Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.2036
Peer-review started: November 29, 2022
First decision: February 14, 2023
Revised: February 20, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 26, 2023
Processing time: 107 Days and 23.5 Hours
Acromicric dysplasia (AD) is a rare skeletal dysplasia. Its incidence is < 1/1000000, and only approximately 60 cases are reported worldwide. It is a disease characterized by severe short stature, short hands and feet, facial abnormalities, normal intelligence, and bone abnormalities. Unlike other skeletal dysplasia, AD has a mild clinical phenotype, mainly characterized by short stature. Extensive endocrine examination has not revealed a potential cause. The clinical effect of growth hormone therapy is still uncertain.
We report a clinical phenotype of AD associated with mutations in the fibrillin 1 (FBN1) (OMIM 102370) gene c.5183C>T (p. Ala1728Val) in three people from a Chinese family. A 4-year-old member of the family first visited the hospital because of slow growth and short stature for 2 years, but no abnormalities were found after a series of laboratory tests, echocardiography, pituitary magnetic resonance imaging, and ophthalmological examination. Recombinant human growth hormone (rhGH) was used to treat the patient for > 5 years. The efficacy of rhGH was apparent in the first year of treatment; the height increased from -3.64 standard deviation score (SDS) to -2.88 SDS, while the efficacy weakened from the second year. However, long-term follow-up is required to clarify the efficacy of rhGH.
FBN1-related AD has genetic heterogeneity and/or clinical variability, which brings challenges to the evaluation of clinical treatment. rhGH is effective for treatment of AD, but long-term follow-up is needed to clarify the effect.
Core Tip: Acromicric dysplasia (AD) is a rare skeletal dysplasia, and its incidence is < 1 in 1000000. Here, we report a clinical phenotype of AD associated with mutations in the fibrillin 1 (OMIM 102370) gene c.5183C>T (p.Ala1728Val) in three people from a Chinese family. A 4-year-old boy was treated with recombinant human growth hormone (rhGH) for > 5 years. The efficacy of rhGH was clear in the first year of treatment; his height increased from -3.64 standard deviation score (SDS) to -2.88 SDS, while the efficacy weakened from the second year. However, long-term follow-up is required to clarify the efficacy of rhGH.
- Citation: Shen R, Feng JH, Yang SP. Acromicric dysplasia caused by a mutation of fibrillin 1 in a family: A case report. World J Clin Cases 2023; 11(9): 2036-2042
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/2036.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.2036
Geleophysic dysplasia (GD), acromicric dysplasia (AD), and Weill–Marchesani syndrome (WMS) are all acrodysplasia syndromes caused by mutations in the fibrillin 1 (FBN1) gene. AD is a rare heterogeneous skeletal dysplasia characterized by short stature, short hands and feet, stiff joints, thickened skin, facial abnormalities, normal intelligence, and skeletal abnormalities, with a prevalence of < 1 in 1000000[1-3]. Although these diseases are similar in physical features, each disease has its own unique clinical phenotype. GD is characterized by happy facial features, progressive valvular heart disease, progressive hepatomegaly, tracheal stenosis, and tiptoe gait. Although AD shows similar characteristics as GD, AD usually does not manifest heart valve abnormalities, hepatomegaly, tracheal stenosis, or eye disease[4]. WMS is distinguished from AD by ocular abnormalities, including lens myopia, lens ectopia, glaucoma, and spherical lenses. In contrast to the genetic heterogeneity of GD and WMS, AD is caused only by mutations in FBN1 and is an autosomal dominant disorder[5]. Here, we report the clinical phenotype and genetic characteristics of three cases of AD associated with a mutation in the FBN1 (OMIM 102370) gene c.5183C>T (p. Ala1728Val) in a Chinese family, to enhance clinicians’ understanding of the disease and to share the experience of a young patient receiving long-term recombinant human growth hormone (rhGH) therapy.
A 4-year-old boy presented with slow growth and short stature for 2 years.
Two years ago, the patient was found to have short stature, normal mental capacity, liver enlargement, dyspnea, myopia, glaucoma, and other symptoms.
The patient was healthy in the past and had no history of skin, heart, lung, liver, kidney or eye diseases.
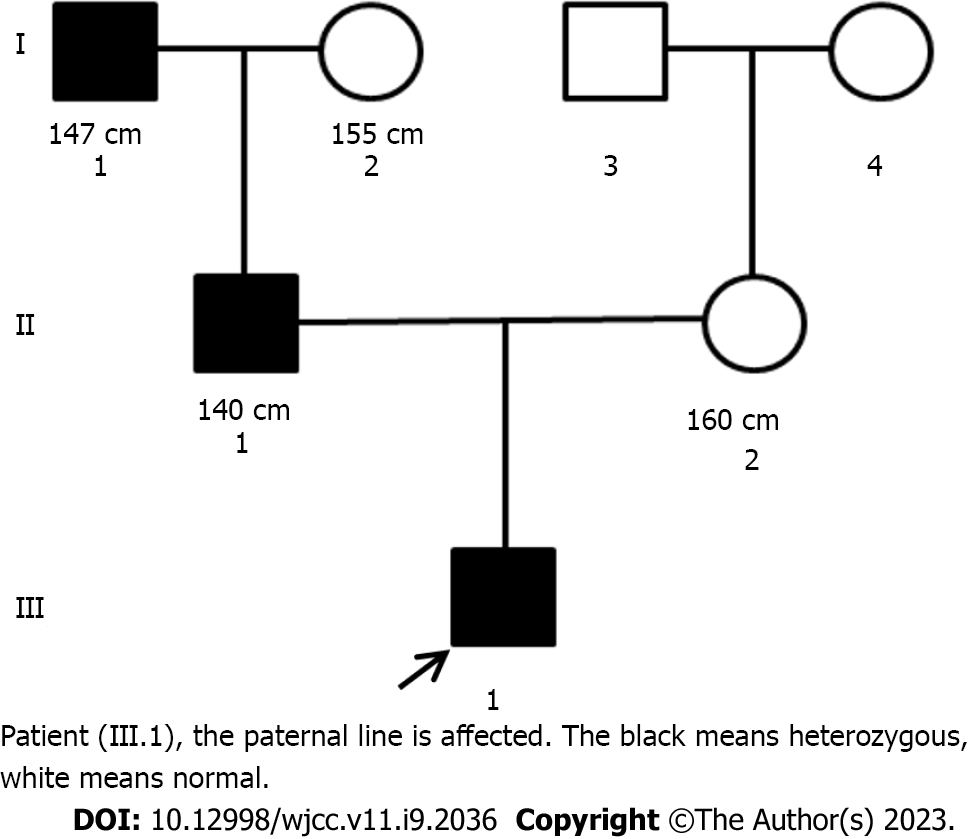
The patient was born in Zhejiang Province of China, of Han nationality. The patient was the first birth of the second pregnancy, born by cesarean section at 40 wk’ gestation, with a birth weight of 3500 g and length of 50 cm, and without a history of birth trauma asphyxia at birth. In the family, the height of the father was 140 cm [< -5 standard deviation score (SDS)][6] tall and the grandfather was 147 cm (< -4 SDS)[6] tall, the mother and grandmother were of normal height, and neither parents nor grandparents were consanguineous (Figure 1).
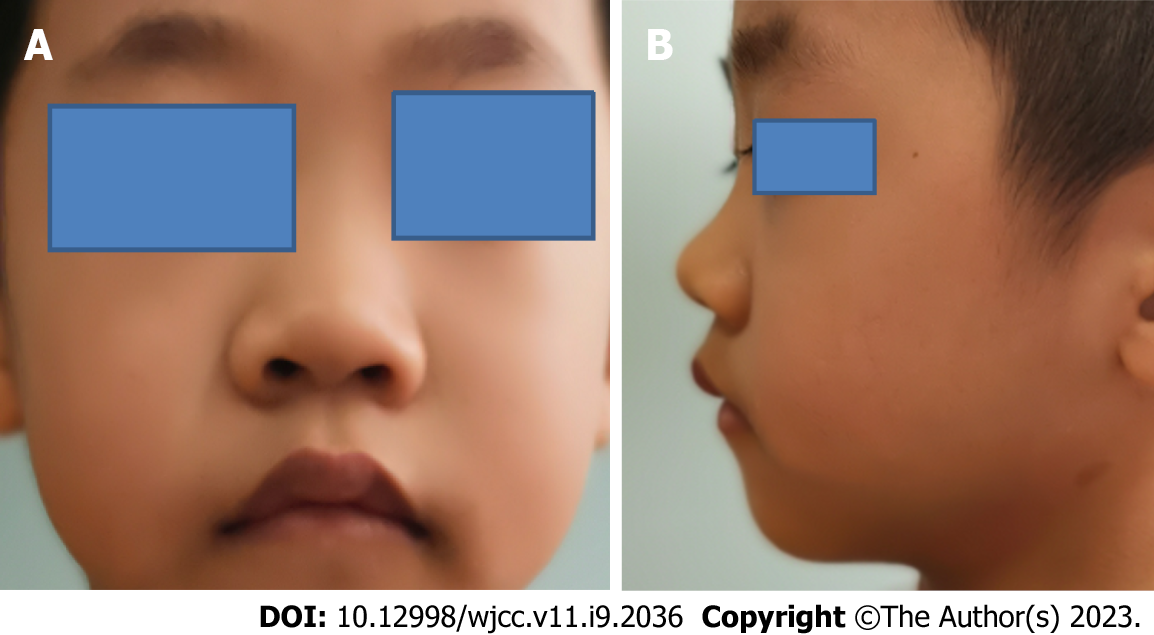
The patient was short (90.5 cm, < -3 SDS)[6], with low weight (12.5 kg, < -2 SDS)[6], round face, stubby nose, forward-leaned nostrils, long eyelashes and thick lips (Figure 2).
There were no obvious abnormalities in sex hormones, insulin-like growth factor (IGF)-1, IGF binding protein-3, thyroid function, biochemistry, trace elements, routine blood tests, routine urine tests, growth hormone challenge tests, cardiac ultrasound, pituitary-enhanced magnetic resonance imaging or karyotyping (550 bands, G bands).
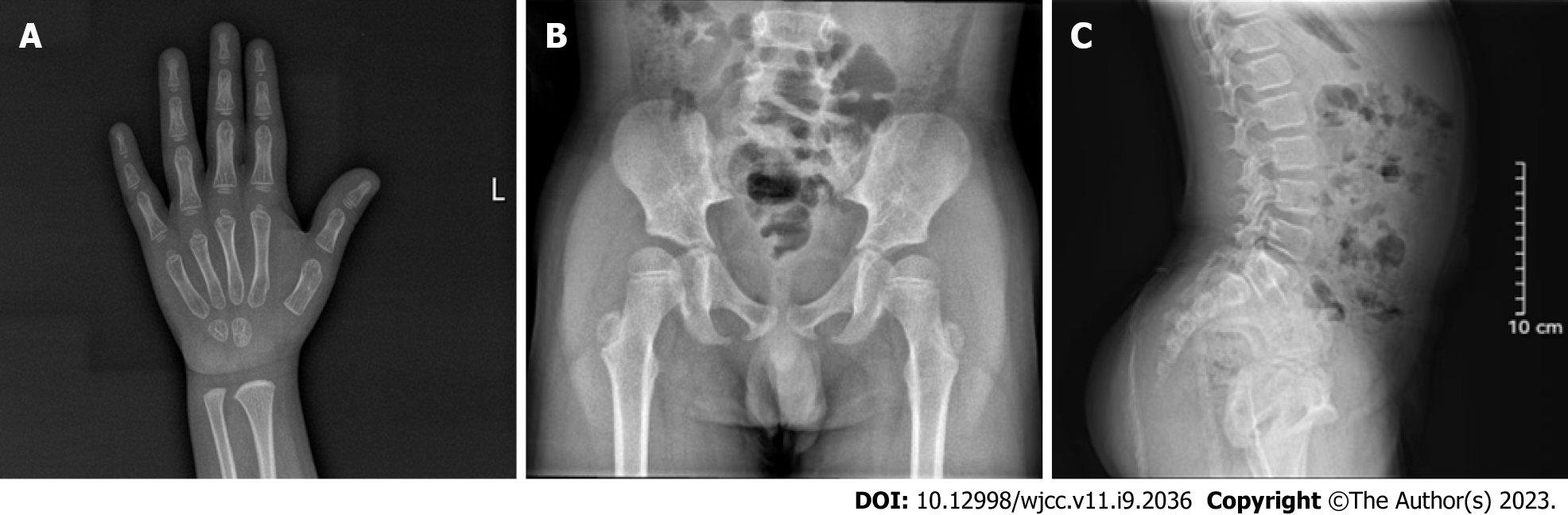
Radiography showed delayed bone age (2.4 years) (Figure 3A). Plain pelvic radiography showed a beak-like femur head (Figure 3B), and the lumbar lateral radiograph showed lumbar lordosis (Figure 3C).
The patient, his father and grandfather all showed significant familial short stature. We suspected the possibility of hereditary short stature and recommended that the patient, his parents and his grandfather underwent whole-exome sequencing. On October 17, 2019, whole-exome sequencing of the patient, his father and grandfather showed a mutation of the FBN1 gene (OMIM 102370) c.5183C>T (p. Ala1728Val), while whole-exome sequencing of the patient’s mother showed no abnormalities. The pathogenicity criteria of the mutation according to the American College of Medical Genetics and Genomics, and the pathogenicity rating was likely pathogenic[7].
Combined with the patient’s medical history, physical examination and whole-exome sequencing, the final diagnosis was AD.
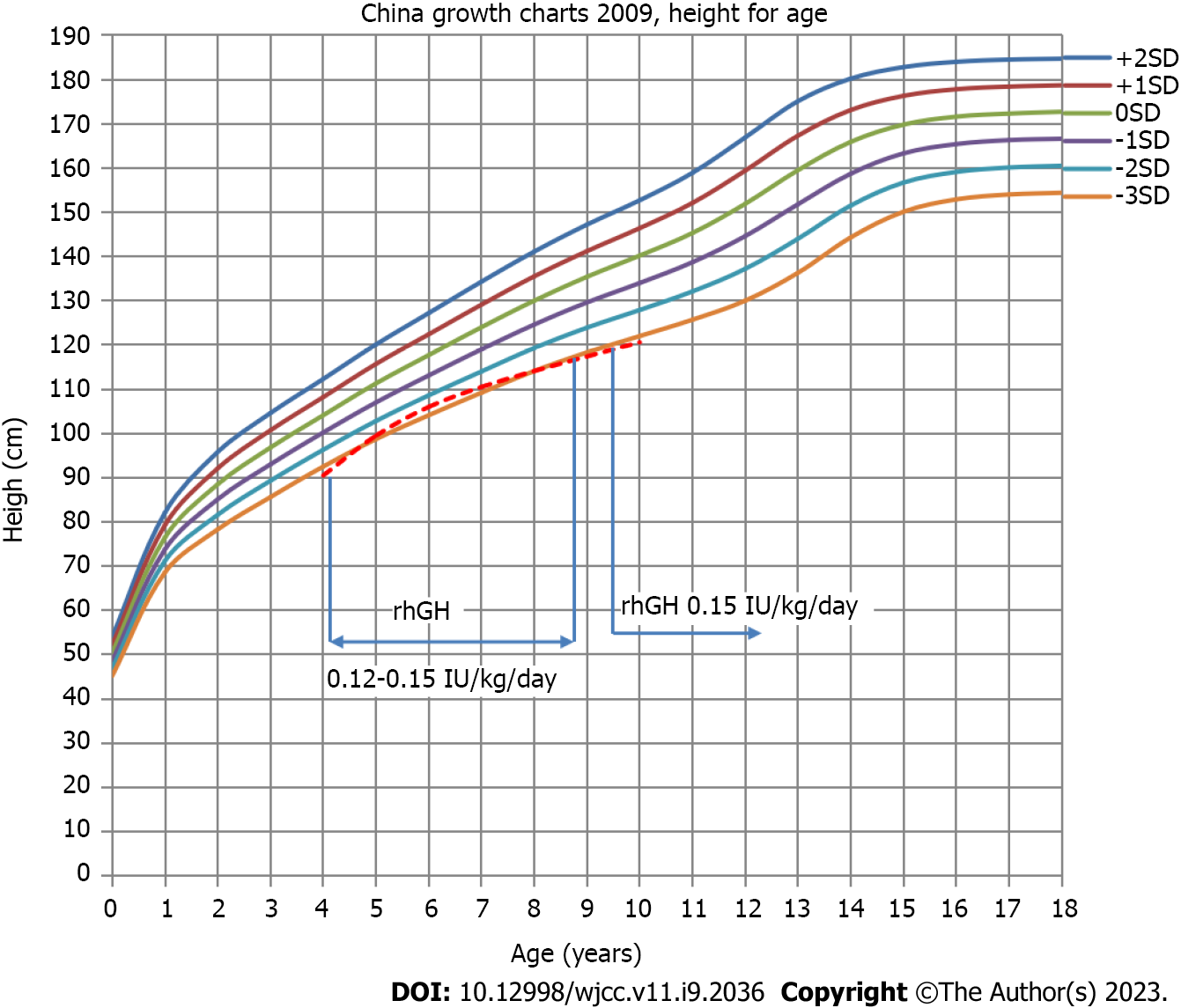
Before whole-exome sequencing, the initial diagnosis was idiopathic short stature. From October 2016 to October 2019, rhGH at a dose of 0.12–0.15 IU/kg/d was used to treat the patient. After whole-exome sequencing, the final diagnosis was AD. Although the current case reports suggested that rhGH treatment had limited benefits on the final height of the children, the parents requested to continue rhGH treatment after informed consent was obtained. From October 2019 to present, rhGH at a dose of 0.15 IU/kg/d was used to treat the patient (it was suspended from July 2021 to February 2022) (Figure 4).
The height at the beginning of treatment was 90.5 cm (-3.64 SDS), and the height after treatment in the first year (October 2016 to September 2017) was 99.5 cm (-2.88 SDS). Thus, the annual growth rate during the first year was 9.0 cm/year. The height after treatment in the second year (October 2017 to September 2018) was 106 cm (-2.60 SDS); thus, the annual growth rate during the second year was 6.5 cm/year. The height after treatment in the third year (October 2018 to September 2019) was 110.5 cm (-2.80 SDS); thus, the annual growth rate during the third year was 4.5 cm/year. The height after treatment in the fourth year (October 2019 to September 2020) was 114.1 cm (-3.04 SDS); thus, the annual growth rate during the fourth year was 3.6 cm/year. The height after treatment in the fifth year (October 2020 to July 2021) was 117.1 cm (-3.09 SDS), yielding an annual growth rate during the fifth year of 3.0 cm/year. Considering the poor efficacy, it is recommended to stop rhGH treatment. After stopping rhGH for 7 mo, the height was 118.5 cm (-3.17 SDS) in February 2022; thus, the annual growth rate was 2.4 cm/year. The parents requested reuse of rhGH. Afterward, the height was 119.6 cm (-3.20 SDS) in June 2022 and 121.7 cm (-3.11 SDS) in October 2022, indicating an annual growth rate of 4.8 cm/year. To date, the patient has been treated and followed up for 6 years (Figure 4). The efficacy of rhGH treatment was clear in the first year, and the height increased from -3.64 SDS to -2.88 SDS. However, the efficacy of the treatment weakened from the second year. At the request of the parents, the patients would continue to be treated with rhGH and be followed up.
In 1986, Maroteaux et al[8] first described a novel type of osteodysplasia in six children with short stature, short hands and feet, normal intelligence, mild facial deformities, and hand X-ray abnormalities. They called the disease AD and could not clarify the cause further because of the limitations of the genetic diagnostic technology at that time.
In 1991, Dietz et al[9] first identified the FBN1 gene as the causative gene of Marfan syndrome (MS). In 2011, Le Goff et al[10] found that the FBN1 gene was associated with GD and AD autosomal dominant inheritance, which showed the opposite phenotype of MS. Both AD and MS have FBN1 gene mutations, so why do the two diseases have opposite phenotypes? Mutations in FBN1 leading to MS exist throughout the entire length of the gene (mainly in exon regions 13, 15, 24–28, 32 and 43)[11], while FBN1 mutations that result in AD are mainly limited to the hotspot regions of exons 41 and 42[5]. These findings indicated that the clinical phenotype could be related to the type and location of the gene variant.
In the 2015 revision of the classification of hereditary bone diseases, acral dysplasia included 10 diseases, among which the FBN1 gene mutation causes AD, GD, and WMS, and specific mutations in the FBN1 gene have been found in AD, GD and WMS patients[1,4,12]. The FBN1 gene is located in the long arm of human chromosome 15 (15q15–q21.1) and contains 66 exons, encoding a 2871-aa (350 kDa) structural protein called fibrillin (FBN)1[13]. FBN1 consists of 47 epidermal growth factor-like domains and seven transforming growth factor (TGF)-β1-binding-protein-like domains[14]. FBN1 is the only gene associated with AD that is inherited in an autosomal dominant manner[5]. The FBN1 gene can participate in the TGF-β signaling pathway by regulating the bioavailability and local activity of TGF-β, which is an important pathway for linear growth regulation. AD and GD2 (ADAMTSL2-negative) are caused by TGF-β5 region heterozygous missense mutations in the FBN1 gene, and disruption of TGF-β signaling is a common underlying mechanism in different types of patients with acrodysplasia[15].
In the present case, the patient’s father and grandfather all had mutations in the FBN1 gene c.5183C>T (p. Ala1728Val). This gene mutation was first found in a GD patient with severe short stature and cardiac involvement[10]. We reviewed three previously reported AD cases associated with mutation of FBN1 (OMIM 102370) gene c.5183C>T (p.Ala1728Val). A Brazilian boy developed severe dwarfism at the age of 10 years and 2 mo (-3.9 SDS). He was born with normal birth length, his mother was short at 131cm (-5 SDS), while his father and brothers were normal in height. Physical examination found that fingers and palms were short. His nose bridge was wide, the middle part of his nose was prominent, his lips were thick, and he had obvious genu varus, but his gait was normal. No abnormality was found in endocrine examination. The patient was diagnosed as idiopathic short stature and began to receive rhGH treatment (50-66 mg/kg/d) according to experience, but the overall effect was poor[15]. An African–American girl born in the United States was 107.5 cm (-4 SDS) tall at the age of 7 years. She was born at 37 wk of gestation, with a birth length of only 43 cm (-2.3 SDS) and a birth weight of 2580 g (-0.9 SDS). The height of both parents was normal. The physical examination found that the bridge of the nose was broad, and she had slight osteoporosis, thick lips, small hands and feet, but no other abnormalities. Endocrine examination was normal, and bone age was delayed by about 2 years. At the age of 8 years and 9 mo, recombinant IGF-1 was administered according to experience, and reached a maximum dose of 90 mg/kg/d. The growth rate increased significantly. However, this growth acceleration was confused with early puberty development at the age of 9 years and 1 mo. After the start of leuprorelin treatment, the growth rate decreased to 4–5 cm/year before treatment. Treatment with recombinant IGF-1 was stopped at the age of 9 years and 4 mo[15]. A 5 years and 7 month old Chinese boy was 100.3 cm tall (< -3 SDS). The birth length was 49 cm. His father was 148 cm tall (< -4 SDS). His mother was 160 cm tall. Physical examination showed that the hands were wide and short, but there was no joint stiffness, and there were no abnormality in cardiovascular, abdominal or endocrine examination. The X-ray examination showed that the tubular bone of the hands were shortened and the femoral head was beaked[13]. Although these cases had the same gene mutation site, the clinical phenotypes varied. This site variant might be associated with the autosomal dominant AD (OMIM: 102370), GD (OMIM: 614185) and WMS (OMIM: 608328)[1]. In our case, the three patients showed severe short stature as the main manifestation, and none of them had hepatomegaly, heart valve disease, joint stiffness or eye disease. Meanwhile, the disease condition was milder compared with GD or WMS, neither the quality of the study or quality of life was affected, and the adults are doing well in their professions; thus, all the clinical conditions support the diagnosis of AD. The same mutation at the FBN1 gene locus could lead to a variety of different clinical phenotypes, and the discovery of multiple phenotypes could help to conduct further research on the pathogenesis of the diseases.
GH therapy has not been widely used in patients with skeletal dysplasia because of genetic heterogeneity and/or clinical variability, which pose challenges in assessing treatment effectiveness. The efficacy of GH in the treatment of AD is still unclear. Faivre et al[16] reported one case of AD given rhGH treatment, but there was no significant effect on final height. Jin et al[17] found that a patient with AD received rhGH treatment of 0.1 IU/kg/d for 3.5 years, and during treatment, his growth rate remained at 5 cm/year, and his height was -2.23 SDS after 3.5 years. However, long-term follow-up is needed to verify the effect of rhGH treatment. The effects of GH treatment in our present case were similar to those in the patient just described. In our case, the patient’s height increased by 9.0 cm in the first year of rhGH treatment, 6.5 cm in the second year, 4.5 cm in the third year, 3.6 cm in the fourth year, and 3 cm in the fifth year. In the first year of treatment, the annual growth rate was 9.0 cm/year, and the height increased from -3.64 SDS to -2.88 SDS, an increase of 0.76 SDS, suggesting that rhGH therapy was effective[18]. In the patient's family, the height of the father was 140 cm (< -5 SDS) tall and the grandfather was 147 cm (< -4 SDS) tall. In the first 5 years of treatment, the growth rate of the patient remained at 5.3 cm/year, and the height increased from -3.64 SDS to -3.09 SDS. It seems that the treatment had some effect, and the first year was the most significant. However, whether it would have a positive effect on the final height remains unclear, and long-term follow-up observation is needed.
AD is a rare skeletal dysplasia and an autosomal dominant disease. FBN1 is the only gene related to AD. FBN1-related acral dysplasia has genetic heterogeneity and/or clinical variability, which brings challenges to the evaluation of clinical treatment. rhGH therapy appears to have some effect on growth, but long-term follow-up is needed to clarify the effect.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhai JF, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Bonafe L, Cormier-Daire V, Hall C, Lachman R, Mortier G, Mundlos S, Nishimura G, Sangiorgi L, Savarirayan R, Sillence D, Spranger J, Superti-Furga A, Warman M, Unger S. Nosology and classification of genetic skeletal disorders: 2015 revision. Am J Med Genet A. 2015;167A:2869-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 398] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 2. | Globa E, Zelinska N, Dauber A. The Clinical Cases of Geleophysic Dysplasia: One Gene, Different Phenotypes. Case Rep Endocrinol. 2018;2018:8212417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Quitter F, Flury M, Waldmueller S, Schubert T, Koehler K, Huebner A. Acromicric dysplasia due to a novel missense mutation in the fibrillin 1 gene in a three-generation family. J Pediatr Endocrinol Metab. 2022;35:1443-1447. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Zhang H, Ye J, Han L, Gu X. Three novel mutations of the FBN1 gene in Chinese children with acromelic dysplasia. J Hum Genet. 2014;59:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (2)] |
| 5. | Sakai LY, Keene DR, Renard M, De Backer J. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene. 2016;591:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 6. | Li H, Ji CY, Zong XN, Zhang YQ. [Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years]. Zhonghua Er Ke Za Zhi. 2009;47:487-492. [PubMed] |
| 7. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22559] [Article Influence: 2255.9] [Reference Citation Analysis (0)] |
| 8. | Maroteaux P, Stanescu R, Stanescu V, Rappaport R. Acromicric dysplasia. Am J Med Genet. 1986;24:447-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1374] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 10. | Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, Brady AF, Cordier MP, Devriendt K, Genevieve D, Kiper PÖ, Kitoh H, Krakow D, Lynch SA, Le Merrer M, Mégarbane A, Mortier G, Odent S, Polak M, Rohrbach M, Sillence D, Stolte-Dijkstra I, Superti-Furga A, Rimoin DL, Topouchian V, Unger S, Zabel B, Bole-Feysot C, Nitschke P, Handford P, Casanova JL, Boileau C, Apte SS, Munnich A, Cormier-Daire V. Mutations in the TGFβ binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am J Hum Genet. 2011;89:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Collod-Béroud G, Le Bourdelles S, Ades L, Ala-Kokko L, Booms P, Boxer M, Child A, Comeglio P, De Paepe A, Hyland JC, Holman K, Kaitila I, Loeys B, Matyas G, Nuytinck L, Peltonen L, Rantamaki T, Robinson P, Steinmann B, Junien C, Béroud C, Boileau C. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat. 2003;22:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Le Goff C, Cormier-Daire V. From tall to short: the role of TGFβ signaling in growth and its disorders. Am J Med Genet C Semin Med Genet. 2012;160C:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Shan YC, Yang ZC, Ma L, Ran N, Feng XY, Liu XM, Fu P, Yi MJ. A Review of Three Chinese Cases of Acromicric/Geleophysic Dysplasia with FBN1 Mutations. Int J Gen Med. 2021;14:1873-1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Sakai LY, Keene DR. Fibrillin protein pleiotropy: Acromelic dysplasias. Matrix Biol. 2019;80:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | de Bruin C, Finlayson C, Funari MF, Vasques GA, Lucheze Freire B, Lerario AM, Andrew M, Hwa V, Dauber A, Jorge AA. Two Patients with Severe Short Stature due to a FBN1 Mutation (p.Ala1728Val) with a Mild Form of Acromicric Dysplasia. Horm Res Paediatr. 2016;86:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Faivre L, Le Merrer M, Baumann C, Polak M, Chatelain P, Sulmont V, Cousin J, Bost M, Cordier MP, Zackai E, Russell K, Finidori G, Pouliquen JC, Munnich A, Maroteaux P, Cormier-Daire V. Acromicric dysplasia: long term outcome and evidence of autosomal dominant inheritance. J Med Genet. 2001;38:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Jin HS, Song HY, Cho SY, Ki CS, Yang SH, Kim OH, Kim SJ. Acromicric Dysplasia Caused by a Novel Heterozygous Mutation of FBN1 and Effects of Growth Hormone Treatment. Ann Lab Med. 2017;37:92-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, Chernausek SD, Savage MO, Wit JM; 2007 ISS Consensus Workshop participants. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93:4210-4217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 448] [Article Influence: 26.4] [Reference Citation Analysis (0)] |