The slices were then washed again with PBS again before the addition of 50 μL of 5% bovine serum albumin, which was incubated at room temperature for 10 min. After adding the primary antibody against MKI67 (1:200 dilution), the slices were incubated overnight at 4 °C and washed with PBS. Following the addition of the secondary antibody, the slices were incubated at room temperature for 60 min and washed again with PBS. The sections were then treated with the DAB color reagent for 5 min, re-stained with hematoxylin, dehydrated using a graded ethanol series, and sealed with neutral resin for microscopic analysis.
Criteria for determining results: Given that MKI67 is primarily expressed in the nucleus, the appearance of pale yellow, brown-yellow, or brown granules in the nuclei at 200-fold magnification was considered positive. For a section to be judged positive, ten microscopic views at 400-fold magnification were randomly observed and scored according to the proportion of positive cells and staining intensity[17].
General information: The clinical data for patients with PCa included age, body mass index (BMI), diabetes and hypertension, preoperative PSA level, postoperative Gleason score, IHC findings, pathological staging, and follow-up time, with a follow-up cutoff date of December 31, 2021. The endpoint event was progression-free interval (PFI), which was defined as a decrease in PSA level to < 0.2 ng/mL in patients six weeks after RP, and a PSA ≥ 0.2 ng/mL was monitored during the follow-up. Herein, we included 271 patients with an average age of 69.50 ± 6.74 years, mean BMI of 23.94 ± 3.22 kg/m2, and mean preoperative PSA of 19.15 ± 16.81 ng/mL. MKI67 expression ranged from 1% to 95%, with a mean of 9.38% ± 11.87%, and biochemical recurrence was observed in 37 cases. The duration of follow-up varied from 5 to 44 mo, with an average of 34.83 ± 8.30 mo. Overall, 141 patients with hypertension and 40 with diabetes mellitus were enrolled.
Sample size calculation: Calculations using R software and verification by statisticians from the First Affiliated Hospital of Soochow University confirmed that the minimum sample size required for this study was 38. A total of 271 patients were included in this study, providing statistical validity for the conclusions of the study. The statistical code is as follows[18]: “pA = 0.04, pB = 0.26, kappa = 1, alpha = 0.05, beta = 0.20, nB = [pA × (1 - pA)/kappa + pB × (1 - pB)] × [qnorm(1-alpha/2) + qnorm(1-beta)]/(pA-pB)2, ceiling(nB) # 38, z = (pA-pB)/sqrt[pA × (1 - pA)/nB/kappa + pB × (1-pB)/nB], power = pnorm[z - qnorm(1-alpha/2)] + pnorm[-z - qnorm(1-alpha/2)]”.
Data analyses were performed using the R software (version 3.6.3; The R Foundation for Statistical Computing, Vienna, Austria). Enumeration data are shown as n (%), and measurement data are shown as the mean ± SD. For analysis, patients were divided into two PSA groups (< 4 ng/mL group and ≥ 4 ng/mL group) and six TNM groups (T2 and T3-4, N0 and N1, M0 and M1). Patients were also classified into low/medium-(≤ 7 points) and high-risk (> 7 points) Gleason score groups. Low and high MKI67 expression groups were classified based on the median value of MKI67 expression. Differences between MKI67 expression and clinicopathological features were compared using the Wilcoxon rank-sum test [ggplot2 (version 3.3.3)]. The receiver operating characteristic (ROC) curve [pROC (version 1.17.0.1)] was used to determine the efficacy of MKI67 expression diagnosis. Kaplan-Meier method [survminer (version 0.4.9)] was performed to reveal the survival rates of PFI for patients with PCa. A time-ROC curve was used to predict the 1-, 2-, and 3-year survival rates of PFI for PCa [timeROC (version 0.4)]. The correlation between genetic and clinicopathological features was evaluated using Cox regression in both univariate and multivariate analyses (survival version 3.2-10). A nomogram was constructed using the rms package (version 6.2-0). A at P < 0.05 was deemed statistically significant, and not significant at P > 0.05.
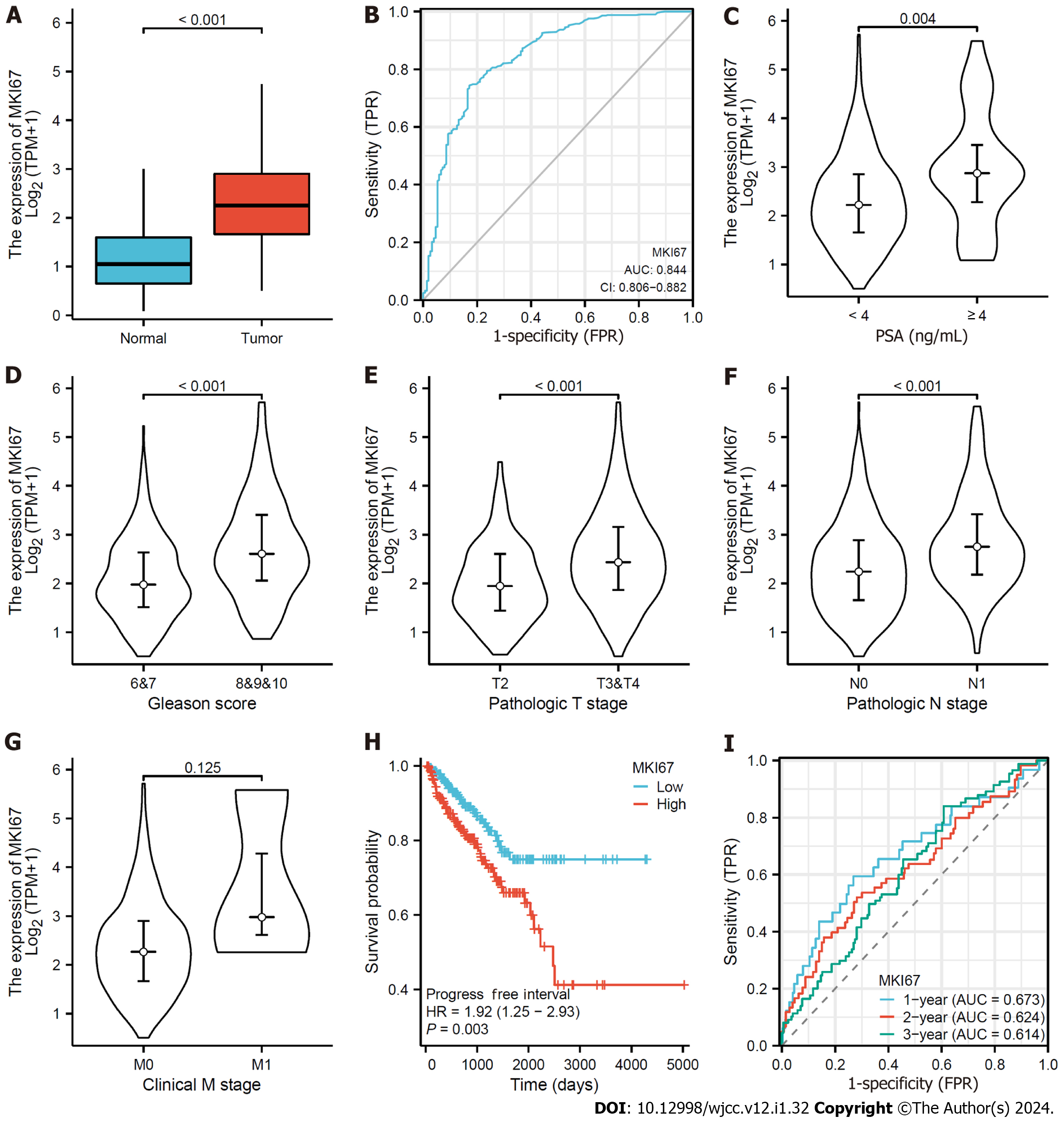
We found that MKI67 expression exceeded normal levels in PCa tissues (Figure 1A). ROC curves were then plotted to analyze the effect of MKI67 expression in distinguishing PCa tissues from normal samples, confirming the moderate ability of MKI67 expression to diagnose PCa, with an area under the curve (AUC) of 0.844 (Figure 1B). Next, the data were classified into two groups based on the median value of MKI67 expression. The association between MKI67 expression and clinicopathological features, including PSA, Gleason Score, T Stage, N Stage, and M Stage, were evaluated, and PSA, Gleason Score, T Stage, and N Stage were found to be positively associated (P < 0.05) (Figures 1C-G). According to the Kaplan-Meier method, the MKI67 high-expression group exhibited a lower PFI survival rate than the MKI67 low-expression group [hazard ratio (HR) = 1.92, P = 0.003] (Figure 1H). Moreover, we plotted a time-ROC curve to examine the capability of MKI67 expression to predict the 1-, 2-, and 3-year survival rates of PFI, resulting in a weak prognosis with AUC values of 0.672, 0.624, and 0.613, respectively.
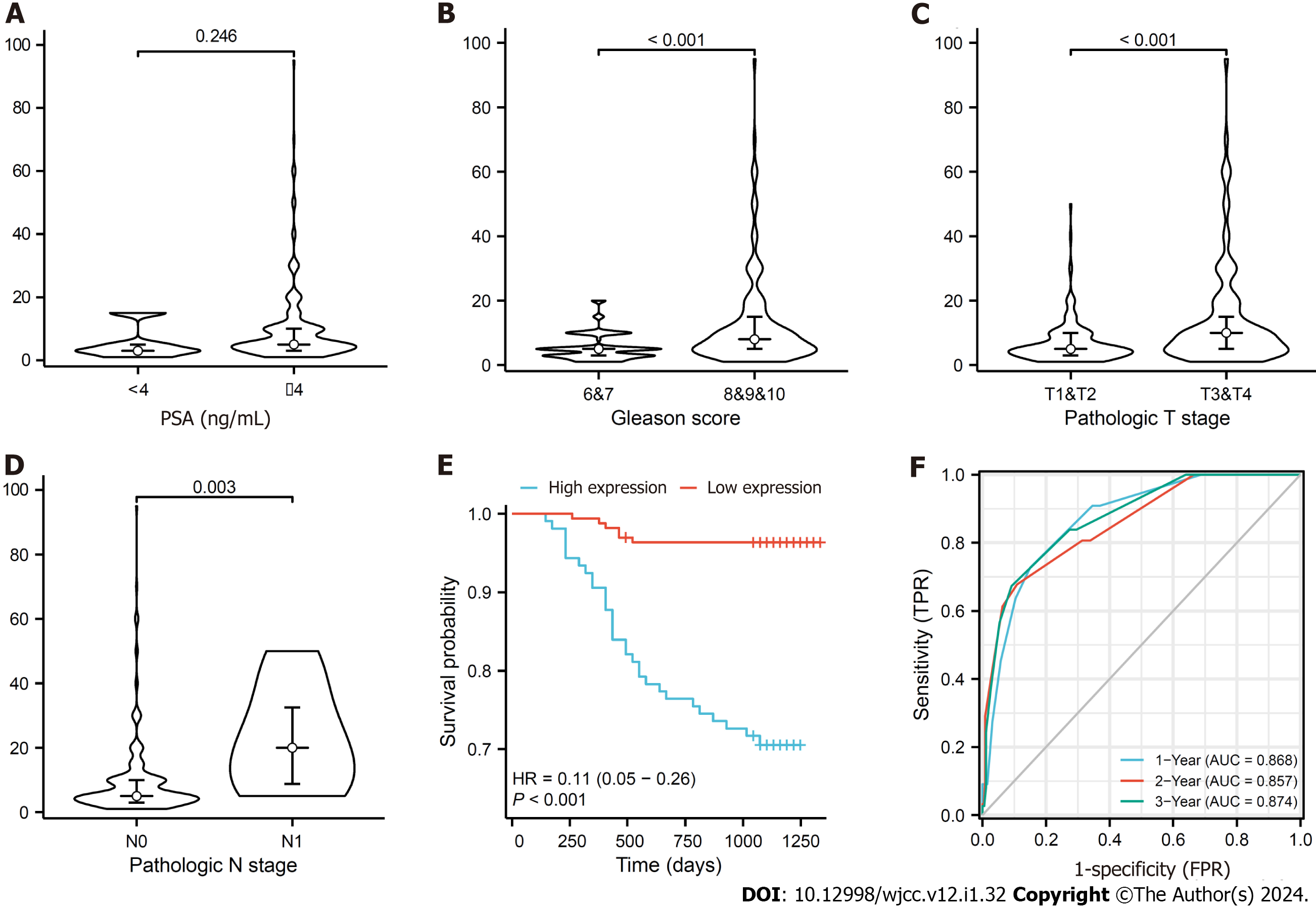
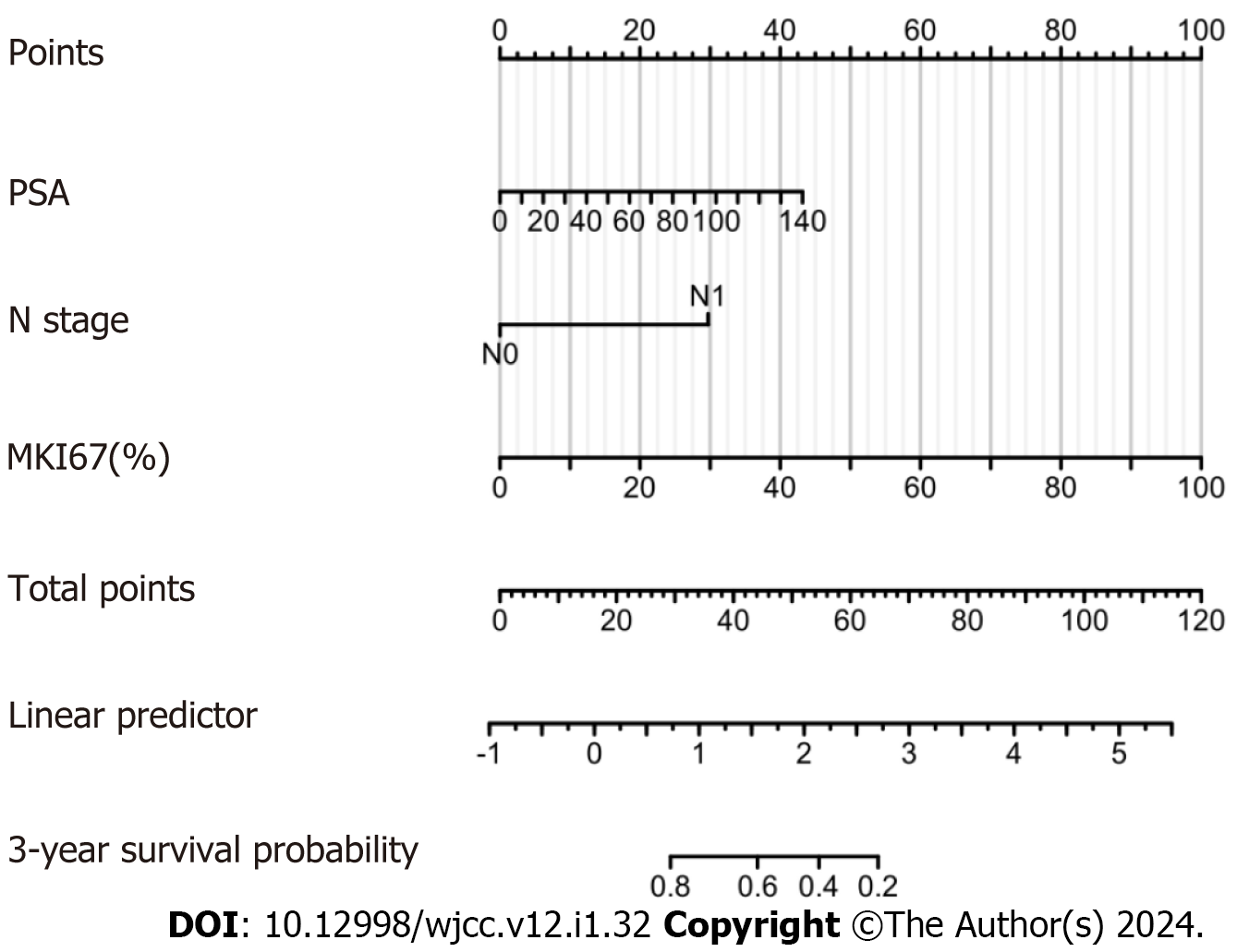
The expression of MKI67 in prostate tissue is shown in Figure 2. We found that MKI67 expression was significantly and positively associated with Gleason score, T stage, and N stage (P < 0.05) but negatively correlated with PFI (P < 0.001) (Figure 3A-D). The Kaplan-Meier method showed that the MKI67 high-expression group had a lower PFI survival rate in than the low-expression group (HR = 0.11, P < 0.001) (Figure 3E). Moreover, the time-ROC curves showed that MKI67 expression had robust prognostic power in PCa (1 year, AUC = 0.868; 2 years, AUC = 0.857; 3 years, AUC = 0.874) (Figure 3F). Multivariate Cox regression analysis was performed to identify risk factors, including PSA level, N stage, and MKI67 expression (P < 0.05) (Table 1). Based on these results, a nomogram for predicting the 3-year PFI was established for clinical use (Figure 4).
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | ||
| Age | 271 | 0.965 (0.921-1.012) | 0.140 | ||
| HBP | 271 | ||||
| Yes | 141 | Reference | |||
| No | 130 | 0.905 (0.474-1.729) | 0.763 | ||
| DM | 271 | ||||
| Yes | 40 | Reference | |||
| No | 231 | 0.873 (0.364-2.093) | 0.761 | ||
| PSA | 271 | 1.021 (1.008-1.035) | 0.002 | 1.017 (1.000-1.033) | 0.045 |
| T stage | 271 | ||||
| T3&T4 | 98 | Reference | |||
| T1&T2 | 173 | 0.349 (0.181-0.673) | 0.002 | 0.824 (0.373-1.819) | 0.631 |
| N stage | 271 | ||||
| N0 | 263 | Reference | |||
| N1 | 8 | 10.022 (3.885-25.850) | < 0.001 | 5.165 (1.756-15.194) | 0.003 |
| M stage | 271 | ||||
| M0 | 270 | Reference | |||
| M1 | 1 | 73.441 (8.365-644.773) | < 0.001 | 0.912 (0.081-10.244) | 0.941 |
| Gleason score | 271 | ||||
| 6&7 | 202 | Reference | |||
| 8&9&10 | 69 | 3.940 (2.063-7.525) | < 0.001 | 1.024 (0.401-2.615) | 0.961 |
| MKI67(%) | 271 | 1.057 (1.044-1.071) | < 0.001 | 1.056 (1.037-1.074) | < 0.001 |
PCa is well-known to pose a serious threat to the lives and health of males. Although the prevalence of PCa in China is substantially lower than that in Western countries, it is growing annually owing to changes in diet and an aging population. Currently, the commonly used diagnostic approaches for PCa include PSA, prostate puncture biopsy, and pathological diagnosis, with prognosis mostly based on the PSA level, TNM stage, and Gleason score. However, serum PSA levels are markedly limited in the diagnosis of early PCa, which may result in the overdiagnosis or overtreatment of patients and other situations[19]. Prostate biopsy has certain requirements regarding the tumor sampling site and the physician’s operating technique, and the potential for misdiagnoses or missed diagnoses cannot be excluded. Furthermore, owing to the heterogeneity of tumors, a single prognostic factor appears to be insufficient to predict patient prognosis. Molecular markers can be used to reduce overtreatment. Despite the widespread interest in different biomarkers and accumulating evidence, especially in the genomic signature era[20], a limited number of biomarkers have survived the test of reproducibility to enter clinical application.
Compared with other prostate biomarkers, MKI67 is an attractive biomarker owing to its universal applicability and reproducibility[21]. MKI67 is widely used in routine practice and is measured in a semi-quantitatively manner with a low failure rate. Moreover, MKI67 is highly expressed in circulating cells but strongly downregulated in resting G0 cells[22], making MKi67 a clinically important marker of cell proliferation for grading several cancers. MKI67 has demonstrated a good prognostic value in breast, lung, and cervical cancers[23-25]. In addition, because the PCa grading system does not consider the cell proliferation rate, detecting the proliferation rate could yield additional prognostic information. Several studies have shown that MKI67 is a promising early diagnostic and prognostic biomarker for PCa[14,26-28]. However, there was substantial heterogeneity among the studies, including different endpoints, cohort types, sample sizes, and cutoff values, as well as a lack of further assessment of MKI67 expression for PCa diagnosis and prognosis. Notably, the results of the current study have several important implications. First, analyzing and validating both cohorts, we found that MKI67 had a high diagnostic value for PCa, with markedly higher expression in PCa tissues than that in normal tissues and positively related to the Gleason score, T stage, and N stage, but not the M stage; these findings are consistent with those reported previously[29]. Collectively, these findings suggest that MKI67 protein expression is associated with aggressiveness and metastasis. Moreover, our results suggest that immunohistochemical techniques can be used to detect the expression of markers to locate the clinical stage of patients, facilitating the development of more appropriate treatment plans to increase the benefits for patients with PCa and improve their survival prognosis. Second, MKI67 expression negatively correlated with PFI in the latest clinical data validation, which is consistent with findings reported previously[27,30-33]. In addition, MKI67 expression was strongly associated with the prediction of 3-year PFI, facilitating the identification of patients with poor prognosis in the clinic and the tailoring of intensive treatment strategies within a reasonable clinical turnaround time. Third, Cox regression analysis identified PSA level, N stage, and MKI67 expression as risk factors affecting PCa prognosis and established a nomogram to predict the 3-year PFI for clinical application.
Although several independent studies and accumulating evidence persistently propose new biomarkers, MKI67, the most widely studied molecular biomarker in PCa, should be investigated in prospective studies to establish personalized cancer treatment and clinically effective molecular prognosis. In the future, we plan to further elucidate the mechanistic pathways underlying the role of MKI67 in the development of PCa to identify potential targets for the next step in targeted therapy.
This study had some limitations. First, the sample volume of the clinical cohort in this study was small, including only one case with the M stage, although MKI67 expression did not correlate with the M stage in the bioinformatics analysis. Age, clinical stage, Gleason score, and PSA levels have been found to affect the prognosis of patients with PCa[34]. Age was not associated with patient prognosis in the univariate analysis in this study, whereas PSA level, N stage, and MKI67 expression were related to prognosis in the Cox multifactorial analysis, which may be linked to the small sample size of our study. Meanwhile, the predictive capacity of MKI67 expression on the 3-year PFI rate tended to differ between the two cohorts; therefore, the observed results need to be validated in multicenter trials. Second, the present study showed a positive association between MKI67 expression and well-established prognostic variables, such as PSA, Gleason score, and disease stage; however, our findings were insufficient to precisely quantify the extent of the relationship between MKI67 and outcome. Finally, although we presented a 3-year PFI, the follow-up period was short, and no prospective follow-up was conducted; therefore, other hard endpoints could not be stated, which could have biased the results. Despite limitations, our findings could facilitate further investigations among patients with PCa, both prospectively and as archival materials.
By comparatively analyzing bioinformatics databases and clinical data, we found that MKI67 expression was positively correlated with the Gleason score, T stage, and N stage, which would help in establishing the clinical stage of patients and thus develop more appropriate treatment plans. MKI67 is a highly effective diagnostic and prognostic parameter for PCa, and a nomogram for predicting the 3-year PFI was established to facilitate its clinical application. To overcome the limitations of this study, prospective validation of our findings is required to verify their clinical validity.
Prostate cancer (PCa) represents a serious health threat to elderly men as a malignant tumor. Presently, methodologies available for the diagnosis and treatment of PCa are, regrettably, still lacking. Given that marker Ki-67 (MKI67) has been linked with the proliferation and metastasis of PCa cells, it holds substantial clinical meaning to apply both bioin
By establishing the link between MKI67 and PCa, we pave the way for innovative molecular targets and therapeutic approaches for the future diagnosis and treatment of PCa.
To investigate the efficacy of antigen identified by MKI67 expression in the diagnosis and prognosis of PCa.
This study undertook a retrospective analysis utilizing both bioinformatics and clinical data. The association between MKI67 expression and various clinicopathological features was assessed using the Wilcoxon rank-sum test. The diagnostic efficacy of MKI67 expression was conveyed via the receiver operating characteristic (ROC) curve. The Kaplan-Meier method was employed to elucidate the progression-free interval (PFI) survival rates in PCa patients. Meanwhile, the time-ROC curve was utilized to predict the 1-, 2-, and 3-year survival rates of the PFI in PCa. Both univariate and multivariate Cox regressions were performed to evaluate the relationship between genetic and clinicopathological characteristics. Lastly, a nomogram was constructed using the rms package.
In the bioinformatics data, MKI67 expression demonstrated a significant correlation with prostate-specific antigen (PSA), Gleason Score, T stage, and N stage. The ROC curve pointed to a robust diagnostic capacity, while the Kaplan-Meier method indicated that MKI67 expression had a negative correlation with PFI. Moreover, the time-ROC curve exhibited a modest prognostic capability of MKI67 in PCa. In the clinical data, MKI67 expression was significantly tied to the Gleason score, T stage, and N stage, and it was negatively linked to PFI. The time-ROC curve displayed a more substantial prognosis for MKI67 in PCa. A multivariate COX regression analysis was conducted to pinpoint risk factors, which included PSA, N stage, and MKI67 expression. A nomogram was subsequently developed to project 3-year PFI.
Through comparative analysis of bioinformatics databases and clinical data, MKI67 expression positively correlated with Gleason score and T and N stages, aiding in pinpointing patient’s clinical stages for better treatment planning. MKI67 serves as an efficient diagnostic and prognostic tool for PCa, and a nomogram was constructed for predicting 3-year PFI, enhancing its clinical utility.
In light of the limitations of this study, future prospective validation is necessitated to confirm the clinical relevance of MKI67 in relation to PCa.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He L, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
| 1. | Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, Bryce A, Chapin B, Cheng HH, D'Amico AV, Desai N, Dorff T, Eastham JA, Farrington TA, Gao X, Gupta S, Guzzo T, Ippolito JE, Kuettel MR, Lang JM, Lotan T, McKay RR, Morgan T, Netto G, Pow-Sang JM, Reiter R, Roach M, Robin T, Rosenfeld S, Shabsigh A, Spratt D, Teply BA, Tward J, Valicenti R, Wong JK, Berardi RA, Shead DA, Freedman-Cass DA. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J Natl Compr Canc Netw. 2022;20:1288-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 2. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15476] [Article Influence: 2579.3] [Reference Citation Analysis (2)] |
| 3. | Pinsky PF, Parnes H. Screening for Prostate Cancer. N Engl J Med. 2023;388:1405-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 4. | Marsh HP, Bowler IC, Watson CJ. Successful treatment of Rhodococcus equi pulmonary infection in a renal transplant recipient. Ann R Coll Surg Engl. 2000;82:107-108. [PubMed] |
| 5. | Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E, Buckley DI, Griffin JC, Cookson MS. Updates to Advanced Prostate Cancer: AUA/SUO Guideline (2023). J Urol. 2023;209:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 130] [Reference Citation Analysis (0)] |
| 6. | Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, Jarrard DF, Kibel AS, Morgan TM, Morgans AK, Oh WK, Resnick MJ, Zietman AL, Cookson MS. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J Urol. 2021;205:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 7. | Brajtbord JS, Leapman MS, Cooperberg MR. The CAPRA Score at 10 Years: Contemporary Perspectives and Analysis of Supporting Studies. Eur Urol. 2017;71:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol. 2003;170:1872-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Egevad L, Delahunt B, Yaxley J, Samaratunga H. Evolution, controversies and the future of prostate cancer grading. Pathol Int. 2019;69:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Schiliro C, Firestein BL. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 327] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 11. | Lindsay CR, Le Moulec S, Billiot F, Loriot Y, Ngo-Camus M, Vielh P, Fizazi K, Massard C, Farace F. Vimentin and Ki67 expression in circulating tumour cells derived from castrate-resistant prostate cancer. BMC Cancer. 2016;16:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Karakas C, Nead MA, Velez MJ. Diffuse pulmonary meningotheliomatosis with pan-TRK expression by immunohistochemistry: a novel finding and potential pitfall. Diagn Pathol. 2023;18:22. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Wang J, Yan K, Lin J, Zheng Z, Bi J. Identification of core genes associated with prostate cancer progression and outcome via bioinformatics analysis in multiple databases. PeerJ. 2020;8:e8786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Hammarsten P, Josefsson A, Thysell E, Lundholm M, Hägglöf C, Iglesias-Gato D, Flores-Morales A, Stattin P, Egevad L, Granfors T, Wikström P, Bergh A. Immunoreactivity for prostate specific antigen and Ki67 differentiates subgroups of prostate cancer related to outcome. Mod Pathol. 2019;32:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 15. | Berlin A, Castro-Mesta JF, Rodriguez-Romo L, Hernandez-Barajas D, González-Guerrero JF, Rodríguez-Fernández IA, González-Conchas G, Verdines-Perez A, Vera-Badillo FE. Prognostic role of Ki-67 score in localized prostate cancer: A systematic review and meta-analysis. Urol Oncol. 2017;35:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, Pfeil J, Narkizian J, Deran AD, Musselman-Brown A, Schmidt H, Amstutz P, Craft B, Goldman M, Rosenbloom K, Cline M, O'Connor B, Hanna M, Birger C, Kent WJ, Patterson DA, Joseph AD, Zhu J, Zaranek S, Getz G, Haussler D, Paten B. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol. 2017;35:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 865] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 17. | Zhang Z, Zhou Q, Ouyang J, Pu J, Hou J, Zhang J. Expression and clinical significance of interleukin-9 in renal tumors. Transl Androl Urol. 2020;9:2657-2664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Wang X, Ji X. Sample Size Estimation in Clinical Research: From Randomized Controlled Trials to Observational Studies. Chest. 2020;158:S12-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 19. | Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, Davis M, Peters TJ, Turner EL, Martin RM, Oxley J, Robinson M, Staffurth J, Walsh E, Bollina P, Catto J, Doble A, Doherty A, Gillatt D, Kockelbergh R, Kynaston H, Paul A, Powell P, Prescott S, Rosario DJ, Rowe E, Neal DE; ProtecT Study Group. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1896] [Article Influence: 210.7] [Reference Citation Analysis (0)] |
| 20. | Spratt DE, Zumsteg ZS, Feng FY, Tomlins SA. Translational and clinical implications of the genetic landscape of prostate cancer. Nat Rev Clin Oncol. 2016;13:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Maia R, Santos GAD, Reis S, Viana NI, Pimenta R, Guimarães VR, Recuero S, Romão P, Leite KRM, Srougi M, Passerotti CC. Can we use Ki67 expression to predict prostate cancer aggressiveness? Rev Col Bras Cir. 2022;49:e20223200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Sun X, Kaufman PD. Ki-67: more than a proliferation marker. Chromosoma. 2018;127:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 581] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 23. | Ishibashi N, Nishimaki H, Maebayashi T, Hata M, Adachi K, Sakurai K, Masuda S, Okada M. Changes in the Ki-67 labeling index between primary breast cancer and metachronous metastatic axillary lymph node: A retrospective observational study. Thorac Cancer. 2019;10:96-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Atari M, Imai K, Nanjo H, Wakamatsu Y, Takashima S, Kurihara N, Kuriyama S, Suzuki H, Demura R, Harata Y, Hiroshima Y, Sato Y, Nomura K, Minamiya Y. Rapid intraoperative Ki-67 immunohistochemistry for lung cancer using non-contact alternating current electric field mixing. Lung Cancer. 2022;173:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Li C, Wu H, Guo L, Liu D, Yang S, Li S, Hua K. Single-cell transcriptomics reveals cellular heterogeneity and molecular stratification of cervical cancer. Commun Biol. 2022;5:1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 26. | Caputo A, D'Antonio A, Memoli D, Sabbatino F, Altieri V, Zeppa P. Ki67 in Gleason Pattern 3 as a Marker of the Presence of Higher-Grade Prostate Cancer. Appl Immunohistochem Mol Morphol. 2021;29:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Zhang Y, Chen J, Fang W, Liang K, Li X, Zhang F, Pang Y, Fang G, Wang X. Kaempferol suppresses androgen-dependent and androgen-independent prostate cancer by regulating Ki67 expression. Mol Biol Rep. 2022;49:4607-4617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Berney DM, Gopalan A, Kudahetti S, Fisher G, Ambroisine L, Foster CS, Reuter V, Eastham J, Moller H, Kattan MW, Gerald W, Cooper C, Scardino P, Cuzick J. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study. Br J Cancer. 2009;100:888-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Porter CR, Suardi N, Kodama K, Capitanio U, Gibbons RP, Correa R, Jeldres C, Perrotte P, Montorsi F, Karakiewicz PI. A nomogram predicting metastatic progression after radical prostatectomy. Int J Urol. 2008;15:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Fantony JJ, Howard LE, Csizmadi I, Armstrong AJ, Lark AL, Galet C, Aronson WJ, Freedland SJ. Is Ki67 prognostic for aggressive prostate cancer? A multicenter real-world study. Biomark Med. 2018;12:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Kammerer-Jacquet SF, Ahmad A, Møller H, Sandu H, Scardino P, Soosay G, Beltran L, Cuzick J, Berney DM. Ki-67 is an independent predictor of prostate cancer death in routine needle biopsy samples: proving utility for routine assessments. Mod Pathol. 2019;32:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Green WJ, Ball G, Hulman G, Johnson C, Van Schalwyk G, Ratan HL, Soria D, Garibaldi JM, Parkinson R, Hulman J, Rees R, Powe DG. KI67 and DLX2 predict increased risk of metastasis formation in prostate cancer-a targeted molecular approach. Br J Cancer. 2016;115:236-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Tretiakova MS, Wei W, Boyer HD, Newcomb LF, Hawley S, Auman H, Vakar-Lopez F, McKenney JK, Fazli L, Simko J, Troyer DA, Hurtado-Coll A, Thompson IM Jr, Carroll PR, Ellis WJ, Gleave ME, Nelson PS, Lin DW, True LD, Feng Z, Brooks JD. Prognostic value of Ki67 in localized prostate carcinoma: a multi-institutional study of >1000 prostatectomies. Prostate Cancer Prostatic Dis. 2016;19:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Jang WS, Yoon CY, Kim KH, Kang YJ, Shin SJ, Cho NH, Lee JY, Cho KS, Ham WS, Rha KH, Hong SJ, Choi YD. Prognostic Significance of Vas Deferens Invasion After Radical Prostatectomy in Patients with Pathological Stage T3b Prostate Cancer. Ann Surg Oncol. 2017;24:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |