Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.2029
Peer-review started: December 1, 2022
First decision: January 2, 2023
Revised: January 14, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 26, 2023
Processing time: 105 Days and 21.2 Hours
The standard treatment for advanced T2 gastric cancer (GC) is laparoscopic or surgical gastrectomy (either partial or total) and D2 lymphadenectomy. A novel combined endoscopic and laparoscopic surgery (NCELS) has recently been proposed as a better option for T2 GC. Here we describe two case studies demo
Two T2 GC cases were both resected by endoscopic submucosal dissection and full-thickness resection and laparoscopic lymph nodes dissection. This method has the advantage of being more precise and minimally invasive compared to current methods. The treatment of these 2 patients was safe and effective with no complications. These cases were followed up for nearly 4 years without recu
This novel method provides a minimally invasive treatment option for T2 GC, and its potential indications, effectiveness and safety needs to be further eva
Core Tip: We propose a new method of combining endoscopic local lesion resection and laparoscopic lymph node dissection for 2 cases of advanced T2 gastric cancer (GC). This new method has the advantages of precision and being minimally invasive with similar outcomes as surgical resection. It is a novel method providing a minimally invasive treatment option for T2 GC.
- Citation: Dai JH, Qian F, Chen L, Xu SL, Feng XF, Wu HB, Chen Y, Peng ZH, Yu PW, Peng GY. Novel combined endoscopic and laparoscopic surgery for advanced T2 gastric cancer: Two case reports. World J Clin Cases 2023; 11(9): 2029-2035
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/2029.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.2029
The standard treatment for advanced gastric cancer (GC) is laparoscopic or surgical gastrectomy (either partial or total) and D2 lymphadenectomy[1]. Here, we report 2 cases indicating that endoscopic local lesion resection combined with laparoscopic lymph node dissection may be an effective treatment for T2 GC. These cases included a 23-year-old male with a 2.0 cm × 2.0 cm T2 poorly differentiated gastric antrum adenocarcinoma and a 54-year-old female with a 2.0 cm × 2.5 cm T2 poorly differentiated gastric body adenocarcinoma. Both were resected by endoscopic submucosal dissection and full-thickness resection (ESDFTR) and laparoscopic lymph node dissection with good postoperative recovery. This method has the advantages of being more precise and minimally invasive compared to current methods. With this in mind, its potential indications, effectiveness and safety need to be further evaluated in controlled studies.
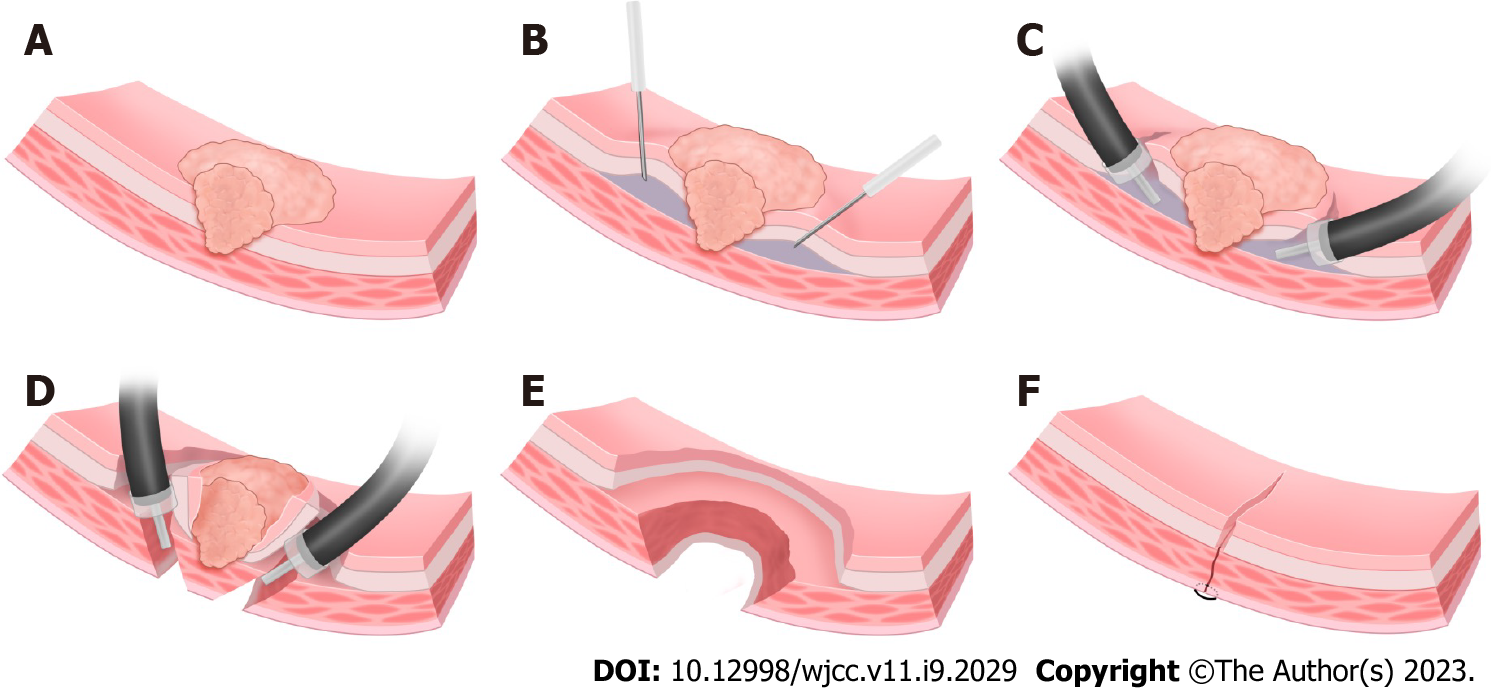
At present, partial or total gastrectomy is used in the minimally invasive treatment of advanced GC. However, this procedure has the drawback that it destroys the normal anatomical structure of the stomach seriously affecting its physiological function. It is often associated with substantial trauma, slower recovery, a higher complication rate and decreased postoperative quality of life. With advances in endoscopic resection technology, endoscopic submucosal dissection (ESD) or closed laparoscopic and endoscopic cooperative surgery is increasingly applied for T1 early cancer[2]. Endoscopic full-thickness resection technology can completely remove benign tumors of the muscularis propria[3]. For treatment of T2 GC, we reported a novel combined endoscopic and laparoscopic surgery (NCELS) to resect local lesions with ESDFTR (Figure 1) along with lymph node removal by laparoscope, which together achieved complete resection of the lesion and thorough clearing of the lymph nodes. This procedure also allowed preservation of the anatomical structure and physiological function of the stomach thereby enhancing the postoperative quality of life of the patients.
A 23-year-old male and a 54-year-old female were admitted to our hospital with poorly differentiated gastric adenocarcinoma.
No specific medical history.
Carcinoembryonic antigen (CEA) test values were in the normal range.
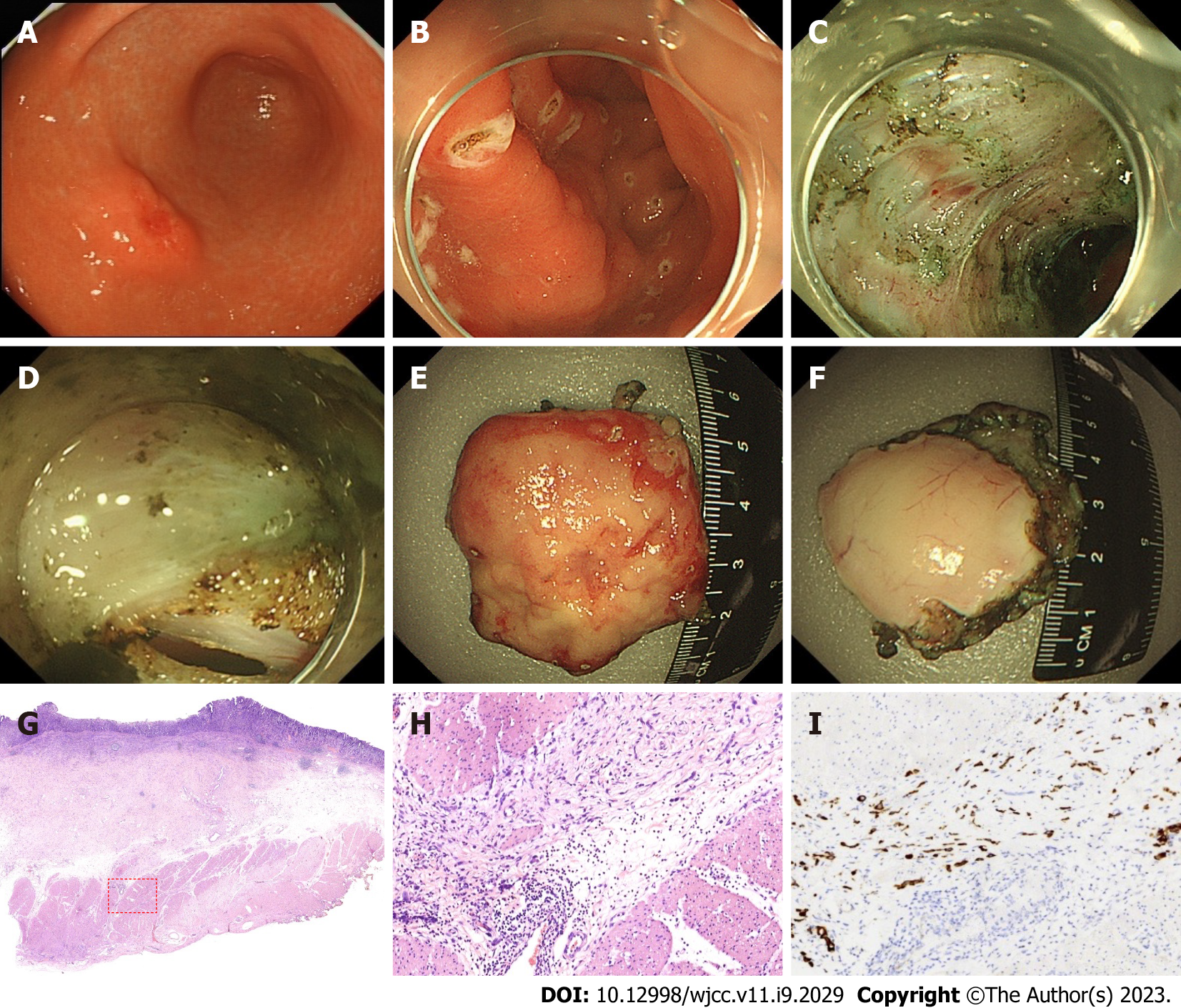
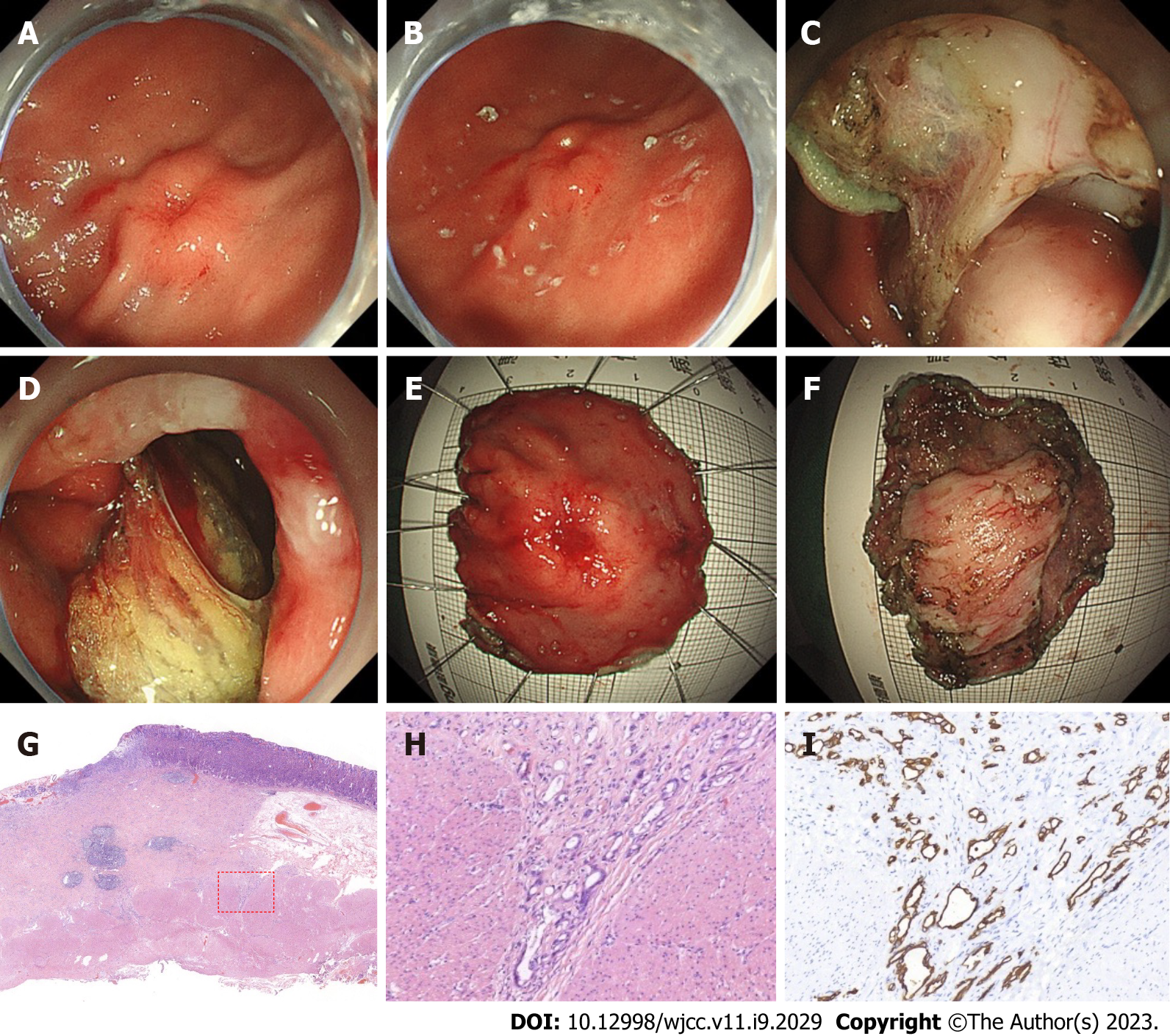
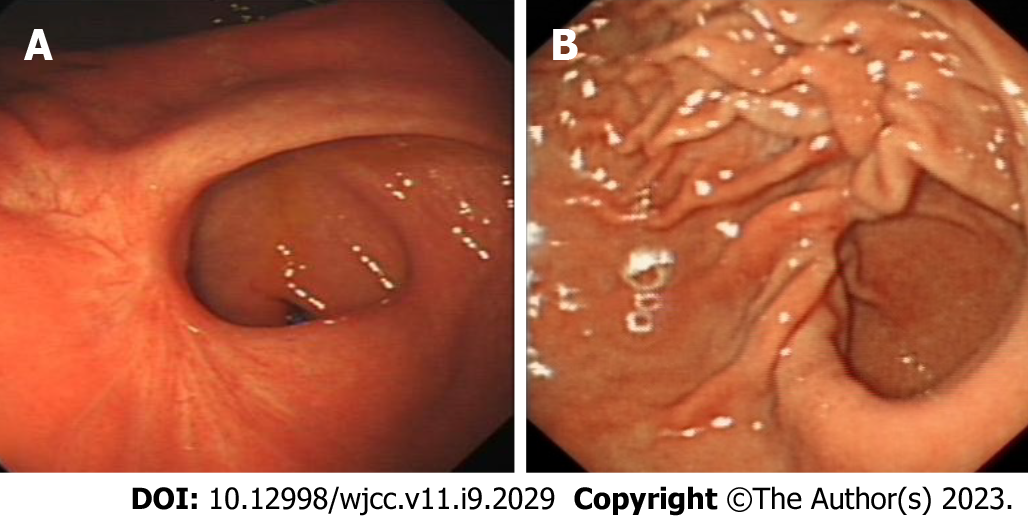
Gastroscopy revealed a 2.0 cm × 2.0 cm protuberant lesion on the greater curvature of the anterior wall of the gastric antrum in the male patient (Figure 2) and a 2.0 cm × 2.5 cm 0-IIa + IIc type lesion on the greater curvature of the lower gastric body in the female patient (Figure 3). Pathological examination showed poorly differentiated adenocarcinoma. Contrast-enhanced computed tomography (CT) of the abdomen showed that areas of the antrum and gastric body were slightly thickened. No metastasis to the lymph nodes or other organs was observed on the chest and abdomen CT scans. Endoscopic ultrasonography revealed invasion of the muscularis propria.
The clinical diagnosis in both cases was T2 GC without lymph node and other organ metastasis. Postoperative pathological diagnosis was T2 GC for both cases.
The patients were placed under general anesthesia with endotracheal intubation. The procedure included four steps as follows:
Step 1: Determine the demarcation line. Magnifying endoscopy with narrow band imaging was used to determine the demarcation line of the lesion. Marking was set to 1.5 cm outside the lesion.
Step 2: ESDFTR. After submucosal injection, the lesion was dissected by circumferential ESD until the tumor infiltrated deeper (the submucosa was thin or unclear), then dental floss was used to traction the lesion. A whole layer incision was performed at 0.5-1.0 cm lateral to the deep infiltration, followed by circumferential full-thickness resection, and the resected lesion was removed.
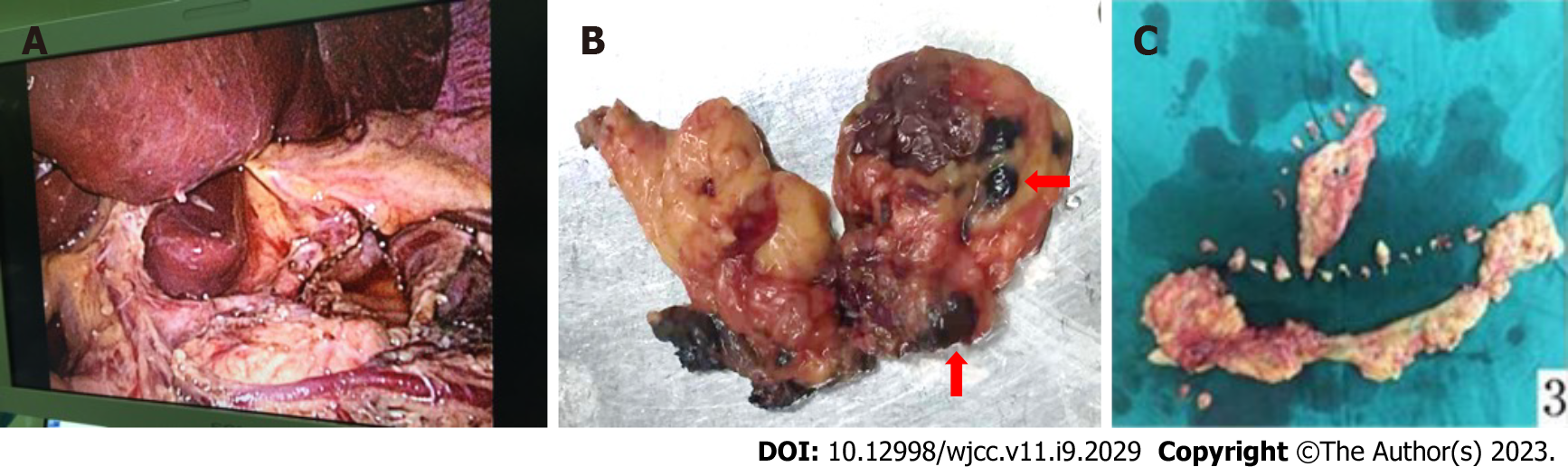
Step 3: Gastric wound suture and lymph node dissection. The gastric wound was sutured with an endoscope or laparoscope. Then regional lymph nodes were radically dissected (Figure 4).
Step 4: Pathological examination. The resected gastric specimens and lymph nodes were pathologically examined to determine the depth of invasion and lymph node metastasis.
The 2 cases were characterized as poorly differentiated adenocarcinoma, infiltrating into the superficial layer of the muscularis propria. One case had negative lymph node metastasis, and the other case had nerve infiltration. Postoperative pathological diagnosis was T2 GC for both cases.
The patients were discharged 8 d and 10 d after operation. No local or metastatic recurrence of lymph nodes and organs were found in regular review of gastroscopy (Figure 5) and enhanced CT of the abdomen within 4 years for the male patient and 3 years for the female patient following the operation. CEA test values remained in the normal range. Their postoperative quality of life was good, with no significant gastrointestinal symptoms, and they retained a normal diet and weight.
At present, there are two main techniques for minimally invasive treatment of GC: endoscopic resection and laparoscopic radical resection. Both endoscopic and laparoscopic therapies have their own advantages and limitations in the treatment of T2 GC. The advantages of endoscopic resection are that the lesion can be observed under direct vision, the resection range of the lesion can be accurately determined, the lesion can be completely removed, and the normal gastric anatomy and gastric physiological function can be preserved, thus ensuring postoperative quality of life of the patient. However, the limitations are that the lymph nodes cannot be cleared and the risk of lymph node metastasis cannot be eliminated[4]. The advantage of laparoscopic surgery is that it can completely remove the lesion and thoroughly clear the lymph nodes. However, because T2 cancers grow in the lumen, the extent of the lesion cannot be accurately determined outside the gastric wall. Therefore, laparoscopic surgery alone requires partial or total gastrectomy, which destroys the normal anatomical structure of the stomach and affects the physiological function of the stomach, thus seriously affecting postoperative quality of life of the patient, combined with a risk of local residue and recurrence[5,6].
T2 GC often has deep infiltration in the central part of the lesion, while the surrounding region is shallow, with mucosal or submucosal infiltration. Using ESDFTR technology, submucosal dissection can be used to completely remove the mucosal and submucosal infiltrating lesion, followed by full-thickness resection to remove the lesion in the deep submucosa and muscularis propria, which can minimize the damage to the gastric anatomical structure providing a more precise and minimally invasive treatment option. In addition, laparoscopy is a more reliable procedure to seal the gastric wound. Furthermore, the lymph nodes can be thoroughly cleared of metastatic tumor tissue. Therefore, the NCELS can both provide a cure and be precise and minimally invasive. Compared with traditional surgery, NCELS for T2 GC achieved complete resection of the lesion, thorough clearing of the lymph nodes and preservation of the anatomical structure and physiological function of the stomach. It has the advantages of less trauma, fewer complications, lower cost and better postoperative quality of life.
The novel method described here provides a minimally invasive treatment option for T2 GC. However, the selection of standard indications, surgical methods, short-term and long-term complications, long-term recurrence rate and prognosis still need further evaluation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YH, China; Murariu MS, Romania S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 2. | Saito H, Nishimura A, Sakimura Y, Tawara H, Hayashi K, Kato K, Tsuji T, Yamamoto D, Kitamura H, Kadoya S, Bando H. Closed laparoscopic and endoscopic cooperative surgery for early gastric cancer with difficulty in endoscopic submucosal dissection: a report of three cases. Surg Case Rep. 2020;6:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Bhagat VH, Kim M, Kahaleh M. A Review of Endoscopic Full-thickness Resection, Submucosal Tunneling Endoscopic Resection, and Endoscopic Submucosal Dissection for Resection of Subepithelial Lesions. J Clin Gastroenterol. 2021;55:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Eisenberg D, Bell R. Intraoperative endoscopy: a requisite tool for laparoscopic resection of unusual gastrointestinal lesions--a case series. J Surg Res. 2009;155:318-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Matsuda T, Hiki N, Nunobe S, Aikou S, Hirasawa T, Yamamoto Y, Kumagai K, Ohashi M, Sano T, Yamaguchi T. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc. 2016;84:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Hu J, Or BH, Hu K, Wang ML. Comparison of the post-operative outcomes and survival of laparoscopic versus open resections for gastric gastrointestinal stromal tumors: A multi-center prospective cohort study. Int J Surg. 2016;33 Pt A:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |