Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.224
Peer-review started: November 5, 2023
First decision: November 22, 2023
Revised: November 30, 2023
Accepted: December 20, 2023
Article in press: December 20, 2023
Published online: January 6, 2024
Processing time: 57 Days and 19.1 Hours
Kidney transplantation is the best option for patients with end-stage renal disease. However, the need for lifelong immunosuppression results in renal transplant recipients being susceptible to various infections. Rhodococcus equi (R. equi) is a rare opportunistic pathogen in humans, and there are limited reports of infection with R. equi in post-renal transplant recipients and no uniform standard of treat
Here, we present the case of a 25-year-old man who was infected with R. equi 21 mo after renal transplantation. Although the clinical features at the time of presentation were not specific, chest computed tomography (CT) showed a large volume of pus in the right thoracic cavity and right middle lung atelectasis, and fiberoptic bronchoscopy showed an endobronchial mass in the right middle and lower lobe orifices. Bacterial culture and metagenomic next-generation sequen
Infection with R. equi in renal transplant recipients is rare and complex, and the clinical presentation lacks specificity. Elaborate antibiotic therapy is required, and adequate abscess drainage and surgical excision are necessary. Given the recurrent nature of R. equi, patients need to be followed-up closely.
Core Tip: Infection with Rhodococcus equi (R. equi) is rare in renal transplant recipients. To date, no cases of pleural empyema and endobronchial mass have been reported in renal transplant recipients infected with R. equi. We report the diagnosis and management of a renal transplant recipient infected with R. equi at 21 mo postoperatively and incorporate a review of the literature to illustrate the characteristics of R. equi infection in renal transplant recipients.
- Citation: Liang GF, Chao S, Sun Z, Zhu KJ, Chen Q, Jia L, Niu YL. Pleural empyema with endobronchial mass due to Rhodococcus equi infection after renal transplantation: A case report and review of literature. World J Clin Cases 2024; 12(1): 224-231
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/224.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.224
Kidney transplantation is an optimal choice for patients with end-stage renal disease (ESRD), which improves quality of life and prolongs life expectancy compared to other treatments[1,2]. To prevent graft rejection, kidney transplant recipients need to take immunosuppressive drugs long-term, and the use of immunosuppressive drugs can cause them to be susceptible to various infections. The development of rare, hard-to-diagnose infections after transplantation may be a serious threat to survival for kidney transplant recipients[3]. Rhodococcus equi (R. equi) is a zoonotic bacterium that causes infections in a wide variety of animals such as horses, cattle, pigs, and sheep[4]. R. equi is a rare opportunistic pathogen in humans that occurs mainly in immunocompromised populations such as those with human immunodeficiency virus (HIV)-infection, malignancy, and/or organ transplant recipients[5]. As immunosuppressive regimens used in organ transplantation therapy become more complex and widespread, further understanding of this pathogen is critical. We report the diagnosis and treatment of a case of pleural empyema and endobronchial mass caused by R. equi infection in a renal transplant recipient 21 mo after the transplantation. We also summarize the characteristics and treatment of R. equi infection in renal transplant recipients through a comprehensive literature review to provide clinicians with some experience in the diagnosis and treatment of this rare disease.
Cough and sputum for one week, and fever, chest pain, and chest tightness for one day.
A 25-year-old man underwent allogeneic kidney transplantation in May 2020 for ESRD secondary to chronic glomerulonephritis. The kidney was obtained from a brain-dead donor. His immune-induction regimen was basiliximab and anti-human T-cell porcine immunoglobulin, and his immunization maintenance regimen was tacrolimus (0.5 mg, bid), mycophenolate mofetil (0.5 g, bid), and methylprednisolone (16 mg, qd). One month after surgery, the patient experienced acute rejection, which improved after steroid pulse therapy, and he did not experience delayed graft function. Postoperatively, cotrimoxazole was given to prevent Pneumocystis carinii pneumonia, voriconazole to prevent fungal infections, and ganciclovir to prevent viral infections. The other medications he took were nifedipine and ir
The patient had a history of hypertension with blood pressure levels of up to 182/90 mmHg managed through the long-term use of controlled-release nifedipine and irbesartan tablets. He denied any history of diabetes mellitus, coronary artery disease, or tuberculosis.
The patient was a smoker for 5 years, but had now quit smoking and had no history of alcohol consumption. He denied any history of exposure to tuberculosis. He also denied any recent contact with farm animals such as horses, pigs, and cows and had not recently visited any farm. He did not have any clinically relevant family history.
The initial checkup indicated that the temperature was 38.2 °C, pulse 112/min, respiration 23/min, and blood pressure 144/112 mmHg. Physical examination showed that his general condition was poor: Respiratory movements were slightly rapid, right lower lung fibrillation was weakened, there was no pleural friction, turbidity was noted on percussion in the right lower lungs, right lung respiratory sounds were low. Cardiac and abdominal examination showed no abnormality, and both lower limbs were mildly swollen.
White blood cell count, 9.79 × 109/L; neutrophil percentage, 77.8%; absolute lymphocyte value, 1.27; lymphocyte percentage, 13.00%; absolute monocyte value, 0.88; and monocyte percentage, 9.00%. Furthermore, serum creatinine was 526.40 μmol/L, procalcitonin was 3.40 ng/mL, and ultrasensitive C-reactive protein was > 20 mg/L.
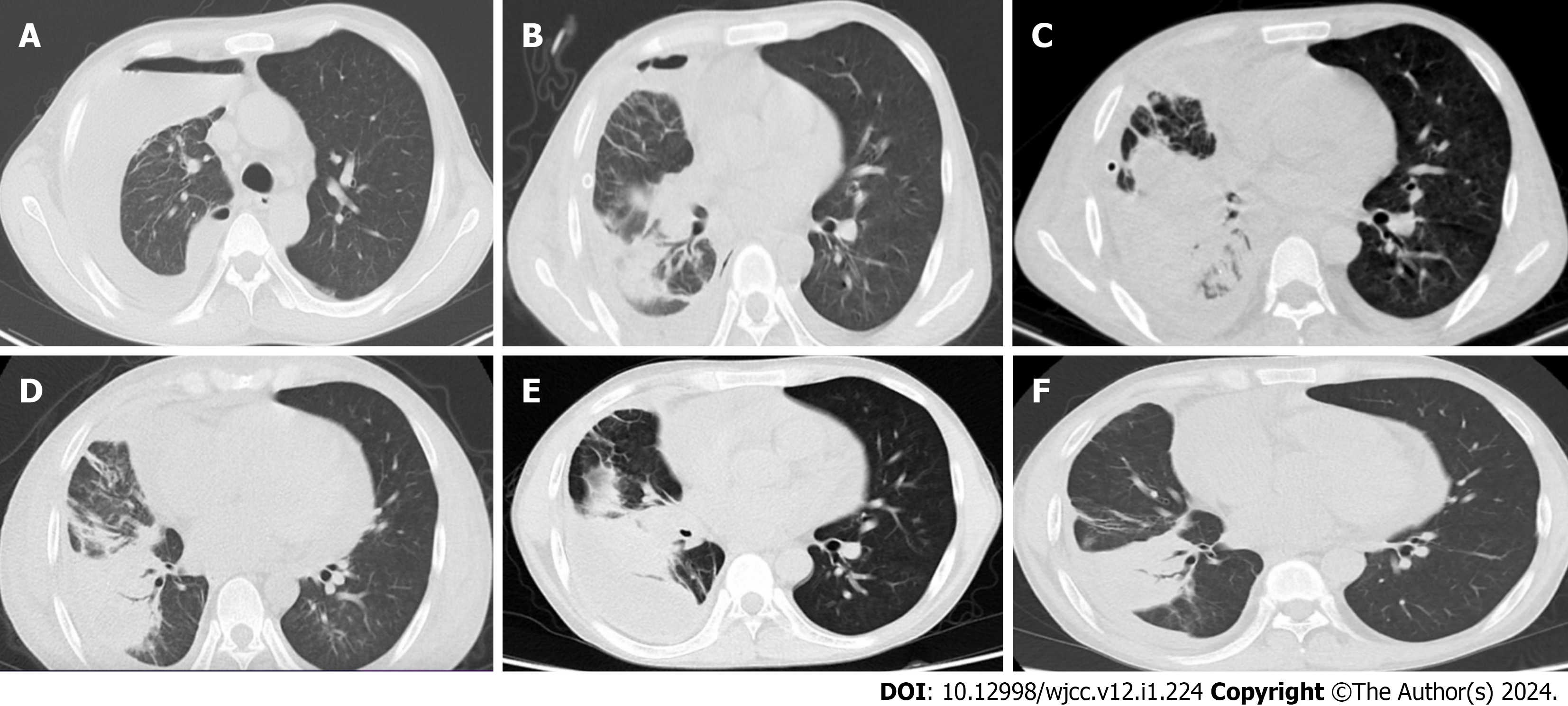
Chest CT suggested the following: Right-sided pneumothorax with multiple fluid within it; right lung with multiple exudates, and right lower lobe abscesses (Figure 1A).
Pleural empyema and endobronchial mass.
Yellow pus was withdrawn during diagnostic thoracentesis at the time of the patient’s admission. Combined with the chest CT findings, the patient was initially considered to have a pleural empyema, and closed thoracic drainage was performed. To analyze the pathogenic bacteria, the drainage fluid was subjected to bacterial and fungal cultures, acid-fast staining, and Gram’s staining, as well as metagenomic next-generation sequencing (mNGS) sequencing. Given the severe infection, the patient was given a suspension of immunosuppressants and empirical anti-infective therapy with piperacillin-tazobactam (4.5 g, q8h). After 3 d of treatment, the patient’s symptoms did not improve, his highest recorded temperature was 39.0 °C, and his cough and sputum symptoms were worse than before, with yellow mucus in the sputum. Bacterial culture and mNGS sequencing of the drainage fluid were suggestive of R. equi infection. Our department had no experience in the treatment of this disease owing to the rarity of this organism, and there are no relevant treatment guidelines as yet for reference. Therefore, after reviewing the literature and referring to The Sanford Guide to Antimicrobial Therapy, we adjusted the antibiotics to vancomycin (0.5 g, bid) and azithromycin (0.5 g, qd) for intravenous infusion. Afterwards, the patient’s clinical symptoms improved, and the cough and sputum were better than before with only occasional fever. After 10 d of antibiotic-altered treatment, the patient’s inflammatory indices and renal function improved, and the white blood cell count reduced to 5.64 × 109/L, neutrophil percentage was 83.70%, and the serum creatinine was 366.00 μmol/L. Chest CT suggested improvement of the right pleural empyema and right lower lobe abscess, but development of right middle lobe atelectasis (Figure 1B). Drainage fluid culture was performed again, and the culture result was still R. equi. The patient’s clinical symptoms resolved, and the antibiotic regimen was switched to ciprofloxacin (200 mg, qd) and azithromycin (0.5 g, qd) for intravenous infusion. After a month of treatment, the patient’s cough and sputum improved; he had no fever, chest pain, or chest tightness; and the bacterial culture of drainage fluid was negative. Therefore, the treatment plan was simplified to oral ciprofloxacin and azithromycin. The patient was restarted on low-dose immunosuppression [mycophenolate mofetil 180 mg (bid), tacrolimus 0.5 mg (qd)]. However, chest CT suggested that the right lung middle lobe atelectasis was worse than before (Figure 1C). In view of the patient’s right middle lung atelectasis and right lower lung abscess, after multidisciplinary consultation with thoracic surgery, infectious disease, respiratory medicine, and clinical pharmacy, fiberoptic bronchoscopy, instead of surgery, was recommended to identify the cause of lung atelectasis. Interestingly, fiberoptic bronchoscopy revealed a nascent mass in the right middle and lower lobe orifices and symptomatic hypertrophic luminal narrowing of the right pulmonary mucosa (Figure 2A). The mass was biopsied, and histopathology suggested that the mucosa of the right middle and lower lobes of the mouth showed chronic inflammation with fibrous hyperplasia in the lamina propria, with a large number of histiocyte-like cells, but no malignant tumor cells (Figures 2B and C). Immunohistochemistry suggested chronic inflammation of the right middle and lower lobe orifice mucosa with fibrous granulation tissue proliferation (Figure 2D). Taken together, the pulmonary atelectasis was considered to be due to this nascent mass, and a bronchoscopic right middle and lower lobe hyperplastic sarcoidectomy, balloon dilatation, and cryosurgery were performed on the 44th d after admission.
The patient’s condition improved, and chest CT suggested the following: The right pleural encapsulated effusion was absorbed compared to before; right lower lobe abscess and multiple right lung exudates were reduced compared to before; and right middle lobe atelectasis was the same as before (Figure 1D). He was discharged on April 20, 2022. He was instructed to continue oral anti-infective treatment with ciprofloxacin and azithromycin for 6 months after discharge.
Two weeks after the patient was discharged from the hospital, his chest CT suggested that the right-sided encapsulated effusion was significantly larger than before (Figure 1E), and he was readmitted to the hospital on May 6, 2022. He had no symptoms of fever or cough and sputum. Closed drainage of the right thoracic cavity was performed again and yellow-green pus was drained. Bacterial cultures of the drainage fluid were negative, so mNGS of the drainage fluid was performed, which suggested that this purulent infection was still dominated by R. equi. Ciprofloxacin infusion and oral azithromycin were given. After treatment, the right-sided pleural encapsulated effusion was significantly reduced from the previous level (Figure 1F). The patient was discharged on May 25, 2022. His chest CT performed one month after discharge suggested a continued decrease in the right-sided pleural encapsulated effusion.
We searched the Embase, PubMed, Web of Science, and Cochrane Library databases for articles related to infection with R. equi after renal transplantation in humans, from 1977 to the present. Information collected included year of publication, age, sex, time interval between kidney transplantation and R. equi infection, disease diagnosis, mode of diagnosis of R. equi infection, treatment, and outcomes. After searching, a total of 17 articles were available, all of which were case reports. Among them, 10 patients were male and 7 were female. The age distribution ranged from 38 to 67 years, and the infections occurred 5-228 mo after transplantation. Most of them had pulmonary involvement, presenting as lung masses, cavities, lung abscesses, and pleural effusions. A small number of concurrent cases presented with subcutaneous abscesses, brain abscesses, and osteomyelitis. Confirmation of the diagnosis of R. equi included bacterial culture of blood, sputum, pus, and broncho-alveolar lavage; fine needle aspiration cytology; biopsy of diseased tissue; and 16s rRNA sequencing. All were treated with a combination of two or more antibiotics and some cases were treated with surgical resection. Twelve patients improved after treatment, four died (one of whom died after recurrence), and one had recurrence (which improved after treatment). Details of these cases are summarized in Table 1.
| Number | Year | Age | Sex | Post-transplant (mo) | Radiographic diagnosis | Bacteriological diagnosis | Treatment | Outcome | Ref. |
| 1 | 2019 | 67 | F | 7 | Right perihilar mass | BLA culture; TB | Abs | Improved | [17] |
| 2 | 2021 | 60 | M | 108 | LUL mass | BC; TB | Abs; surgery | Died | [18] |
| 3 | 2014 | 52 | F | 72 | RUL mass | BC, TB | Abs; surgery | Improved | [19] |
| 4 | 2012 | 57 | M | 24 | RUL cavity | FNAC | Abs; surgery | Relapsed and then died | [16] |
| 5 | 2016 | 49 | M | 5 | SA | PC | Abs | Improved | [20] |
| 6 | 2015 | 57 | M | 7 | LLL mass | BC | Abs | Improved | [21] |
| 7 | 2009 | 42 | F | 60 | SA, BA | 16s rRNA sequencing | Abs | Died | [22] |
| 8 | 2009 | 60 | M | 60 | LUL mass | 16s rRNA sequencing | Abs | Improved | [23] |
| 9 | 2008 | 42 | M | 48 | PA | PC, TB | Abs | Improved | [24] |
| 10 | 2004 | 42 | M | 19 | LLL mass | BLAC and FNAC | Abs | Improved | [25] |
| 11 | 2008 | 52 | M | 228 | LUL mass, LPE | SC, BLAC, BC | Abs; surgery | Improved | [26] |
| 12 | 2002 | 38 | F | 108 | LLL mass | FNAC | Abs | Improved | [27] |
| 13 | 2000 | 58 | M | NR | LUL mass | FNAC | Abs | Improved | [28] |
| 14 | 2001 | 43 | M | 12 | SPN | FNAC | Abs | Improved | [29] |
| 15 | 1997 | 48 | M | 46 | Left lung abscess | Blood culture | Abs | Relapsed and then improved | [30] |
| 16 | 1977 | 45 | F | 120 | LUL abscess | SC; bronchial brush biopsy | Abs | Died | [31] |
| 17 | 1988 | 57 | M | 72 | Osteomyelitis | Bone biopsy | Abs; surgery | Improved | [32] |
Kidney transplantation is the most effective treatment for patients with ESRD. After kidney transplantation, patients are at risk of severe illness and death due to R. equi infections owing to impaired immune function. R. equi is a gram-positive surface intracellular parasitic bacterium commonly found in farm animal feces, soil, and water[6], and is a relatively rare human pathogen. It can infect several parts of the body, with the most common being the lung, and is manifested as cavitary lung lesions, lung abscesses, pyothorax, pneumothorax, and invasion of adjacent chest structures[7]. The clinical manifestations lack specificity and mainly include cough, sputum, chest pain, dyspnea, and persistent high fever[8]. When infected with R. equi, the mortality rate has been reported to be about 11% in immunocompetent patients, 20%-25% in immunocompromised patients (non-HIV), and up to 50%-55% in patients with HIV infection[9]. This case, combined with literature review, showed four deaths out of 18 patients with a mortality rate of 22%, which is consistent with previous reports. Therefore, prompt diagnosis of R. equi infection and early treatment is crucial.
Of note, when our patient underwent fiberoptic bronchoscopy, a nascent mass was found at the mouth of the middle and lower lobes of the right lung, which was diagnosed as an inflammatory granulomatous hyperplasia on histopathologic biopsy. We hypothesized that the endobronchial mass was associated with R. equi infection, which has been previously reported as an associated endobronchial mass in addition to pulmonary manifestations in HIV patients infected with R. equi[10-12]. To our knowledge, this is the first reported case about a renal transplant recipient who developed pleural empyema combined with endobronchial mass after infection with R. equi. Unfortunately however, because of the rarity of the disease and clinical inexperience, the patient’s endobronchial mass was detected late, and the affected lung tissue appeared to have potentially irreversible pathologic changes, resulting in poor recovery of pulmonary atelectasis even after surgical resection and treatment. The nascent endobronchial mass may lead to bronchial lumen obstruction and pulmonary atelectasis, which in turn may affect the patient’s respiratory function. This em
At present, there are no guidelines for the treatment of patients infected with R. equi, as R. equi is an intracellular parthenogenetic parasitic bacterium, in that phagocytosis into and destruction of host macrophage is the basis of its pathogenicity[13]. Therefore, treatment with antibiotics with high intracellular permeability is recommended. According to the latest Sanford Guidelines for Antimicrobial Therapy in the United States, the preferred regimen for the treatment of R. equi infections is a combination of at least two of the following drugs, namely azithromycin, levofloxacin, and rifampicin, and the second preferred regimen is the combination antimicrobial treatment of vancomycin or imipenem with any of the following drugs-azithromycin, levofloxacin, or rifampicin. The duration of therapy for R. equi depends on the site of infection, the extent of tissue involvement, and the patient’s immune function. Renal transplant recipients tend to require longer antibiotic therapy because of immunosuppression, with most reports advocating a minimum of 6 mo of two or more combined antibiotic agents[14]. The initial treatment regimen for R. equi in this case was vancomycin combined with azithromycin infusion, and after clinical symptoms resolved, the patient was given an antibiotic regimen of ciprofloxacin combined with azithromycin infusion, which was changed to oral ciprofloxacin and azithromycin for close to one month and was scheduled to be taken orally for up to 6 mo. The patient’s symptoms, signs, and chest imaging showed improvement, suggesting that the treatment regimen was feasible and effective. In addition, adequate abscess drainage and surgical resection of the lesion are necessary in addition to proper antibiotic selection[15]. It has been reported that one quarter of organ transplant recipients infected with R. equi have relapsed before cure during the treatment[16]. In this case, the patient experienced a relapse 2 wk after discharge from the hospital. Therefore, considering the nature of recurrence of R. equi, we should closely follow-up discharged patients.
We report the diagnosis and management of a renal transplant recipient with an abscessed chest and endobronchial mass due to R. equi infection at 21 mo postoperatively. Since this is a single case report, it is insufficient to establish treatment guidelines for those infected with R. equi after renal transplantation. Despite this, we believe that our case report will provide a valuable reference for transplant physicians to help identify post-transplant R. equi infections and guide potential treatment.
We would like to thank all the medical staff of the Organ Transplantation Department of the Affiliated Hospital of Guizhou Medical University for their help, and the patient for his cooperation and support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G, Albania S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | Strohmaier S, Wallisch C, Kammer M, Geroldinger A, Heinze G, Oberbauer R, Haller MC. Survival Benefit of First Single-Organ Deceased Donor Kidney Transplantation Compared With Long-term Dialysis Across Ages in Transplant-Eligible Patients With Kidney Failure. JAMA Netw Open. 2022;5:e2234971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 2. | Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1054] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 3. | Pfirmann P, Garrigue I, Chauveau B, Rondeau V, Tumiotto C, Weinmann L, Dubois V, Couzi L, Merville P, Kaminski H, Taton B. Trends in epidemiology and risk factors of opportunistic infections in kidney transplant recipients between 2004-2022. Nephrol Dial Transplant. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 4. | Vázquez-Boland JA, Giguère S, Hapeshi A, MacArthur I, Anastasi E, Valero-Rello A. Rhodococcus equi: the many facets of a pathogenic actinomycete. Vet Microbiol. 2013;167:9-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Lin WV, Kruse RL, Yang K, Musher DM. Diagnosis and management of pulmonary infection due to Rhodococcus equi. Clin Microbiol Infect. 2019;25:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Prescott JF. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991;4:20-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 460] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Gundelly P, Suzuki Y, Ribes JA, Thornton A. Differences in Rhodococcus equi Infections Based on Immune Status and Antibiotic Susceptibility of Clinical Isolates in a Case Series of 12 Patients and Cases in the Literature. Biomed Res Int. 2016;2016:2737295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Vergidis P, Ariza-Heredia EJ, Nellore A, Kotton CN, Kaul DR, Morris MI, Kelesidis T, Shah H, Park SY, Nguyen MH, Razonable RR. Rhodococcus Infection in Solid Organ and Hematopoietic Stem Cell Transplant Recipients(1). Emerg Infect Dis. 2017;23:510-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Kedlaya I, Ing MB, Wong SS. Rhodococcus equi infections in immunocompetent hosts: case report and review. Clin Infect Dis. 2001;32:E39-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Akilesh S, Cross S, Kimmelshue K, Kirmani N, Dehner LP, El-Mofty SK. Pseudotumor of the tracheal-laryngeal junction with unusual morphologic features caused by Rhodococcus equi infection. Head Neck Pathol. 2011;5:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Canfrere I, Germaud P, Roger C. Another cause of endobronchial lesions found in HIV patients. Chest. 1995;108:587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Pardo Mateu L, Faubel Serra M, Llavero Segovia MT, Cano Cuenca B, Pérez Climent F, Giménez Vaillo F, Grau Alario E, Lazaro Santander R. [Laryngeal infection by Rhodococcus equi in patient with AIDS]. Acta Otorrinolaringol Esp. 2002;53:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Dawson DR, Nydam DV, Price CT, Graham JE, Cynamon MH, Divers TJ, Felippe MJ. Effects of opsonization of Rhodococcus equi on bacterial viability and phagocyte activation. Am J Vet Res. 2011;72:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Weinstock DM, Brown AE. Rhodococcus equi: an emerging pathogen. Clin Infect Dis. 2002;34:1379-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 182] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Azzam O, Crowe A, Sajiv C, Pawar B. Rhodococcus equi peritonitis in continuous ambulatory peritoneal dialysis: a first in Australia. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Menon V, Gottlieb T, Gallagher M, Cheong EL. Persistent Rhodococcus equi infection in a renal transplant patient: case report and review of the literature. Transpl Infect Dis. 2012;14:E126-E133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Alfano G, Ventura P, Fontana F, Marcacci M, Ligabue G, Scarlini S, Franceschini E, Codeluppi M, Guaraldi G, Mussini C, Cappelli G. Rhodococcus equi Pneumonia in Kidney Transplant Recipient Affected by Acute Intermittent Porphyria: A Case Report. Transplant Proc. 2019;51:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Shah P, Rojas-Moreno C, Alexander J, Vasudevan A, Nguyen V. Rhodococcus equi: Another great masquerader. IDCases. 2021;24:e01144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Ursales A, Klein JA, Beal SG, Koch M, Clement-Kruzel S, Melton LB, Spak CW. Antibiotic failure in a renal transplant patient with Rhodococcus equi infection: an indication for surgical lobectomy. Transpl Infect Dis. 2014;16:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Varotti G, Barabani C, Dodi F, Bertocchi M, Mondello R, Cupo P, Santori G, Palombo D, Fontana I. Unusual Extrapulmonary Rhodococcus Equi Infection in a Kidney Transplant Patient. Exp Clin Transplant. 2016;14:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Macken E, de Jonge H, Van Caesbroeck D, Verhaegen J, Van Kerkhoven D, Van Wijngaerden E, Kuypers D. Rhodococcus equi Sepsis in a Renal Transplant Recipient: A Case Study. Transplant Direct. 2015;1:e11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Rahamat-Langendoen JC, van Meurs M, Zijlstra JG, Lo-Ten-Foe JR. Disseminated Rhodococcus equi infection in a kidney transplant patient without initial pulmonary involvement. Diagn Microbiol Infect Dis. 2009;65:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | El Karoui K, Guillet C, Sekkal N, Lanternier F, Méchaï F, Hue K, Hiesse C, Mamzer Bruneel MF, Catherinot E, Viard JP, Mainardi JL, Lecuit M, Ferroni A, Lortholary O. Synergistic effect of carbapenem-teicoplanin combination during severe Rhodococcus equi pneumonia in a kidney transplant recipient. Transpl Infect Dis. 2009;11:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Tse KC, Tang SC, Chan TM, Lai KN. Rhodococcus lung abscess complicating kidney transplantation: successful management by combination antibiotic therapy. Transpl Infect Dis. 2008;10:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Arya B, Hussian S, Hariharan S. Rhodococcus equi pneumonia in a renal transplant patient: a case report and review of literature. Clin Transplant. 2004;18:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Speck D, Koneth I, Diethelm M, Binet I. A pulmonary mass caused by Rhodococcus equi infection in a renal transplant recipient. Nat Clin Pract Nephrol. 2008;4:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | González-Roncero FM, Gentil MA, Rodriguez-Algarra G, Pereira P, Cisneros JM, Castilla JJ, Rocha JL, Mateos J. Medical management of pneumonia caused by Rhodococcus equi in a renal transplant recipient. Am J Kidney Dis. 2002;39:E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Marsh HP, Bowler IC, Watson CJ. Successful treatment of Rhodococcus equi pulmonary infection in a renal transplant recipient. Ann R Coll Surg Engl. 2000;82:107-108. [PubMed] |
| 29. | Simsir A, Oldach D, Forest G, Henry M. Rhodococcus equi and cytomegalovirus pneumonia in a renal transplant patient: diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 2001;24:129-131. [PubMed] [DOI] [Full Text] |
| 30. | Barsotti M, Cupisti A, Morelli E, Meola M, Barsotti G. Sepsis from Rhodococcus equi successfully treated in a kidney transplant recipient. Nephrol Dial Transplant. 1997;12:2002-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Savdie E, Pigott P, Jennis F. Lung abscess due to Corynebacterium equi in a renal transplant recipient. Med J Aust. 1977;1:817-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Novak RM, Polisky EL, Janda WM, Libertin CR. Osteomyelitis caused by Rhodococcus equi in a renal transplant recipient. Infection. 1988;16:186-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |