Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.2074
Peer-review started: December 16, 2022
First decision: January 12, 2023
Revised: January 27, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 26, 2023
Processing time: 90 Days and 23.4 Hours
Infarction of the conus medullaris is a rare form of spinal cord infarction. The first symptom is usually acute non-characteristic lumbar pain, followed by lower limb pain, saddle numbness, fecal incontinence, and sexual dysfunction. Spontaneous conus infarction with "snake-eye appearance" on magnetic resonance imaging has rarely been reported.
We report a 79-year-old male patient with spontaneous conus infarction who had acute lower extremity pain and dysuria as the first symptoms. He did not have any recent history of aortic surgery and trauma. Magnetic resonance imaging revealed a rare "snake-eye appearance." In addition, we reviewed the literature on 23 similar cases and summarized the clinical features and magnetic resonance manifestations of common diseases related to the "snake-eye sign" to explore the etiology, imaging findings, and prognosis of spontaneous conus infarction.
We conclude that acute onset of conus medullaris syndrome combined with "snake-eye appearance" should be strongly suspected as conus medullaris infarction caused by anterior spinal artery ischemia. This special imaging manifestation is helpful in the early diagnosis and treatment of conus infarction.
Core Tip: Infarction of the conus medullaris is a rare form of spinal cord infarction, and there is no specific examination method in clinic. On the other hand, the "Snake-Eye Appearance" on the diffusion-weighted imaging sequence of magnetic resonance imaging highly suggests spinal cord infarction, although few cases of conus infarction have been reported. This special imaging manifestation is helpful in the early diagnosis and treatment of conus infarction.
- Citation: Zhang QY, Xu LY, Wang ML, Cao H, Ji XF. Spontaneous conus infarction with "snake-eye appearance" on magnetic resonance imaging: A case report and literature review. World J Clin Cases 2023; 11(9): 2074-2083
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/2074.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.2074
Spinal cord infarctions account for approximately 1.2% of all ischemic strokes[1], and less than 10% of acute non-traumatic myelopathies[2]. The most common infarct sites are the cervical and thoracic segments[3]. The common clinical manifestations of spinal cord infarction are motor and sensory disorders with impaired autonomic nerve function, and the specific manifestations are related to the affected sites[4]. Combined with the observation of the clinical manifestations, magnetic resonance imaging (MRI) and cerebrospinal fluid examination are helpful in distinguishing this condition from optic neuromyelitis pedigree diseases, acute disseminated encephalomyelitis, spinal cord tumors, multiple sclerosis, intervertebral disc herniation, and other spinal cord diseases. However, the lack of specific MRI features in conus medullaris infarction increases the difficulty of early diagnosis and recognition. In this paper, we report a case of spontaneous conus medullaris infarction. Simultaneously providing an analysis of the literature, we focus on the special imaging manifestations and etiological mechanisms of this condition to improve clinicians’ understanding of this disease.
A 71-year-old Chinese man with a complaint of sudden onset of pain in both lower extremities and urination disorders for 2 d.
The patient experienced continuous pain on the back of both lower limbs when standing up from the sofa before 2 d, accompanied by muscle spasm, increased pain while sitting, urinary incontinence, and difficulty defecating, but no erectile dysfunction.
The patient had a history of hypertension for 5 years and diabetes for 2 years. In the last year, the patient regular oral administration of Amlodipine besylate tablets was 5 mg qd, and the blood pressure was stable at 17-20/9-11 kPa; Metformin 0.5 g tid and the glycosylated hemoglobin was 6.8%.
The patient has been smoking for 43 years, about 10 cigarettes a day. The patient denies any family history of disease.
After 48 h of onset, neurological examination showed normal muscle strength and muscle tension in both lower limbs. The bilateral knee reflex was symmetrical, with no hyperactivity and weakness, the bilateral Achilles tendon reflex disappeared, the saddle area sense was normal, anal reflex was weakened, the urinary retention was moderate, and lack of the bilateral Babinski sign.
No obvious abnormalities were found in the test results of perfect nail work, serum immunology, tumor markers, or coagulation images.
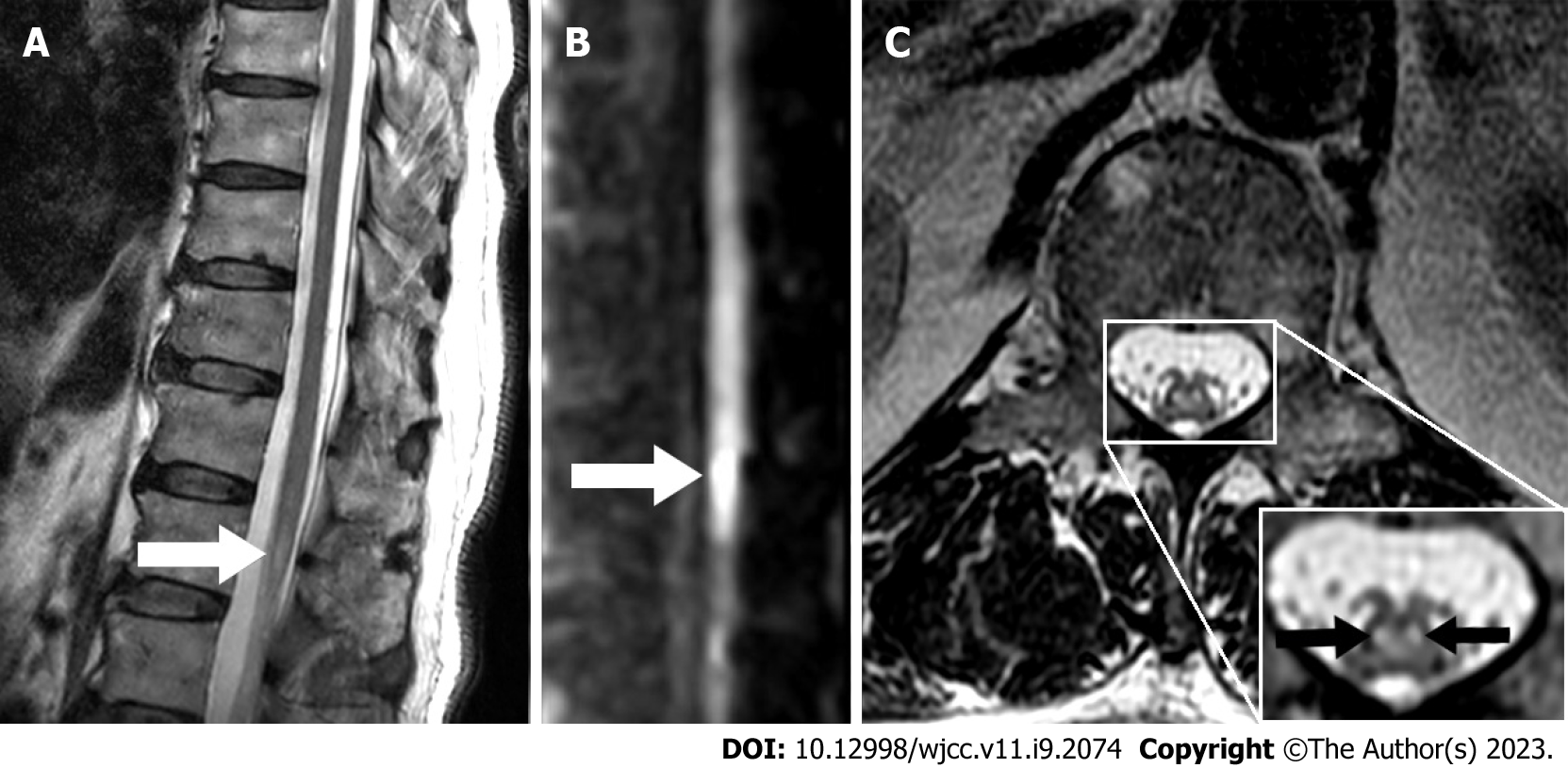
On the 4th day of onset, lumbar MRI revealed a high signal on the L1 horizontal axial T2-weighted image with a "snake-eye appearance", and conus infarction was thus considered. On the 5th day of onset, lumbar MRI showed a high signal on L1 horizontal sagittal diffusion-weighted imaging (DWI) (Figure 1).
Spinal angiography and aortic angiography (CTA) improved, but no definite vascular stenosis or malformation was identified. On the 7th day of onset, the measurement of nerve conduction velocity revealed that the F-wave latency of the left tibial nerve was prolonged.
We used the key terms "conus medullaris infarction,” "conus medullaris syndrome,” "spinal cord ischemia,” "spinal cord infarction,” "snake-eye appearance" and "owl-eye appearance" to search the literature on PubMed. We thus identified and summarized 23 cases of conus medullaris infarction from January 1971 to January 2021 (Table 1)[5-21]. The male to female ratio was 1.3:1. The median age at onset was 63 years. Eleven cases (47.8%) were complicated by cardiovascular risk factors, 7 (30.4%) were related to aortic disease, and 3 (13.0%) were secondary to postural changes, similar to the present case. Other causes included dural arteriovenous fistulas, spinal cord venous thrombosis, fibrocartilage embolism, and so on; 9 cases (39.1%) showed a high signal on axial T2-weighted imaging, of which 2 cases showed the typical "snake-eye appearance” high signal on sagittal T2-weighted imaging in the T10 to L1 vertebral segments, 3 cases (13.0%) showed limited DWI, 5 cases (21.7%) involved the vertebral body/muscle/ligament at the same time, and 16 cases (69.6%) showed a partial recovery of neurological deficit, with sequelae of varying degrees, while 5 cases (21.7%) had no improvement, and the overall prognosis was poor.
| Ref. | Age/gender | Risk factors | Pathogeny/mechanism | Prognosis | MRI findings | |||
| T2WI I high signal (Axial) | T2WI high signal (Sagittal) | DWI high signal | Involve centrum/muscle/ligament | |||||
| Herrick et al[5] | 84/M | NA | Aortic dissection aneurysm | Partial improvement, died of rupture of aortic dissection aneurysm on the 18th day of admission | NA | NA | NA | NA |
| 79/M | Heart failure | Aortic atherosclerosis | No improvement, died of acute myocardial infarction on the 25th day of admission | NA | NA | NA | NA | |
| Anderson et al[6] | 54/M | Coronary diseaseHeart failure | Aortic balloon pump implantation | Some improved strength in the legs before death 7 wk after the ictus | NA | NA | NA | NA |
| 75/M | Smoking | Repair operation of abdominal aortic aneurysm | Persistent urinary incontinence with some improvement in bowel function and in motor and sensory signs 16 mo after the ictus | NA | NA | NA | NA | |
| 66/M | Smoking | Aortic atherosclerosis | Some functions recovered 2 mo after the ictus | NA | NA | NA | NA | |
| 51/M | Smoking | NA | Persistent urinary incontinence with some functions recovered 28 mo after the ictus | NA | NA | NA | NA | |
| 47/F | NA | NA | No improvement in 2 yr | NA | NA | NA | NA | |
| Ohbu et al[7] | 69/F | Hypertension | NA | NA | NA | NA | NA | NA |
| Andrews et al[8] | 71/F | NA | NA | Walking independently, mild hypoesthesia, but persistent urinary incontinence 2 mo after the ictus | NA | NA | NA | NA |
| Mhiri et al[9] | 28/M | NA | Dural arteriovenous fistula | No improvement | NA | NA | NA | NA |
| Sinha et al[10] | 63/M | HypertensionCoronary disease | Coronary artery bypass grafting (CABG) | persistent urinary incontinence 5 yr after the ictus | NA | NA | NA | NA |
| Greiner-Perth et al[11] | 66/M | NA | NA | No improvement in 8 mo | NA | T12-L1 | NA | NA |
| Combarros et al[12] | 69/F | Hypertension | NA | The bladder function returned to normal and can walk with a walker 2 mo after the ictus | NA | NA | NA | NA |
| Wildgruber et al[13] | 44/F | NA | Spinal venous thrombosis | Motor function recovered partially and leaving hypoesthesia 6 mo after the ictus | Bilateral anterior horn of gray matter (Snake-eye appearance) | T12-L1 | NA | NA |
| Wong et al[14] | 79/F | Coronary disease | Aortic atherosclerosis | Partial neurologic recovery | Bilateral gray matter and central white matter | T12-L1 | Yes | NA |
| Konno et al[15] | 77/F | Hypertension | Spinal venous thrombosis | Symptoms improved rapidly | Diffuse | L1 | NA | Yes |
| Diehn et al[16] | 24/M | NA | Fibrocartilage embolism | No improvement | Bilateral anterior horn of gray matter | T10-L1 | NA | Yes |
| Alanazy[17] | 48/M | NA | Overstretch | Walking resumed on day 105 | Diffuse | T11-L1 | NA | NA |
| Hor et al[18] | 51/F | NA | NA | NA | Bilateral gray matter and central white matter | T12 | NA | NA |
| Kamimura et al[19] | 70/F | NA | Spinal venous thrombosis | Sensory disturbance improved, leaving numbness in the sellar area and urinary incontinence | Bilateral posterior funiculus, right posterior horn, right lateral funiculus | T12 | NA | Yes |
| Weng et al[20] | 55/M | Hyperlipidemia | Sofa sedentary | Calf muscle atrophy, perianal hypoesthesia and neurogenic bladder 3 yr after ictus | Bilateral anterior horn of gray matter | T11-12 | Yes | Yes |
| 34/F | NA | Toilet sedentary | Calf muscle atrophy, perianal hypoesthesia and neurogenic bladder 4 yr after ictus | NA | T12 | Yes | NA | |
| Breitling et al[21] | 52/M | NA | NA | Motor function recovered partially, leaving bladder and rectum dysfunction | Bilateral anterior horn of gray matter (Snake-eye appearance) | L1 | NA | Yes |
Combined with the patient’s medical history, spontaneous spinal cord infarction was confirmed.
The patient was admitted to the hospital and administered clopidogrel to facilitate antiplatelet aggregation and atorvastatin calcium tablets to reduce blood lipid levels.
After 10 d of treatment, the pain in both lower extremities was relieved, and the symptoms of urinary retention were relieved. After discharge, the patient continued to take clopidogrel and atorvastatin calcium tablets orally. After 3 mo of telephone follow-up, the patient complained of left lower limb pain and prolonged urination time.
Conus medullaris infarction is rare, and its incidence is unclear. A study on the clinical and magnetic resonance imaging manifestations and short-term prognosis of patients with spinal cord infarction showed that only 12.5% had isolated conus medullaris infarction[22]. The blood supply to the conus medullaris is very rich and mainly supplied by the anterior spinal artery, posterior spinal artery, and nerve root medullary artery. The anterior spinal artery supplies the first two-thirds of the conus medullaris, and the posterior spinal artery supplies the last one-third. These form a coronary artery ring at the level of the conus medullaris, which then branches from the artery ring into the conus medullaris. In addition, the thick nerve root medullary artery (Adamkiewicz artery) from the intercostal or lumbar artery from T9 to T12 and the desproges gotteron artery originating from the iliolumbar artery are also involved in the blood supply to the spinal conus[6,23-25]. At present, the diagnosis of conus medullaris infarction is mainly based on clinical manifestations and MRI findings. Lumbar puncture cerebrospinal fluid examination is helpful in distinguishing between inflammatory and demyelinating diseases. In January 2019, Zalewski et al[26] proposed the diagnostic criteria for spontaneous spinal cord infarction, emphasizing that the high signal on a MRI intramedullary T2-weighted image is evidence of acute spinal cord infarction, while the DWI/apparent diffusion coefficient diffusion is limited, accompanied by corresponding pyramidal infarction, arterial dissection, or occlusion near the lesion. However, it is important to note that T2-weighted magnetic resonance imaging has a low sensitivity.
In a clinical study[27], only 45% of patients with acute spinal cord infarction showed a high signal on T2-weighted magnetic resonance imaging. The volume of the conus medullaris is smaller, the magnetic sensitivity artifact tendency of DWI is higher, and the detection sensitivity is much lower than those of acute cerebral infarction. Therefore, early neurological function evaluation is very important in identifying patients with negative MRI findings. The MRI results of this patient were consistent with the above standards. It is worth noting that the patient had an isolated "snake-eye appearance" high signal on axial T2-weighted MRI at the level of the spinal cord cone. This "snake-eye appearance" was first described in the results of delayed myelography in 7 patients with compressive cervical spondylotic myelopathy in 1986[28], and is also known as "owl-eye appearance". The main pathological changes which can result in this appearance are cystic necrosis of the central gray matter of the ventrolateral column of the spinal cord and loss of neurons in the anterior horn of the spinal cord[29]. It is usually related to lower motor neuron syndrome, such as Hirayama disease[30,31], spinal muscular atrophy syndrome[32], cervical spondylotic myelopathy[33], amyotrophic lateral sclerosis[34], and anterior spinal artery ischemia[35-37], Detailed identification is shown in Table 2[34,38-41]. There is a watershed area between the sulcus commissural artery and the coronary artery ring sent by the anterior spinal artery. The anterior horn cells of the spinal gray matter in this area are highly sensitive to hypoxia; when local or overall perfusion is insufficient, they are prone to degeneration and necrosis, forming a "snake-eye appearance" high signal limited to the anterior horn of spinal gray matter on the T2-weighted image of the magnetic resonance imaging axis[42,43]. When this "snake-eye appearance" appears during acute onset myelopathy, a vascular origin should be highly suspected. The most commonly used method is aortic CTA. Thoracic and abdominal CTA can help detect aortic atherosclerotic plaques, dissection, aneurysm, and mural thrombosis. If the CTA result is negative, spinal angiography is necessary to exclude dural arteriovenous fistula and spinal intramedullary arteriovenous malformation[4]. Wildgrube et al[13] previously reported a case of conus medullaris infarction with "snake-eye appearance", caused by spinal cord venous thrombosis. Thus, it is necessary to evaluate thrombophilic factors and improve spinal cord angiography to distinguish between venous and arterial conus medullaris infarction. The cell number and IgG index of cerebrospinal fluid in patients with spinal conus infarction are usually normal. There is no oligoclonal band, and the protein content in cerebrospinal fluid can be slightly increased in some patients[6-7,20,26]. A previous study[44] proposed two mechanisms of spinal cord infarction: (1) Infarction triggered by mechanical factors (bilateral anterior or posterior spinal cord artery infarction and unilateral infarction); and (2) infarction caused by long-term hypotension or arterial insufficiency (central spinal cord artery infarction and transverse spinal cord infarction). In this case, the patient was associated with cardiovascular risk factors, such as hypertension, diabetes, and smoking, but had no definite history of trauma before disease onset. However, upon presentation, he complained of suffering from bilateral lower extremity pain after completing mechanical action from the sofa, and urination disorder was observed. We speculated that the possible mechanism was based on atherosclerosis in the anterior spinal cord. Mechanical stress can lead to anterior spinal artery ischemia. At present, the treatment principles for spinal cord infarction mainly refer to the guidelines for acute ischemic stroke. Old age, severe initial neurological deficit, and long segment lesions are considered to be related to poor prognosis[22,27,45,46]. The prognostic value of the "snake-eye appearance" on magnetic resonance imaging in acute myelopathy is unclear, but may be related to the poor prognosis of chronic myelopathy[29,47,48].
| Disease | Clinical features | Magnetic resonance performance | Neuroelectrophysiological manifestations |
| Conus medullaris infarction[38] | The main manifestations are sensory disturbance in the sellar region, bladder and rectal incontinence, bulbar anal reflex weakening or disappearing, erectile dysfunction, root neuralgia and lower limb motor neuron paralysis when combined with cauda equina damage | T12-L1 horizontal magnetic resonance T2WI and DWI high signal, T1W1 low signal | There are few reports about the neurophysiological characteristics of conus medullaris infarction. The reappearance of F wave after infarction may mark the improvement of clinical prognosis |
| Hirayama disease[39] | The self-limited disease, which is mainly characterized by unilateral muscle atrophy of the distal end of the upper limb, mainly affects the intrinsic muscles of the hand and forearm muscle groups. Typical clinical manifestations also include "cold paralysis", "finger extension tremor" and "muscle bundle tremor" | Asymmetric cervical spinal cord flattening, atrophy and intramedullary T2W1 high signal in cervical flexion position, disappearance of cervical physiological flexion, expansion and increase of epidural venous plexus, and anterior displacement of dural sac after over-extension and over-flexion position | The neurogenic damage of the affected muscle group mainly occurred in the C7-8 sarcomere and T1 sarcomere, while the C5-6 sarcomere (i.e. deltoid, biceps brachii and radial brachii) was not affected |
| Amyotrophiclateralsclerosis[34] | Malignant degenerative motor neuron disease characterized by multiple or localized progressive muscular atrophy and apraxia is characterized by limb spasms, tendon hyperreflexia, localized or multiple muscle weakness, muscular atrophy and fascicular tremor | T2WI, FLAIR and DWI can find symmetrical high signal in the pyramidal tract of the brain. In a few patients, SWI can see the deposition of hemosiderin along the motor cortex | The muscles innervated by different nerve segments of medulla oblongata, neck, chest and lumbosacral appear progressive denervation and chronic nerve regeneration |
| Cervical spondylotic myelopathy[40] | Based on cervical degeneration, the main manifestation is atrophy of the proximal or distal muscles of the upper limb, which usually occurs in one side, usually without sensory abnormalities | It is usually manifested as spinal cord thinning, intervertebral disc protrusion or prolapse. Due to long-term compression of the spinal cord, venous hyperemia and infarction can be caused, which can be secondary to cystic necrosis of the anterior horn of the spinal cord, forming T2WI snake-eye sign | Segmental neurogenic damage consistent with the distribution of the injured nerve root |
| Spinal muscular atrophy syndrome[41] | The most common autosomal recessive disease in childhood is progressive and symmetrical weakness and atrophy of limbs and trunk muscles | Anterior horn of spinal cord α- Degeneration and degeneration of motor neurons led to T2WI snake-eye sign | Typical neuronal damage, fiber fibrillation wave and positive sharp wave can be seen at rest, bundle fibrillation potential can be seen occasionally, and regular spontaneous motor unit activity potential is the characteristic manifestation of its EMG |
Conus medullaris infarction is rare in the clinic and has a high misdiagnosis rate. Detailed medical history and physical examination were the basis of the diagnosis. Although the "snake-eye appearance" is not specific to spinal cord MRI, acute low back pain or bilateral lower limb pain is usually the first symptom, and its clinical manifestation is conus syndrome or cauda equina syndrome. When the axial T2-weighted image of MRI shows "snake-eye appearance”, it is necessary to differentiate between spinal conus infarction caused by anterior spinal artery ischemia. Improving aortography, spinal angiography, and cerebrospinal fluid examination will help to clarify the etiology.
The limitations of this report are its short follow-up period and lack of imaging and neurophysiological evaluation results during the follow-up period. Although we have reviewed previously reported cases of conus medullaris infarction, authors may have different descriptions of clinical characteristics and results. More cases need to be analyzed in the future to improve clinicians' understanding of this disease.
We conclude that acute onset of conus medullaris syndrome combined with "snake-eye appearance" should be strongly suspected as conus medullaris infarction caused by anterior spinal artery ischemia. This special imaging manifestation is helpful in the early diagnosis and treatment of conus infarction.
We would like to thank Dr. Bo Sun and Dr. Dan Ren for providing high-resolution MRI and CTA images. Finally, we thank the patient for his participation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Arboix A, Spain, Spain; Ji X, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989;68:282-292. [PubMed] |
| 2. | Rubin MN, Rabinstein AA. Vascular diseases of the spinal cord. Neurol Clin. 2013;31:153-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Weidauer S, Nichtweiß M, Hattingen E, Berkefeld J. Spinal cord ischemia: aetiology, clinical syndromes and imaging features. Neuroradiology. 2015;57:241-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Nasr DM, Rabinstein A. Spinal Cord Infarcts: Risk Factors, Management, and Prognosis. Curr Treat Options Neurol. 2017;19:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Herrick MK, Mills PE Jr. Infarction of spinal cord. Two cases of selective gray matter involvement secondary to asymptomatic aortic disease. Arch Neurol. 1971;24:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Anderson NE, Willoughby EW. Infarction of the conus medullaris. Ann Neurol. 1987;21:470-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Ohbu S, Ishimoto A, Honda M, Fukuda H, Hata Y, Tada S. Infarction of the conus medullaris. Eur Neurol. 1990;30:343-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Andrews BT, Kwei U, Greco C, Miller RG. Infarct of the conus medullaris simulating a spinal cord tumor: case report. Surg Neurol. 1991;35:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Mhiri C, Miladi MI, Triki C, Kechaou MS. Sacral meningeal arteriovenous fistula supplied by branches of the hypogastric artery revealed by conus medullaris infarction. Spinal Cord. 2000;38:711-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Sinha S, Sirigiri SR, Kanakamedala SK, Singh MK, Sharma RM. Duloxetine for treatment of male sphincteric incontinence following partial conus medullaris infarction after coronary bypass surgery. Cases J. 2009;2:9094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Greiner-Perth R, Schenke H, Böhm H, Both R. Spinal cord tumour simulated by an infarction of the conus medullaris. Acta Neurochir (Wien). 1999;141:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Combarroso O, Sánchez-Pernaute R, Orizaola P, Berciano J. Absence of F-waves as an early electrodiagnostic finding in infarction of the conus medullaris. Muscle Nerve. 1995;18:552-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Wildgruber D, Kuntz R, Kermer P, Bartel J, Fetter M, Dichgans J. Elsberg syndrome due to infarction of the conus medullaris associated with a prothrombin mutation. J Neurol. 1999;246:507-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Wong JJ, Dufton J, Mior SA. Spontaneous conus medullaris infarction in a 79 year-old female with cardiovascular risk factors: a case report. J Can Chiropr Assoc. 2012;56:58-65. [PubMed] |
| 15. | Konno T, Suwabe T, Kasahara S, Umeda Y, Oyake M, Fujita N. [A case of conus medullaris infarction expanding to the vertebral bodies, major psoas and erector spinae muscles]. Rinsho Shinkeigaku. 2015;55:661-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Diehn FE, Hunt CH, Lehman VT, Schwartz KM, Eckel LJ, Black DF, Wood CP, Kotsenas AL, Wald JT, Hocker SE. Vertebral body infarct and ventral cauda equina enhancement: two confirmatory findings of acute spinal cord infarct. J Neuroimaging. 2015;25:133-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Alanazy MH. Conus medullaris stroke. Does F wave predict return of ambulation? Neurosciences (Riyadh). 2016;21:260-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Hor H, Nader H, Tarnutzer AA. Absent F-waves in conus medullaris stroke mimicking Guillain-Barré syndrome. BMJ Case Rep. 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Kamimura T, Nezu T, Aoki S, Ueno H, Hosomi N, Maruyama H. Conus Medullaris Infarction Involving the Paraspinal Muscles and Nerve Roots. J Stroke Cerebrovasc Dis. 2020;29:104983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 20. | Weng YC, Chin SC, Wu YY, Kuo HC. Clinical, neuroimaging, and nerve conduction characteristics of spontaneous Conus Medullaris infarction. BMC Neurol. 2019;19:328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Breitling B, Schmeel FC, Radbruch A, Kaut O. Sudden paraparesis due to spinal cord ischemia with initial contrast enhancement of the cauda equina and time-delayed owl-eyes sign on follow-up MR imaging: a case report. Neurol Res Pract. 2021;3:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Masson C, Pruvo JP, Meder JF, Cordonnier C, Touzé E, De La Sayette V, Giroud M, Mas JL, Leys D; Study Group on Spinal Cord Infarction of the French Neurovascular Society. Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. J Neurol Neurosurg Psychiatry. 2004;75:1431-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Yoshioka K, Niinuma H, Ehara S, Nakajima T, Nakamura M, Kawazoe K. MR angiography and CT angiography of the artery of Adamkiewicz: state of the art. Radiographics. 2006;26 Suppl 1:S63-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Martirosyan NL, Kalani MY, Lemole GM Jr, Spetzler RF, Preul MC, Theodore N. Microsurgical anatomy of the arterial basket of the conus medullaris. J Neurosurg Spine. 2015;22:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Montalbano MJ, Loukas M, Oskouian RJ, Tubbs RS. Innervation of the blood vessels of the spinal cord: a comprehensive review. Neurosurg Rev. 2018;41:733-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Zalewski NL, Rabinstein AA, Krecke KN, Brown RD Jr, Wijdicks EFM, Weinshenker BG, Kaufmann TJ, Morris JM, Aksamit AJ, Bartleson JD, Lanzino G, Blessing MM, Flanagan EP. Characteristics of Spontaneous Spinal Cord Infarction and Proposed Diagnostic Criteria. JAMA Neurol. 2019;76:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 27. | Nedeltchev K, Loher TJ, Stepper F, Arnold M, Schroth G, Mattle HP, Sturzenegger M. Long-term outcome of acute spinal cord ischemia syndrome. Stroke. 2004;35:560-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Jinkins JR, Bashir R, Al-Mefty O, Al-Kawi MZ, Fox JL. Cystic necrosis of the spinal cord in compressive cervical myelopathy: demonstration by iopamidol CT-myelography. AJR Am J Roentgenol. 1986;147:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Mizuno J, Nakagawa H, Inoue T, Hashizume Y. Clinicopathological study of "snake-eye appearance" in compressive myelopathy of the cervical spinal cord. J Neurosurg. 2003;99:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Sarawagi R, Narayanan S, Lakshmanan PM, Chakkalakkoombil SV. Hirayama disease: imaging profile of three cases emphasizing the role of flexion MRI. J Clin Diagn Res. 2014;8:RD03-RD04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Xu H, Shao M, Zhang F, Nie C, Wang H, Zhu W, Xia X, Ma X, Lu F, Jiang J. Snake-Eyes Appearance on MRI Occurs during the Late Stage of Hirayama Disease and Indicates Poor Prognosis. Biomed Res Int. 2019;2019:9830243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Hsu CF, Chen CY, Yuh YS, Chen YH, Hsu YT, Zimmerman RA. MR findings of Werdnig-Hoffmann disease in two infants. AJNR Am J Neuroradiol. 1998;19:550-552. [PubMed] |
| 33. | Zhang Z, Wang H. Is the "snake-eye" MRI sign correlated to anterior spinal artery occlusion on CT angiography in cervical spondylotic myelopathy and amyotrophy? Eur Spine J. 2014;23:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Verma A. Clinical Manifestation and Management of Amyotrophic Lateral Sclerosis. In: Amyotrophic Lateral Sclerosis [Internet]. Brisbane (AU): Exon Publications; 2021-Jul-25. [PubMed] |
| 35. | Bekci T, Yucel S, Aslan K, Gunbey HP, Incesu L. "Snake eye" appearance on a teenage girl with spontaneous spinal ischemia. Spine J. 2015;15:e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Alves JN, Machado C, Taveira M, Soares-Fernandes J, Ferreira C, Pinho J. Teaching NeuroImages: Restricted diffusion "snake eyes appearance" in acute spinal cord ischemia. Neurology. 2016;86:e204-e205. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Martínez Tébar MJ, Baeza Román A, Mejia Olmos GJ. «Snake eye» and «pencil-like» signs together with diaphragmatic paralysis in a patient with anterior spinal cord ischemia. Med Intensiva (Engl Ed). 2019;43:452. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Tan YJ, Manohararaj N. Isolated Infarctions of the Conus Medullaris: Clinical Features and Outcomes. J Stroke Cerebrovasc Dis. 2021;30:106055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Iacono S, Di Stefano V, Gagliardo A, Cannella R, Virzì V, Pagano S, Lupica A, Romano M, Brighina F. Hirayama disease: Nosological classification and neuroimaging clues for diagnosis. J Neuroimaging. 2022;32:596-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 40. | Zhang J, Liu MS, Cui LY, Li BH, DU H. [A clinical, electromyographic, and magnetic resonance imaging study of cervical spondylotic myelopathy: analysis of 96 cases]. Zhonghua Yi Xue Za Zhi. 2009;89:328-330. [PubMed] [DOI] [Full Text] |
| 41. | Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, Mazzone ES, Vitale M, Snyder B, Quijano-Roy S, Bertini E, Davis RH, Meyer OH, Simonds AK, Schroth MK, Graham RJ, Kirschner J, Iannaccone ST, Crawford TO, Woods S, Qian Y, Sejersen T; SMA Care Group. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 632] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 42. | Pullicino P. Bilateral distal upper limb amyotrophy and watershed infarcts from vertebral dissection. Stroke. 1994;25:1870-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Gelfan S, Tarlov IM. Differential vulnerability of spinal cord structures to anoxia. J Neurophysiol. 1955;18:170-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 45. | Salvador de la Barrera S, Barca-Buyo A, Montoto-Marqués A, Ferreiro-Velasco ME, Cidoncha-Dans M, Rodriguez-Sotillo A. Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord. 2001;39:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Hsu JL, Cheng MY, Liao MF, Hsu HC, Weng YC, Chang KH, Chang HS, Kuo HC, Huang CC, Lyu RK, Lin KJ, Ro LS. The etiologies and prognosis associated with spinal cord infarction. Ann Clin Transl Neurol. 2019;6:1456-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Fontanella MM, Zanin L, Bergomi R, Fazio M, Zattra CM, Agosti E, Saraceno G, Schembari S, De Maria L, Quartini L, Leggio U, Filosto M, Gasparotti R, Locatelli D. Snake-Eye Myelopathy and Surgical Prognosis: Case Series and Systematic Literature Review. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Funaba M, Imajo Y, Suzuki H, Nishida N, Nagao Y, Sakamoto T, Fujimoto K, Sakai T. Impact of various MRI signal intensity changes on radiological parameters, the neurological status, and surgical outcomes in degenerative cervical myelopathy. Clin Neurol Neurosurg. 2021;207:106802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |