Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.15
Peer-review started: October 30, 2023
First decision: November 8, 2023
Revised: November 23, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: January 6, 2024
Processing time: 64 Days and 0.2 Hours
Colorectal cancer ranks third and second among common and fatal cancers. The treatment of metastatic colorectal cancer (mCRC) is generally based on XELOX in clinical practice, which includes capecitabine (CAP) and oxaliplatin. Serum tumor markers carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125 and CA199 are prognostic factors for various tumors.
To investigate evaluating combined bevacizumab (BEV) and XELOX in advanced colorectal cancer: Serum markers CEA, CA125, CA199 analysis.
In this retrospective study, a total of 94 elderly patients diagnosed with mCRC were recruited and subsequently categorized into two groups based on the distinct treatment modalities they received. The control group was treated with XELOX plus CAP (n = 47), while the observation group was treated with XELOX plus CAP and BEV (n = 47). Several indexes were assessed in both groups, including disease control rate (DCR), incidence of adverse effects, serum marker levels (CEA, CA125, and CA19) and progression-free survival (PFS).
After 9 wk of treatment, the serum levels of CEA, CA199 and CA125 in the observation group were significantly lower than those in the control group (P < 0.05). Moreover, the PFS of the observation group (9.12 ± 0.90 mo) was significantly longer than that of the control group (6.49 ± 0.64 mo). Meanwhile, there was no statistically significant difference in the incidence of adverse reactions and DCR between the two groups during maintenance therapy (P > 0.05).
On the basis of XELOX treatment, the combination of BEV and CAP can reduce serum tumor marker levels and prolong PFS in patients with mCRC.
Core Tip: Colorectal cancer has a high incidence in the population. The clinical treatment of colorectal cancer is basically XELOX intervention. Prognostic determination of serum tumor markers is a common index to evaluate the efficacy of cancer drugs. Therefore, we studied the therapeutic effect and serum tumor markers of patients with colorectal cancer under different treatments. The results showed that the therapeutic effect of XELOX + capecitabine (CAP) + bevacizumab was better than that of XELOX + CAP, which showed that the serum carcinoembryonic antigen, carbohydrate antigen (CA) 199 and CA125 levels were lower and the median survival time was longer, and all of them were statistically significant.
- Citation: Zhou DB, Cheng J, Zhang XH. Evaluating combined bevacizumab and XELOX in advanced colorectal cancer: Serum markers carcinoembryonic antigen, carbohydrate antigen 125, carbohydrate antigen 199 analysis. World J Clin Cases 2024; 12(1): 15-23
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/15.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.15
Colorectal cancer (CRC), a common malignant tumor of the digestive tract, ranks third and second among common and fatal cancers, respectively[1,2]. The increase in the incidence rate of CRC is mainly due to the increased exposure to environmental risk factors caused by the westernization of lifestyle and diet[3,4]. About 25 to 30% of CRC cases are associated with unmodifiable risk factors such as genetic factors, personal history of polyps or adenomas, or family history or genetic risk of CRC[5,6]. Approximately 70%-75% of CRC cases are associated with variable risk factors, such as smoking, alcoholism, unhealthy eating, sedentary behavior, lack of physical activity, and obesity[7,8]. Currently, the rising incidence of early-onset CRC poses a serious challenge to global public health[9]. In 2020, the incidence rate of CRC accounted for 10% of global cancers, and according to the prediction of aging, population growth and human development, it is estimated that the number of new CRC cases in the world will reach 3.2 million in 2040[10]. Patients with early CRC usually have a better prognosis through surgery, but about 25% of CRC patients are diagnosed with advanced CRC and have metastasis to distant organs[11].
For patients with advanced metastatic CRC (mCRC) who misses the opportunity for surgery, they can only be treated with high-intensity chemotherapy[12]. The treatment of mCRC is generally based on XELOX in clinical practice, which includes capecitabine (CAP) and oxaliplatin. However, after XELOX treatment, subsequent maintenance therapy usually uses low-intensity and low-toxicity drugs. Capecitabine is a fluorouracil (FU) analogue that can exert cytotoxic effects on tumor cells, but it cannot benefit overall survival (OS) when used alone, so it needs to be used in combination with other drugs. Related studies have shown that adjuvant therapy with targeted drugs can improve efficacy by effectively reducing the damage of chemotherapy to normal cells in the body[13-15]. Bevacizumab (BEV), a monoclonal antibody with targeted effects, can inhibit tumor growth and metastasis by inhibiting the formation of tumor neovascularization caused by the binding of vascular endothelial growth factor (VEGF) to its receptor[16,17]. Research has provided evidence that the utilization of BEV in conjunction with adjuvant chemotherapy can significantly enhance long-term efficacy[18,19]. At present, exploring the efficacy and prognosis of BEV combined with XELOX adjuvant chemotherapy in the treatment of advanced mCRC patients is indispensable.
Some tumor markers also play an important role in the diagnosis, treatment and prognosis assessment of malignant tumors, with the continuous development of molecular biology research. Tumor markers can improve patient adherence and tolerability and are suitable for large-scale screening compared to routine and invasive tests. Carcinoembryonic antigen (CEA), a tumor marker with wide-ranging applicability, is present in various solid malignancies including lung cancer, esophageal cancer, colorectal cancer, and ovarian epithelial cancer. CEA, a broad-spectrum tumor marker, exists in solid malignant tumors, such as lung cancer, esophageal cancer, cancer, colorectal cancer, and ovarian epithelial cancer. Its high preoperative concentration is associated with poor prognosis for CRC patients, and the continuous measurement of CEA can detect recurrent CRC, with a sensitivity of approximately 80% and specificity of approximately 70%[20]. Carbohydrate antigen (CA) 125, also known as mucin 16 or MUC16, is a protein encoded by the MUC16 gene in humans. According to reports, CA125 is a significant and independent prognostic factor for colorectal cancer patients who outperform CEA[21]. Regardless of peritoneal metastasis, CRC patients with elevated preoperative CA125 Levels have lower OS and CSS rates[22]. CA199 is a diagnostic indicator for various malignant tumors, which belongs to the category of macromolecular glycoproteins and contains mucus[23]. CA199 has been reported as a valuable indicator for predicting the risk of CRC[24]. Therefore, the combined detection of CEA, CA125, and CA199 is of great significance in evaluating the prognosis of mCRC.
This study mainly explores the efficacy and prognosis of BEV combined with XELOX adjuvant chemotherapy in patients with advanced mCRC by detection of serum levels of CEA, CA199 and CA125.
This study encompassed a retrospective analysis of 94 elderly patients diagnosed with advanced mCRC who received treatment at the hospital between November 2020 and November 2022. The baseline characteristics of mCRC patients are given in Table 1. This study was reviewed and approved by the medical ethics committee of the hospital and executed in accordance with the Helsinki Declaration.
| Group | Gender (male/female) | Age (yr) | Average age (yr) | Primary lesion site (n) | Metastasis site (n) | ||||
| Left-sided colorectal | Right-sided colon | Liver | Bone | Lung | Abdominal cavity | ||||
| Observation group (n = 47) | 23/24 | 60-83 | 72.25 ± 6.41 | 31 | 16 | 19 | 12 | 11 | 5 |
| Control group (n = 47) | 22/25 | 60-82 | 72.19 ± 6.37 | 30 | 17 | 18 | 13 | 10 | 6 |
The inclusion criteria were as follows: metastatic lesions in mCRC patients mCRC could not be surgically removed and distant metastasis was limited to a single organ or site; the Eastern Cooperative Oncology Group (ECOG) score of mCRC patients was 0-2 points; bone marrow function met the standard; the survival in patients with mCRC was estimated to be greater than 3 mo and patients with mCRC had stable disease (SD) or above after chemotherapy with 4 XELOX regimens. Exclusion criteria were as follows: patients with mCRC had active infection, other malignancies, severe endocrine disease, intestinal obstruction or large ascites.
All patients were divided into the observation group (n = 47) and the control group (n = 47) according to the different treatment methods. Both groups were treated with XELOX as follows: Oxaliplatin was administered intravenously for 2 h on the first day of the cycle (130 mg/m2) and CAP tablets were orally administered twice a day for 14 d (1000 mg/m2), with discontinuation for 7 d. One chemotherapy cycle was 21 d, and the patient was treated for 4 cycles. After achieving SD or better status through first-line chemotherapy, the control group received maintenance treatment with CAP twice a day for 14 d orally (1000 mg/m2), followed by discontinuation for 7 d, with 21 d as a cycle. The difference was that the observation group was combined with BEV on the basis of the control group. Specifically, BEV was given for 60-90 min intravenously (7.50 mg/kg) on the first day of the cycle, with 21 d as a cycle. Both groups were subjected to maintenance treatment until disease progression or intolerable adverse reactions occurred.
After the end of chemotherapy, efficacy evaluation was carried out based on the evaluation criteria for solid tumor efficacy. Complete remission (CR) referred to the disappearance of all lesions; Partial remission (PR) was a reduction in lesion radius by more than 30%; SD referred to a decrease in lesion radius of less than or equal to 30% or an increase of less than 20%; The progression of disease (PD) was an increase in lesion radius of ≥ 20%. The equation for disease control rate (DCR) was the sum of CR, PR and SD cases/total cases × 100%. Follow up was performed by phone every 2 wk after chemotherapy and PFS was recorded. In addition, the patient’s serum levels of CEA, CA125, and CA199 were detected before and after chemotherapy.
The data analysis was conducted using SPSS 22.0 software. The measurement data expressed as mean ± SD and compared by using Student’s t-tests. The counting data expressed as n (%) and compared by using χ2 test. P < 0.05 had a statistical significance.
A total of 94 patients, who satisfied the predetermined inclusion and exclusion criteria, were incorporated into the present study. As shown in Table 1, The average age of mCRC patients in the observation and control groups was 72.25 ± 6.41 (range 60-82 years) and 72.19 ± 6.37 (range 60-83 years). The male to female distribution ratios of patients with mCRC in the observation group and control group were 23:24 and 22:25, respectively. The primary lesion of both groups of mCRC patients was located in the left-sided colorectal or right-sided colon, with liver, bone, lung, or abdominal metastasis. There was no significant difference in baseline characteristics between the two groups.
After 9 wk of maintenance treatment, there were no cases of CR in both groups. Among them, the PR, SD, and DCR of the observation group were higher than those of the control group, while the PD was lower than those of the control group. Unfortunately, these values were not statistically significant between the two groups (P > 0.05) (Table 2).
| Group | CR | PR | SD | PD | DCR |
| Observation group (n = 47) | 0 | 11 (23.40) | 17 (36.17) | 19 (40.43) | 28 (59.57) |
| Control group (n = 47) | 0 | 10 (21.28) | 16 (34.04) | 21 (44.68) | 26 (55.32) |
| χ2 | 0.174 | ||||
| P value | 0.677 |
There was no significant difference in the severity of hand-foot skin reaction, anemia, hepatic insufficiency, weakness, nausea and vomiting and neutropenia between the observation and control groups during chemotherapy (P > 0.05) (Table 3).
| Group | HFSR | Anemia | Hepatic insufficiency | Weakness | Nausea and vomiting | Neutropenia |
| Observation group (n = 47) | 10 (21.28) | 2 (4.26) | 2 (4.26) | 7 (14.89) | 14 (29.79) | 14 (29.79) |
| Control group (n = 47) | 9 (19.15) | 1 (2.13) | 2 (4.26) | 5 (10.64) | 12 (25.53) | 12 (25.53) |
| χ2 | 0.066 | 0 | 0.261 | 0.382 | 0.213 | 0.213 |
| P value | 0.797 | 1 | 0.609 | 0.536 | 0.645 | 0.645 |
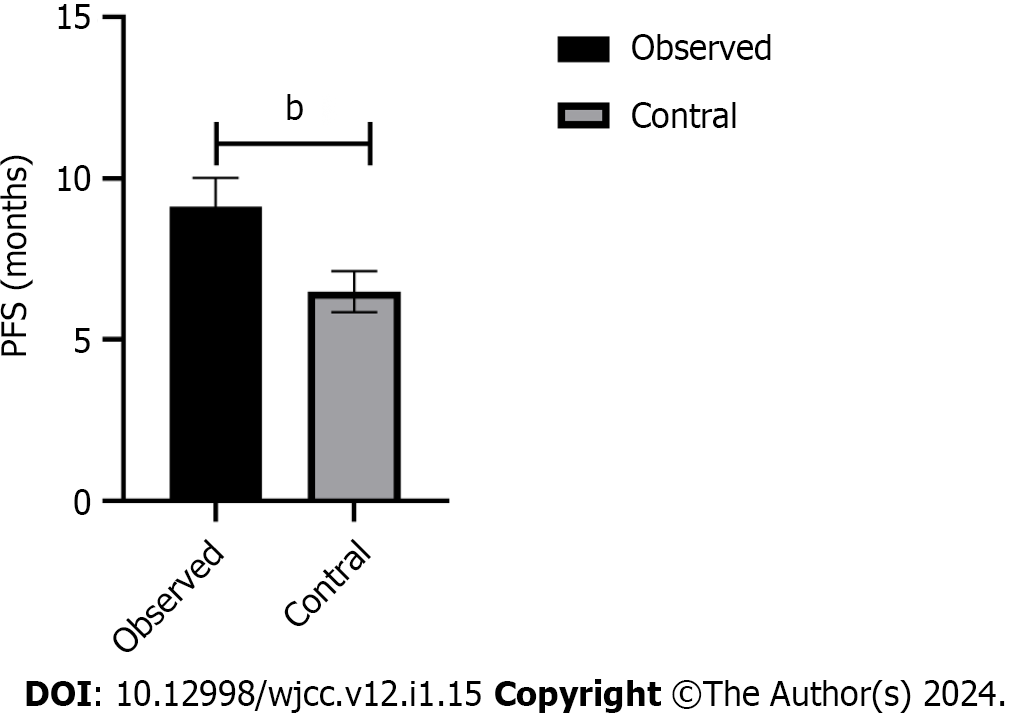
There was no significant difference in serum levels of CEA, CA199 and CA125 between the two groups before maintenance therapy (P > 0.05). After 9 wk of maintenance treatment, the serum levels of CEA, CA199 and CA125 between the two groups were lower than before treatment, and the serum levels of CEA, CA199 and CA125 levels in the observation group were lower than those in the control group after treatment (P < 0.05) (Table 4 and Figure 1). Furthermore, the PFS of the observation group was 9.12 ± 0.90 mo, which was longer than the 6.49 ± 0.64 mo of the control group (P < 0.05) (Figure 2).
| Group | CEA (μg/mL) | CA199 (U/mL) | CA125 (U/mL) | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Observation group (n = 47) | 8.22 ± 0.76 | 1.53 ± 0.12a | 50.63 ± 4.75 | 20.67 ± 2.01a | 72.90 ± 7.04 | 43.09 ± 5.63a |
| Control group (n = 47) | 8.35 ± 0.71 | 3.91 ± 0.19a | 51.22 ± 4.92 | 39.54 ± 2.76a | 72.83 ± 7.12 | 45.13 ± 6.97a |
| χ2 | 0.857 | 72.607 | 0.591 | 37.889 | 0.038 | 2.247 |
| P value | 0.3197 | < 0.001 | 0.278 | < 0.001 | 0.97 | 0.0217 |
There are many factors that contribute to the occurrence of CRC in the body, such as diet, lifestyle habits, environment, and genetics[25]. So far, radical surgery is the only method to cure CRC. However, patients generally develop into distant metastases and are in the advanced stage at the time of diagnosis, which leads to the loss of surgical opportunities for patients[26,27]. Currently, mCRC can be treated with the XELOX regimen, which can inhibit cancer cell proliferation and prolong patient survival time[28,29]. However, oxaliplatin in the XELOX regimen has certain toxicity and cannot be used for a long time, so the significance of taking low-intensity and low-toxicity drugs for treatment is even greater[30].
CAP is a new generation of FU drugs, which can effectively kill tumor cells due to thymidine phosphorylase transformation in the body[31]. At the same time, it has obvious therapeutic effect on malignant tumors, but has no effect on normal tissues basically, which is because thymidine phosphorylase exists in tumor tissues generally[32-34]. BEV is a molecularly targeted drug, which can competitively bind to vascular endothelial growth factor receptors in the body, resulting in slow generation of new blood vessels, and finally allowing the disease to be controlled to a certain extent[35-37]. In this study, there was no statistically significant difference in the incidence of adverse reactions and DCR between the two groups during maintenance treatment for 9 wk, indicating that the two regimens had the same short-term efficacy and safety.
So far, tumor marker indicators can reflect tumor burden to a certain extent, including CEA, CA199, and CA125[38-40]. After 9 wk of maintenance therapy, serum levels of CEA, CA199 and CA125 in the observation group were lower than those in the control group and PFS was longer than in the control group. After XELOX treatment, the tumor cells in the patient’s body were effectively killed and the small metastatic lesions in the body were also cleared. When the disease reaches a stable state, bevacizumab combined with capecitabine acts on tumor cells and vascular endothelial cells to produce sustained anti-tumor effects, which can not only consolidate the efficacy of first-line chemotherapy but also reduce the cumulative toxicity of drugs, delay tumor progression and reduce tumor burden, and ultimately leading to a prolonged survival time for patients. However, there are some limitations in this study. The sample size is a bit small. Further studies were needed to overcome these limitations to make the data more convincing.
In summary, on the basis of XELOX treatment, the maintenance treatment of BEV combined with CAP can reduce serum tumor marker levels of CEA, CA199, and CA125 and prolong PFS.
Colorectal cancer is ranked as the third most common and second most fatal cancer. In clinical practice, the treatment of metastatic colorectal cancer (mCRC) typically relies on the administration of XELOX, a combination therapy involving capecitabine (CAP) and oxaliplatin. Additionally, serum tumor markers such as carcinoembryonic antigen, carbohydrate antigen (CA) 125, and CA199 serve as prognostic indicators for a range of tumors.
The impact of the combination of bevacizumab (BEV) and XELOX chemotherapy on individuals diagnosed with advanced mCRC.
The objective of this study is to examine the impact of the combination of BEV and XELOX chemotherapy on individuals diagnosed with advanced mCRC, as well as the alterations observed in their serum tumor markers.
A comprehensive analysis was conducted on the 94 cases within our hospital, wherein the data was meticulously compared across various treatment modalities.
Multiple indexes were evaluated in both groups.
Based on the utilization of XELOX treatment, the incorporation of BEV and CAP has demonstrated the ability to diminish serum tumor marker levels and extend progression-free survival among patients diagnosed with mCRC.
The addition of Bevacizumab to first-line chemotherapy demonstrated a greater benefit in comparison to chemotherapy alone.
I would like to express my gratitude to all those helped me during the writing of this thesis. I acknowledge the help of my colleagues, they have offered me suggestion in academic studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garcia K, Spain S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, Takeda K, Yonaga K, Masuda Y, Yoshida H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J Nippon Med Sch. 2022;89:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 115] [Reference Citation Analysis (1)] |
| 2. | Talaat IM, Elemam NM, Saber-Ayad M. Complement System: An Immunotherapy Target in Colorectal Cancer. Front Immunol. 2022;13:810993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1587] [Article Influence: 264.5] [Reference Citation Analysis (2)] |
| 4. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 389] [Article Influence: 129.7] [Reference Citation Analysis (6)] |
| 5. | Liu Y, Zhang C, Wang Q, Wu K, Sun Z, Tang Z, Zhang B. Temporal Trends in the Disease Burden of Colorectal Cancer with Its Risk Factors at the Global and National Level from 1990 to 2019, and Projections Until 2044. Clin Epidemiol. 2023;15:55-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 6. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 7. | GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 8. | Sninsky JA, Shore BM, Lupu GV, Crockett SD. Risk Factors for Colorectal Polyps and Cancer. Gastrointest Endosc Clin N Am. 2022;32:195-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 9. | Hua H, Jiang Q, Sun P, Xu X. Risk factors for early-onset colorectal cancer: systematic review and meta-analysis. Front Oncol. 2023;13:1132306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 10. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1356] [Article Influence: 339.0] [Reference Citation Analysis (5)] |
| 11. | Xu W, He Y, Wang Y, Li X, Young J, Ioannidis JPA, Dunlop MG, Theodoratou E. Risk factors and risk prediction models for colorectal cancer metastasis and recurrence: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1443] [Article Influence: 360.8] [Reference Citation Analysis (0)] |
| 13. | van Roessel S, van Veldhuisen E, Klompmaker S, Janssen QP, Abu Hilal M, Alseidi A, Balduzzi A, Balzano G, Bassi C, Berrevoet F, Bonds M, Busch OR, Butturini G, Del Chiaro M, Conlon KC, Falconi M, Frigerio I, Fusai GK, Gagnière J, Griffin O, Hackert T, Halimi A, Klaiber U, Labori KJ, Malleo G, Marino MV, Mortensen MB, Nikov A, Lesurtel M, Keck T, Kleeff J, Pandé R, Pfeiffer P, Pietrasz D, Roberts KJ, Sa Cunha A, Salvia R, Strobel O, Tarvainen T, Bossuyt PM, van Laarhoven HWM, Wilmink JW, Groot Koerkamp B, Besselink MG; European-African Hepato-Pancreato-Biliary Association. Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment. JAMA Oncol. 2020;6:1733-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Zhu J, Jiao D, Wang C, Lu Z, Chen X, Li L, Sun X, Qin L, Guo X, Zhang C, Qiao J, Yan M, Cui S, Liu Z. Neoadjuvant Efficacy of Three Targeted Therapy Strategies for HER2-Positive Breast Cancer Based on the Same Chemotherapy Regimen. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A, Regan MM, Spears PA, Sudheendra PK, Symmans WF, Yung RL, Harvey BE, Hershman DL. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol. 2021;39:1485-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 603] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 16. | Chirio D, Peira E, Sapino S, Chindamo G, Oliaro-Bosso S, Adinolfi S, Dianzani C, Baratta F, Gallarate M. A New Bevacizumab Carrier for Intravitreal Administration: Focus on Stability. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Silva TA, Aguiar RB, Mori M, Machado GE, Hamaguchi B, Machado MFM, Moraes JZ. Potential of an anti-bevacizumab idiotype scFv DNA-based immunization to elicit VEGF-binding antibody response. Gene Ther. 2023;30:598-602. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Wang M, Li J, Xu S, Li Y, Yu J, Tang X, Zhu H. Immunotherapy combined with chemotherapy improved clinical outcomes over bevacizumab combined with chemotherapy as first-line therapy in adenocarcinoma patients. Cancer Med. 2023;12:5352-5363. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Avallone A, Piccirillo MC, Nasti G, Rosati G, Carlomagno C, Di Gennaro E, Romano C, Tatangelo F, Granata V, Cassata A, Silvestro L, De Stefano A, Aloj L, Vicario V, Nappi A, Leone A, Bilancia D, Arenare L, Petrillo A, Lastoria S, Gallo C, Botti G, Delrio P, Izzo F, Perrone F, Budillon A. Effect of Bevacizumab in Combination With Standard Oxaliplatin-Based Regimens in Patients With Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA Netw Open. 2021;4:e2118475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Sørensen CG, Karlsson WK, Pommergaard HC, Burcharth J, Rosenberg J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence - A systematic review. Int J Surg. 2016;25:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Björkman K, Mustonen H, Kaprio T, Kekki H, Pettersson K, Haglund C, Böckelman C. CA125: A superior prognostic biomarker for colorectal cancer compared to CEA, CA19-9 or CA242. Tumour Biol. 2021;43:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Huang JH, Liu HS, Hu T, Zhang ZJ, He XW, Mo TW, Wen XF, Lan P, Lian L, Wu XR. Elevated preoperative CA125 is associated with poor survival in patients with metastatic colorectal cancer undergoing primary tumor resection: a retrospective cohort study. Gastroenterol Rep (Oxf). 2022;10:goac020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Zeng P, Li H, Chen Y, Pei H, Zhang L. Serum CA199 Levels are significantly increased in patients suffering from liver, lung, and other diseases. Prog Mol Biol Transl Sci. 2019;162:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Rao H, Wu H, Huang Q, Yu Z, Zhong Z. Clinical Value of Serum CEA, CA24-2 and CA19-9 in Patients with Colorectal Cancer. Clin Lab. 2021;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Meester RGS, Anderson JC. Long-Term Risk for Colorectal Cancer in Patients With Index Serrated Polyps. Gastroenterology. 2022;162:2108-2110. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Søreide K. Time to halt perioperative chemotherapy for resectable colorectal liver metastasis? Br J Surg. 2022;109:242-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Ando K, Nakanishi R, Oki E. [Ⅱ.Perioperative Therapy for Locally Advanced Rectal Cancer-To Control Distant Metastases]. Gan To Kagaku Ryoho. 2021;48:1343-1348. [PubMed] |
| 28. | Martín-Richard M, Tobeña M. First-Line Maintenance Treatment in Metastatic Colorectal Cancer (mCRC): Quality and Clinical Benefit Overview. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Peng J, Li W, Fan W, Zhou W, Zhu Y, Li X, Pan Z, Lin X, Lin J. Feasibility Study of a Modified XELOX Adjuvant Chemotherapy for High-Recurrence Risk Patients With Operated Stage III Colon Cancer. Front Pharmacol. 2020;11:583091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Argyriou AA, Kalofonou F, Litsardopoulos P, Anastopoulou GG, Kalofonos HP. Oxaliplatin rechallenge in metastatic colorectal cancer patients with clinically significant oxaliplatin-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2021;26:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Lam SW, Guchelaar HJ, Boven E. The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev. 2016;50:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 32. | Hiroi S, Miguchi M, Ikeda S, Nakahara H, Shinozaki K, Nishisaka T, Egi H, Itamoto T. Capecitabine Plus Bevacizumab for Cardiac Metastasis of Sigmoid Colon Cancer: Case Report and Literature Review. In Vivo. 2020;34:3413-3419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Okamoto K, Nozawa H, Emoto S, Murono K, Sasaki K, Ishihara S. Adjuvant Capecitabine and Oxaliplatin for Elderly Patients with Colorectal Cancer. Oncology. 2022;100:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Wu Z, Deng Y. Capecitabine Versus Continuous Infusion Fluorouracil for the Treatment of Advanced or Metastatic Colorectal Cancer: a Meta-analysis. Curr Treat Options Oncol. 2018;19:77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, Chinot OL. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. 2020;86:102017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 729] [Article Influence: 145.8] [Reference Citation Analysis (0)] |
| 36. | Dang A, Jagan Mohan Venkateswara Rao P, Kishore R, Vallish BN. Real world safety of bevacizumab in cancer patients: A systematic literature review of case reports. Int J Risk Saf Med. 2021;32:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Rubinstein MM, Dickinson S, Narayan P, Zhou Q, Iasonos A, Ma W, Lakhman Y, Makker V. Bevacizumab in advanced endometrial cancer. Gynecol Oncol. 2021;161:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Fang T, Wang H, Wang Y, Lin X, Cui Y, Wang Z. Clinical Significance of Preoperative Serum CEA, CA125, and CA19-9 Levels in Predicting the Resectability of Cholangiocarcinoma. Dis Markers. 2019;2019:6016931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Lertkhachonsuk AA, Buranawongtrakoon S, Lekskul N, Rermluk N, Wee-Stekly WW, Charakorn C. Serum CA19-9, CA-125 and CEA as tumor markers for mucinous ovarian tumors. J Obstet Gynaecol Res. 2020;46:2287-2291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 40. | Lin S, Wang Y, Peng Z, Chen Z, Hu F. Detection of cancer biomarkers CA125 and CA199 via terahertz metasurface immunosensor. Talanta. 2022;248:123628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |