Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.2002
Peer-review started: November 8, 2022
First decision: November 25, 2022
Revised: December 2, 2022
Accepted: February 21, 2023
Article in press: February 21, 2023
Published online: March 26, 2023
Processing time: 128 Days and 21.6 Hours
Ralstonia is a Gram-negative non-fermentative bacterium widespread in nature, and includes four species, Ralstonia pickettii, Ralstonia solanacearum, Ralstonia mannitolilytica, and Ralstonia insidiosa, which were proposed in 2003. Ralstonia is mainly found in the external water environment, including municipal and medical water purification systems. This bacterium has low toxicity and is a conditional pathogen. It has been reported in recent years that infections due to Ralstonia are increasing. Previous studies have shown that most cases of infection are caused by Ralstonia pickettii, a few by Ralstonia mannitolilytica, and infections caused by Ralstonia insidiosa are rare.
A 2-year-old Chinese child suffered from intermittent fever and cough for 20 d and was admitted to hospital with bronchial pneumonia. Bronchoscopy and alveolar lavage fluid culture confirmed Ralstonia insidiosa pneumonia. The infection was well controlled after treatment with meropenem and azithromycin.
Ralstonia infections are increasing, and we report a rare case of Ralstonia insidiosa infection in a child. Clinicians should be vigilant about Ralstonia infections.
Core Tip: Ralstonia is a rare type of conditionally pathogenic bacterium found in nature, and its infection incidents have been increasing in recent years. We describe a 2-year-old male baby, who was diagnosed with Ralstonia insidiosa infection after culture of alveolar lavage fluid. The infection was controlled with a combination of two antibiotics. Our report adds to the case reports of rare Ralstonia insidiosa infections and warns doctors to be aware of this rare infection.
- Citation: Lin SZ, Qian MJ, Wang YW, Chen QD, Wang WQ, Li JY, Yang RT, Wang XY, Mu CY, Jiang K. Children with infectious pneumonia caused by Ralstonia insidiosa: A case report. World J Clin Cases 2023; 11(9): 2002-2008
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/2002.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.2002
Ralstonia is a non-fermentative species widespread in nature, it is a Gram-negative bacterium, which was first isolated in 1973 and incorporated into Burkholderia (Burkholderia pickettii and Burkholderia solanacearum). The genus Ralstonia was named separately in 1995 by Yabuuchi et al[1], and it includes Ralstonia pickettii, Ralstonia solanacearum, Ralstonia mannitolilytica, and Ralstonia insidiosa, which were newly introduced in 2003[2]. Ralstonia reproduces in wet conditions and can survive long-term in harsh environments, mainly in external water environments, including municipal water and medical water purification systems[3,4]. Previous reports indicated that the bacterium was less virulent and was an opportunistic pathogen. However, human infections with Ralstonia without exposure to a contaminated solution are rare, thus the bacterium was not considered a major pathogen[5].
However, the incidence of Ralstonia infection is increasing in recent years. Previous studies have shown that most cases of infection are caused by Ralstonia pickettii, and a few by Ralstonia mannitolilytica; these pathogens can cause bloodstream infections, pneumonia, prostatitis, and many other diseases[6]. Ralstonia pickettii, has been isolated from various clinical specimens, such as sputum, blood, infected wounds, urine, ears, nasal swabs, and cerebrospinal fluid[5].
Ralstonia insidiosa is a new species proposed in 2003[7], which is closest to Ralstonia pickettii, and it has been reported to be isolated from the respiratory tract of patients with cystic fibrosis. In addition, Ralstonia insidiosa has been detected in water distribution and laboratory-purified water systems[8]. However, due to the low incidence of this bacterium infection and insufficient clinical awareness, reports of Ralstonia insidiosa infection are infrequent.
A boy aged 2 years and 6 mo presented with intermittent fever and cough for 20 d and attended hospital on January 27, 2019.
The child had a fever without apparent cause which started 20 d previously. His fever was persistent, with yellow-colored sputum but no blood-stained sputum. The clinical diagnosis was bronchial pneumonia, and azithromycin, erythromycin and other drugs were administered for 19 d, and the effect was not satisfactory. On admission, the child still had a fever, and cough with sputum.
The child had previously been physically fit. Family members denied contact with contaminated water in the endemic area or history of surgery and invasive procedures.
The child was gravida 1, para 1, delivered by cesarean section at term, and had no history of related infections, infectious diseases, or history of genetic diseases in the family.
The child’s weight was 16 kg, height 91 cm, admission temperature 39.6 °C, pulse rate 120 bpm, and respiratory rate 28 breaths/min. He had good nutritional status, normal development, no rash on the skin or mucous membranes throughout the body, no pharyngeal hyperemia, and no enlargement and purulent discharge of tonsils, and superficial lymph nodes in the entire body were not palpable. Breath was regular and orderly, breath sounds in both lungs were rough, and crackles could be heard. The heart rhythm was aligned. Pericardial rub, additional heart sounds, and pathological murmur were not heard on auscultation. The abdominal examination was normal. The liver, gallbladder and spleen were not palpable, percussion pain in both kidney areas was negative, and neurological examination was normal.
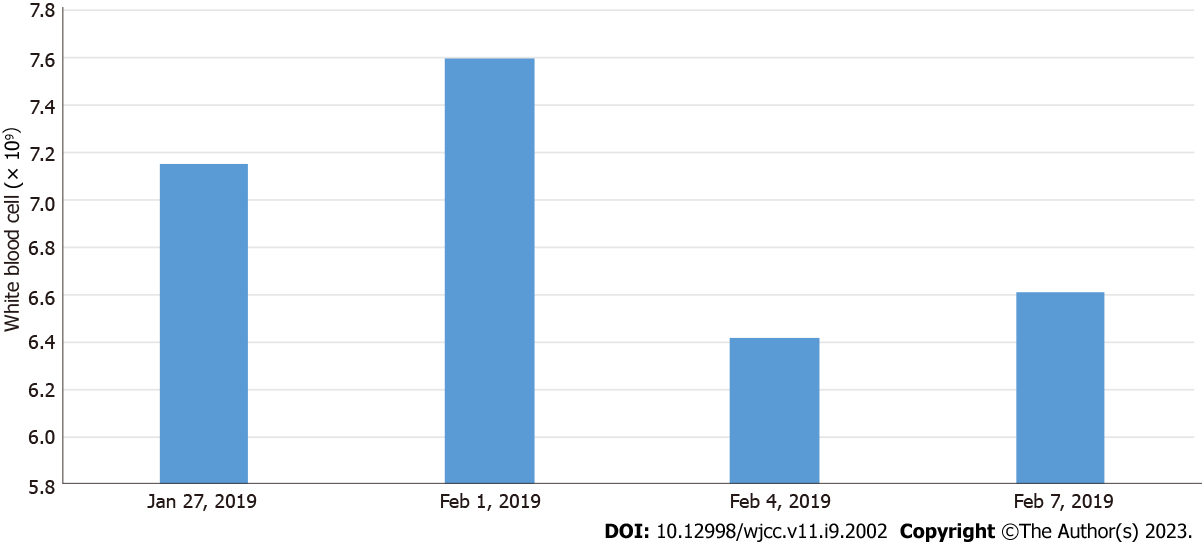
On January 27, 2019, routine blood tests showed white blood cell count (WBC) 7.15 × 109/L, neutrophil (NEU) 59.90%, lymphocyte (LYM) 31.20%, monocyte (MON) ratio 8.50%, red blood cell count (RBC) 4.37 × 1012/L, platelet (PLT) count 159 × 109/L, abnormal LYM (YX) < 10%, and high-sensitivity C-reactive protein (hs-CRP) < 0.50 mg/L.
On January 28, 2019, procalcitonin was < 0.1 ng/mL, immunoglobulin A 0.693 g/L, and complement C3 0.720 g/L. No abnormalities were found in myocardial enzymes, liver and kidney function, and various viral antibody tests. Tuberculosis antibodies, antinuclear antibodies, rheumatoid factor detection and the anti-O test were normal.
On January 30, 2019, thyroid function tests showed T3 0.877 nmol/L, T4 52.92 nmol/L, free triiodothyronine 2.39 pmol/L, free thyroxine 9.43 pmol/L, A-thrombopoietin 96.34 IU/mL, and A-thyroglobulin 328.80 IU/mL.
On February 1, 2019, routine blood tests showed WBC 7.60 × 109/L, NEU 45.80%, LYM 36.70%, MON 8.30%, RBC 4.45 × 1012/L, PLT 253 × 109/L, and hs-CRP 11 mg/L, and D-dimer 932 μg/L. Fungal-D glucan was higher than 162 pg/mL (normal values were < 100.5 pg/mL). No abnormalities in tuberculosis-infected T cells and respiratory virus antibodies were detected.
On February 2, 2019, a blood culture was tested on the next day after admission, and the result was negative on the 7th d.
On February 4, 2019, routine blood tests showed WBC 6.42 × 109/L, NEU 38.64%, LYM 51.44%, MON 6.24%, RBC 4.20 × 1012/L, PLT 277 × 109/L, and hs-CRP < 0.50 mg/L.
On February 7, 2019, routine blood tests showed WBC 6.61 × 109/L, NEU 47.44%, LYM 39.34%, MON 8.04%, RBC 4.39 × 1012/L, PLT 272 × 109/L, and hs-CRP < 0.50 mg/L.
According to the “National Clinical Laboratory Operating Procedures” compiled by the Department of Medical Administration, Ministry of Health, China, The usual WBC count range is (4.0-10.0) × 109, the normal value for the percentage of NEUs is 50%-70%, the normal value for the LYM percentage is 20%-40%, the normal value of the percentage of monocytes is 3%-8%, the normal value of RBCs is (3.5-5.5) × 1012/L, the normal value of PLTs is (100-300) × 109/L, the normal value for the hypersensitive CRP is 0-3 mg/L.
Computed tomography (CT) scanning of the lungs was performed, with the following findings.
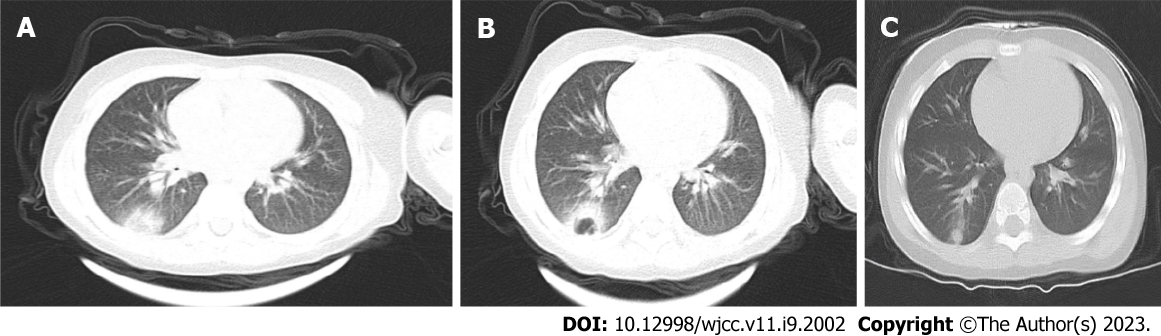
On January 27, 2019, the texture of both lungs was enhanced, the bronchial vascular bundle was thickened and disordered, multiple fuzzy cord shadows were seen, and the lower lobe of the right lung showed patchy dense opacity, with smooth edges and an uneven internal density (Figure 1A).
On January 31, 2019, compared with the previous CT images on January 27, 2019, the density of the lower lobe of the right lung had increased significantly, the edges were blurred, and an irregular cavity was seen inside. A small amount of fluid was noted in the right thorax (Figure 1B).
The patient was eventually diagnosed with Ralstonia insidiosa pneumonia.
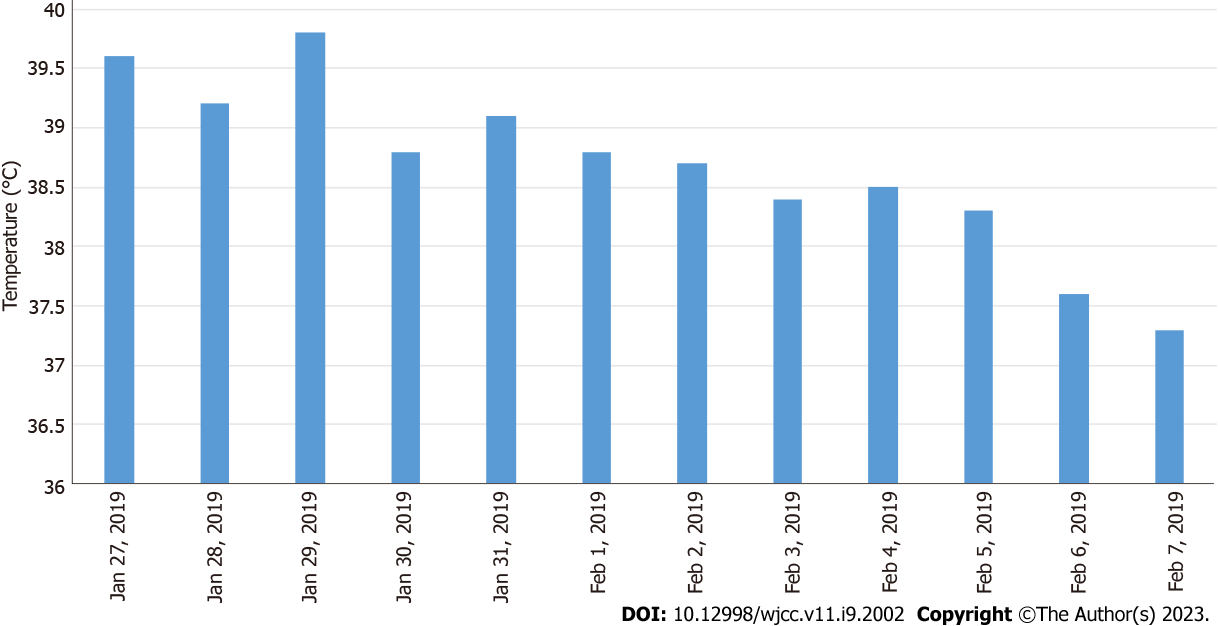
The patient was started on conventional empirical medication with amoxicillin sodium-clavulanate potassium 30 mg/kg 3 times daily and adenosine monophosphate 5 mg/kg once a day. On the third day of admission, the child had increased fever and was given meropenem 20 mg/kg 3 times daily and immunoglobulin 1 g/kg once a day intravenously. Subsequently, meropenem 20 mg/kg was administered 3 times daily combined with azithromycin 10 mg/kg once a day. Based on the timeline of the onset of the infection, a chart of the child’s fever trend (Figure 2) and WBC count (Figure 3) was prepared for comparison.
The child has been followed up, and no disease recurrence has been observed. A pulmonary CT performed 42 d after discharge on March 21, 2019, showed texture enhancement in both lungs. The lower lobe of the right lung showed patchy and cord-like high-density opacities with blurred edges (Figure 1C).
With regard to Ralstonia infections, a review of previous literature showed that the majority of cases were nosocomial infections, with evidence that Ralstonia enters the hospital environment primarily through a contaminated water supply system[9]. Infections are primarily caused by contaminated solutions, including blood products, sterile water, saline, respiratory therapeutic fluids[10-13], and that the most common infected items are blood collection tubes, dialysis machines, and nebulizers, which may eventually lead to different diseases such as bacteremia, sepsis, respiratory infections, and pneumonia[14], with human infections unrelated to contaminated solutions being rare. Infections occur predominantly in weak or immunosuppressed individuals, mainly in the elderly or neonates who are immunocompromised.
The child in the present report was admitted to hospital with intermittent fever and cough for 20 d, with no history of surgery or invasive procedures which excluded contact with medical devices such as ventilators and dialysis machines. The child had a long course of the disease and received antibiotics for 19 d before admission to the hospital (exact dose was unknown). After admission, we ruled out febrile illnesses such as tuberculosis, infectious mononucleosis, Kawasaki disease, rheumatic fever, and epidemic hemorrhagic fever. Physical and chemical examinations showed lower values of immunoglobulin A, complement C3, and serum T3 and T4, suggesting that the child was immunocompromised. On admission, CT showed a patchy dense shadow in the lower lobe of the right lung. A blood culture was tested on the next day after admission, and the result was negative on the 7th d. This also made the diagnosis difficult. The child was initially considered to have lobar pneumonia and was given amoxicillin sodium-clavulanate potassium, immunoglobulin, and meropenem as empirical treatment against infection. The child’s condition did not improve, and a second CT scan showed a significant increase in the hyperdense shadow in the lower lobe of the right lung and a small amount of pleural effusion. The child was also tested for fungal-D glucan, which was higher than 162 mg/L. This also led us to consider the possibility of specific flora infectious pneumonia and fungal pneumonia at the time of diagnosis. As fungal pneumonia does not have characteristic imaging, bronchoalveolar lavage is used for diagnosis[15]. During follow-up, the child underwent bronchoscopy, and an alveolar lavage fluid culture was performed, which eventually confirmed the diagnosis of Ralstonia insidiosa infection based on the lavage fluid culture results.
Reliable therapeutic data on Ralstonia insidiosa infection are lacking. Studies related to drug resistance in Ralstonia insidiosa have shown that this bacterium is highly resistant to the aminoglycoside gentamicin and the β-lactam antibiotic aztreonam, with different drug resistance to ticarcillin-clavulanic acid mixtures[16]. Studies by Ryan and Adley[16] have shown that most Ralstonia strains are sensitive to carbapenem antibiotics. All isolates were sensitive to quinolones (ciprofloxacin and ofloxacin), tetracyclines (tetracycline and minocycline), cephalosporins (cefotaxime and ceftazidime), folate pathway inhibitors (meperidine/sulfamethoxazole) and uropipexicillin broad-spectrum-lactam antibiotics (piperacillin)[17]. Our patient, after 3 d of anti-infective treatment with azithromycin 10 mg/kg once daily combined with meropenem 20 mg/kg three times daily prior to the diagnosis of Ralstonia insidiosa infection, showed a significant reduction in fever and a significant improvement in clinical signs. Therefore, this regimen was continued for 4 d after diagnosis and the infection eventually improved and was controlled.
Ralstonia infections are becoming increasingly common in clinical practice. The reasons for this may be associated with the massive clinical use and partial misuse of antibiotics, the insidious nature of the bacterium, which is easily overlooked in the early stages of infection, and its multi-drug resistance, which makes the infection difficult to control. Our report presents a rare case of childhood Ralstonia insidiosa pneumonia. Clinicians should be vigilant about Ralstonia infections, especially in immunocompromised patients and those with a history of invasive procedures such as ventilators and dialysis machines.
We would like to thank the patient and his family members for their contributions to this report.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Chiu H, Taiwan; Karunanayake A, Sri Lanka S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Yabuuchi E, Kosako Y, Yano I, Hotta H, Nishiuchi Y. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. Nov.: Proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. Nov., Ralstonia solanacearum (Smith 1896) comb. Nov. and Ralstonia eutropha (Davis 1969) comb. Nov. Microbiol Immunol. 1995;39:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 363] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 2. | Sharma D, Sharma P, Soni P, Gupta B. Ralstonia picketti neonatal sepsis: a case report. BMC Res Notes. 2017;10:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Chen YY, Huang WT, Chen CP, Sun SM, Kuo FM, Chan YJ, Kuo SC, Wang FD. An Outbreak of Ralstonia pickettii Bloodstream Infection Associated with an Intrinsically Contaminated Normal Saline Solution. Infect Control Hosp Epidemiol. 2017;38:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Vincenti S, Quaranta G, De Meo C, Bruno S, Ficarra MG, Carovillano S, Ricciardi W, Laurenti P. Non-fermentative gram-negative bacteria in hospital tap water and water used for haemodialysis and bronchoscope flushing: prevalence and distribution of antibiotic resistant strains. Sci Total Environ. 2014;499:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Pan W, Zhao Z, Dong M. Lobar pneumonia caused by Ralstonia pickettii in a sixty-five-year-old Han Chinese man: a case report. J Med Case Rep. 2011;5:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Fang Q, Feng Y, Feng P, Wang X, Zong Z. Nosocomial bloodstream infection and the emerging carbapenem-resistant pathogen Ralstonia insidiosa. BMC Infect Dis. 2019;19:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Coenye T, Goris J, De Vos P, Vandamme P, LiPuma JJ. Classification of Ralstonia pickettii-like isolates from the environment and clinical samples as Ralstonia insidiosa sp. nov. Int J Syst Evol Microbiol. 2003;53:1075-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Ryan MP, Pembroke JT, Adley CC. Genotypic and phenotypic diversity of Ralstonia pickettii and Ralstonia insidiosa isolates from clinical and environmental sources including High-purity Water. Diversity in Ralstonia pickettii. BMC Microbiol. 2011;11:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Tayeb LA, Lefevre M, Passet V, Diancourt L, Brisse S, Grimont PA. Comparative phylogenies of Burkholderia, Ralstonia, Comamonas, Brevundimonas and related organisms derived from rpoB, gyrB and rrs gene sequences. Res Microbiol. 2008;159:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Moreira BM, Leobons MB, Pellegrino FL, Santos M, Teixeira LM, de Andrade Marques E, Sampaio JL, Pessoa-Silva CL. Ralstonia pickettii and Burkholderia cepacia complex bloodstream infections related to infusion of contaminated water for injection. J Hosp Infect. 2005;60:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Labarca JA, Trick WE, Peterson CL, Carson LA, Holt SC, Arduino MJ, Meylan M, Mascola L, Jarvis WR. A multistate nosocomial outbreak of Ralstonia pickettii colonization associated with an intrinsically contaminated respiratory care solution. Clin Infect Dis. 1999;29:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | McNeil MM, Solomon SL, Anderson RL, Davis BJ, Spengler RF, Reisberg BE, Thornsberry C, Martone WJ. Nosocomial Pseudomonas pickettii colonization associated with a contaminated respiratory therapy solution in a special care nursery. J Clin Microbiol. 1985;22:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Yoneyama A, Yano H, Hitomi S, Okuzumi K, Suzuki R, Kimura S. Ralstonia pickettii colonization of patients in an obstetric ward caused by a contaminated irrigation system. J Hosp Infect. 2000;46:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Ryan MP, Pembroke JT, Adley CC. Ralstonia pickettii: a persistent gram-negative nosocomial infectious organism. J Hosp Infect. 2006;62:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Fortún J. Diagnostic and therapeutic approach to fungal pneumonia in the critically ill patient. Rev Esp Quimioter. 2022;35 Suppl 1:97-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Ryan MP, Adley CC. The antibiotic susceptibility of water-based bacteria Ralstonia pickettii and Ralstonia insidiosa. J Med Microbiol. 2013;62:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Girlich D, Naas T, Nordmann P. OXA-60, a chromosomal, inducible, and imipenem-hydrolyzing class D beta-lactamase from Ralstonia pickettii. Antimicrob Agents Chemother. 2004;48:4217-4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |