Published online Mar 26, 2023. doi: 10.12998/wjcc.v11.i9.1992
Peer-review started: October 7, 2022
First decision: November 22, 2022
Revised: December 7, 2022
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 26, 2023
Processing time: 160 Days and 21.2 Hours
Brain arteriovenous malformations (bAVMs) remains one of the most prevalent causes of intracranial hemorrhage and stroke-like syndromes in the young adult population. Although it has been agreed upon that definitive treatment using either single or multi-modal approach is warranted for successful bAVM mana
In this report, we present a case of delayed, definitive endovascular treatment for ruptured bAVM in a 21-year-old female, 3 mo post-ictus. The bAVM, with a left pericallosal feeding artery and cortical draining veins, was successfully obliterated through embolization using the Onyx 18. On follow-up the patient has recommenced her daily activities and experiences only mild occasional headaches with mild motor deficits. The report leads to our review on an important issue regarding the optimal timing of ruptured bAVM definitive mana
Current treatment paradigms of ruptured bAVM remains elusive, with substantial heterogeneity in the current literature. A consensus on the definition of “acute” vs “delayed”, management goal, follow-up length and outcome parameters are required to support formation of a clear paradigm.
Core Tip: In this case report, we present an example of successful delayed management of a ruptured brain arteriovenous malformation (bAVM) in a young female. The case is followed by an updated review regarding current “delayed” and “immediate” definitive interventions for bAVMs and problems associated with the current data on the effectivity of these paradigms.
- Citation: Bintang AK, Bahar A, Akbar M, Soraya GV, Gunawan A, Hammado N, Rachman ME, Ulhaq ZS. Delayed versus immediate intervention of ruptured brain arteriovenous malformations: A case report. World J Clin Cases 2023; 11(9): 1992-2001
- URL: https://www.wjgnet.com/2307-8960/full/v11/i9/1992.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i9.1992
Brain arteriovenous malformations (bAVMs) continue to be one of the most prevalent causes of intracranial hemorrhage and stroke-like syndromes in the young adult population. It is defined as an anomalous network or nidus occurring within the brain parenchyma, in which a high-flow shunt or bypass occurs between the feeding artery to the veins. A large majority of bAVMs manifest as intracranial hemorrhage as the presenting symptom, due to rupture of the malformation. Hemorrhagic bAVMs have been shown to result in a morbidity rate of 30%-50% and mortality rate of 10%-30%[1]. Additionally, hemorrhagic bAVMs have a re-rupture risk of more than 4.8% per year[2,3].
Definitive treatment of bAVMs consist of three main modalities, namely endovascular embolization, stereotactic radiotherapy, and microsurgical resection or extirpation of the malformation, with the goal of complete nidus obliteration and minimized hemorrhage recurrence. Although it has been agreed upon that definitive treatment using either single or multi-modal approach is warranted for successful bAVM management, much debate still revolves regarding the optimal timing of definitive treatment.
Some indications warrant immediate definitive treatment, such as massive hemorrhage that implies a life-threatening mass effect, rapid neurological deterioration, or the presence of high-risk aneurysms, in which surgical evacuation can be performed in conjunction with definitive resection of small, superficial bAVMs. However, other cases may fall into the category of gray areas for immediate treatment. In fact, many interventionists and surgeons prefer delayed definitive treatment, when possible, due to easier visualization and high success rates. The current guideline on the management of bAVMs has acknowledged the lack of evidence to direct decisions in this setting[2], although some reports have suggested an ideal interval of 2-6 wk from rupture for removal of the bAVM[2,4,5], and that immediate removal may be associated with larger mortality and morbidity risk[5]. Unfortunately, more data is needed to prove either the benefits or disadvantages of both approaches.
Here we present a case report of a successful, delayed, exclusively endovascular management in a young, 21-year-old female with a ruptured bAVM of 3 mo onset. We then emphasize on this highly debatable topic with an updated literature review on the current evidence regarding outcomes of definitive treatment using either of the three modalities.
A 21-year-old female presented to Dr. Wahidin Sudirohusodo General Hospital, Makassar, Indonesia, in September 2021 with a chief complaint of weakness on the right side of her body, in addition to right-sided hypoesthesia and occasional headaches.
The complaints were first experienced 3 mo prior, with sudden onset, while the patient was performing her daily activities as a college student. She also recalled other symptoms upon onset, such as headache, transient vision loss, slurred speech, and vomiting. At the time, she experienced a brief period of loss of consciousness and was treated conservatively at her local health care center due to lack of imaging modalities.
The patient had no prior history of trauma, infection, hypertension, diabetes, or stroke.
The patient had no significant personal or family history to disclose.
On presentation at our center, her vital signs were normal. The neurological bedside examination revealed a GCS score of 15, no meningeal signs, and good cognitive function. Her cranial nerve assessment was within normal limits. The motor strength assessment showed weakness on the right side with a strength score of 4 on the right upper and lower extremities. The right extremities also showed increased tone and physiologic reflexes. No pathologic reflexes were observed. The exam also showed right sided hypoesthesia. Autonomic function was within normal limits.
Laboratory tests, chest X-rays, and electrocardiography exams showed no abnormal findings.
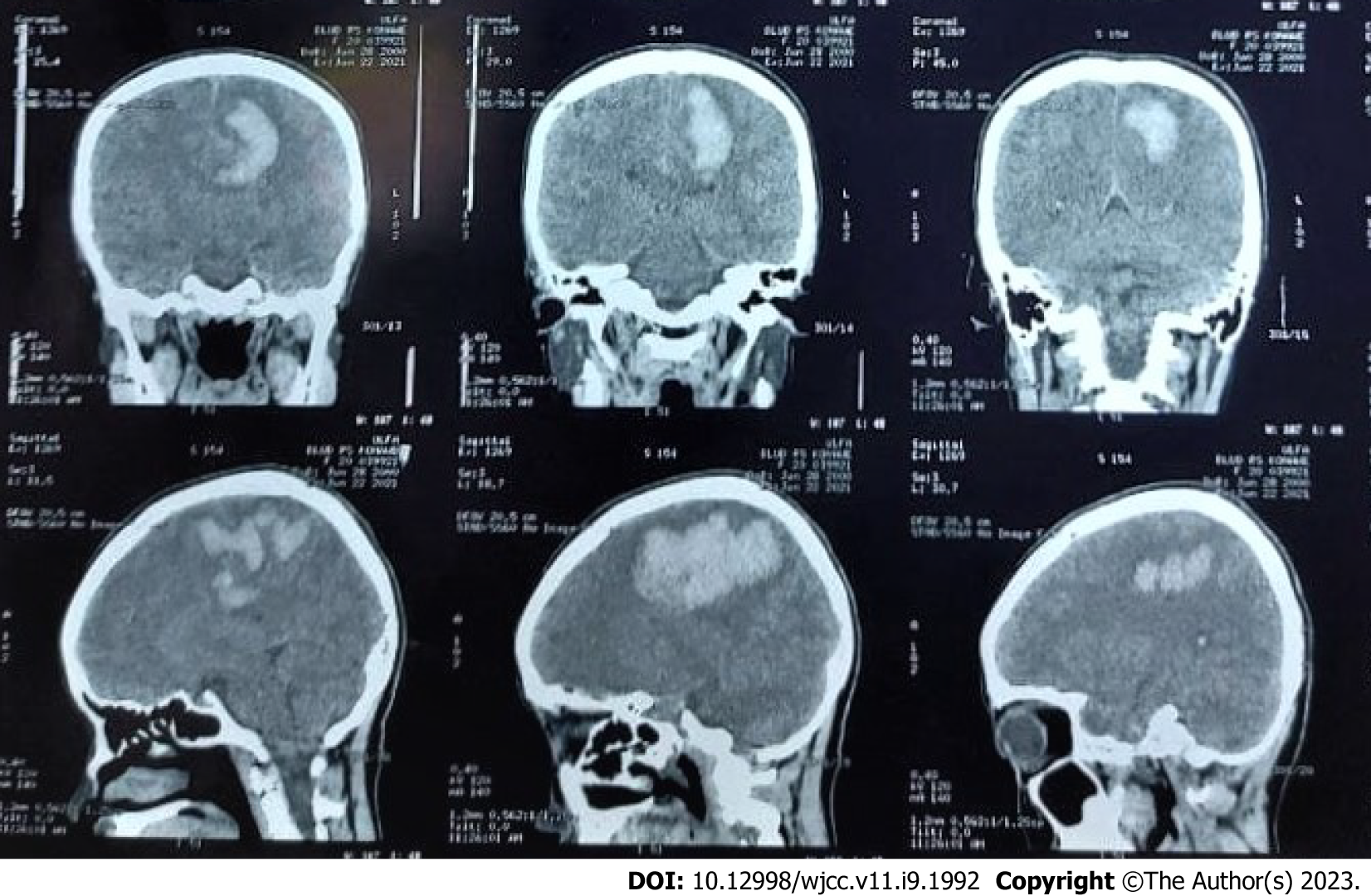
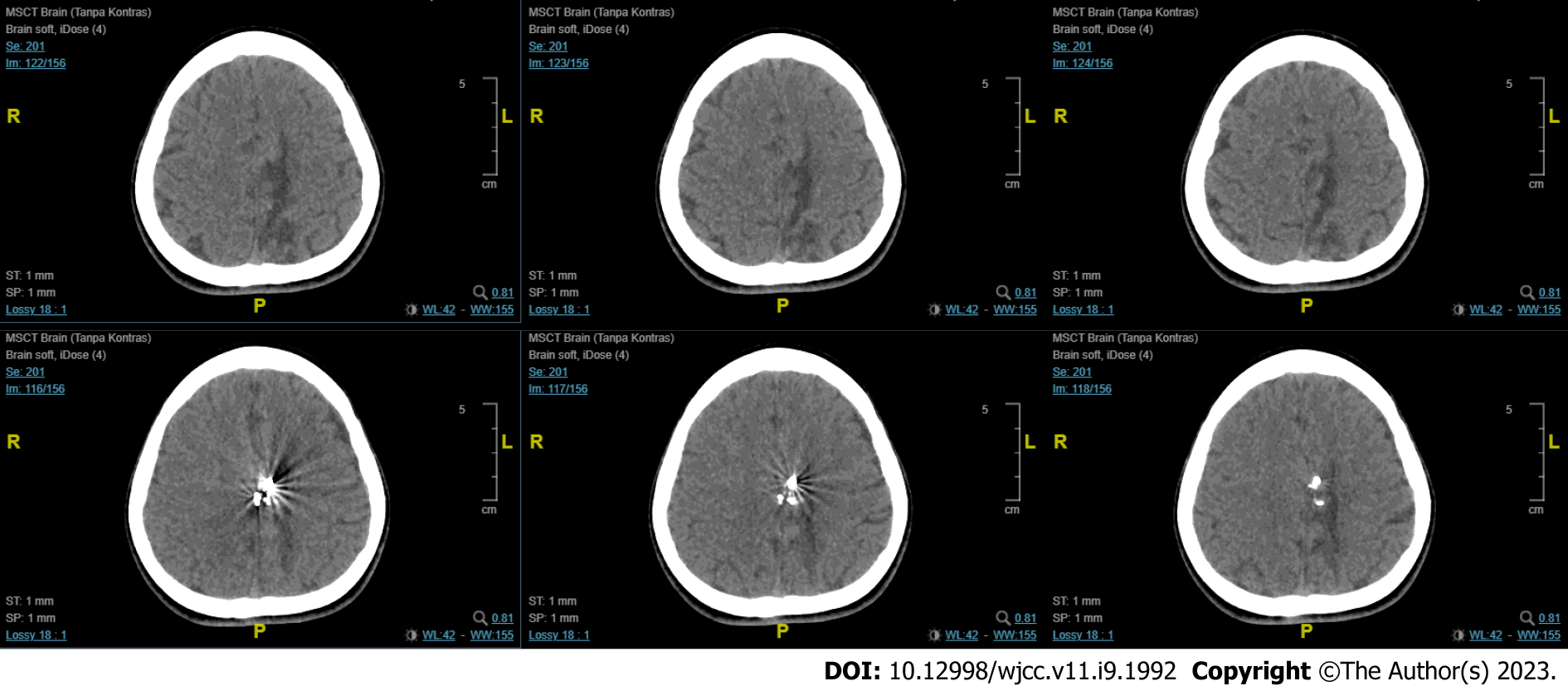
The first imaging examination was a non-contrast head computed tomography (CT) scan performed a few days after the onset, at the patient’s regional hospital. The exam revealed an intracerebral hematoma of the left cerebral hemisphere (Figure 1). Due to the rise in COVID-19 cases at the time, her referral to Dr. Wahidin Sudirohusodo General Hospital for further investigation and definitive therapy was delayed based on her family’s decision, and she was given conservative treatment.
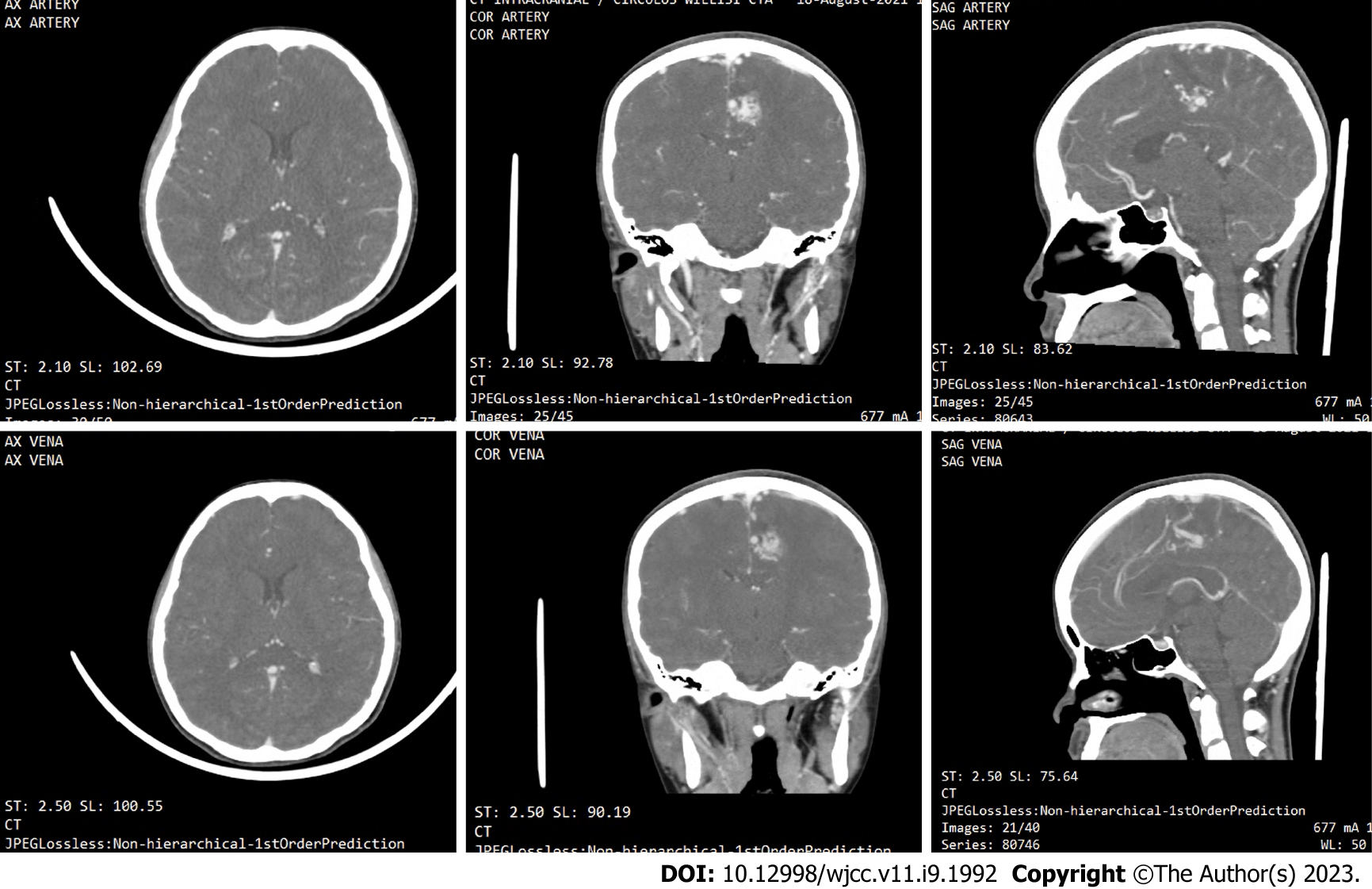
A head CT angiography (Figure 2) was performed approximately 2 mo after onset, revealing the presence of an arteriovenous malformation (AVM) on the left frontal lobe with a feeding artery from the left anterior cerebral artery, draining into the superior sagittal sinus.
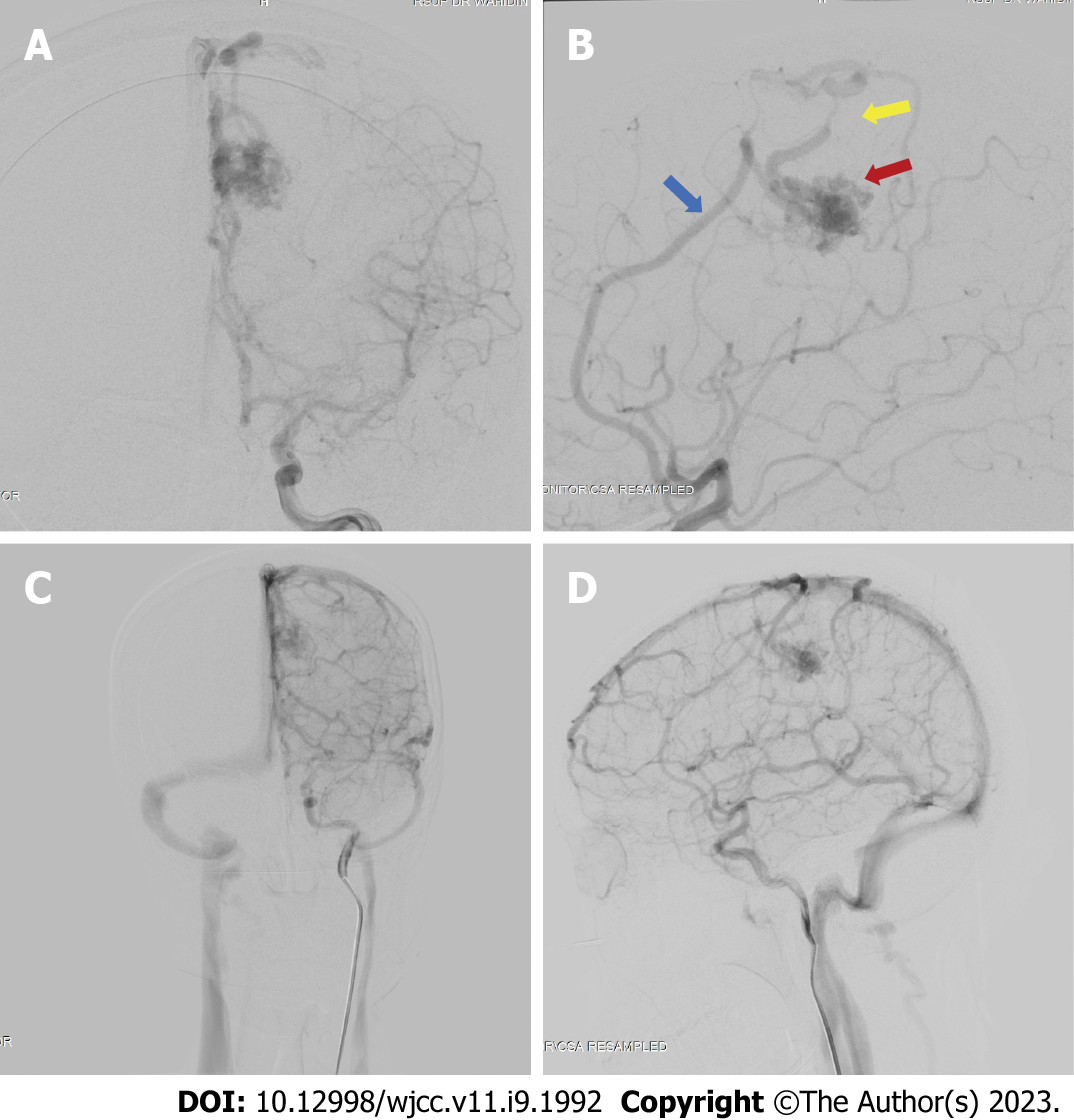
We performed a digital subtraction angiography (DSA) on the patient a few days after her admission in our center (Figure 3). The DSA confirmed that the bAVM fed off the left pericallosal artery, with two draining veins, one towards the cortical vein while the other has undergone stenosis. We graded the AVM as a Spetzler-Martin Grade II AVM.
Based on the clinical findings, the patient was diagnosed with bAVM.
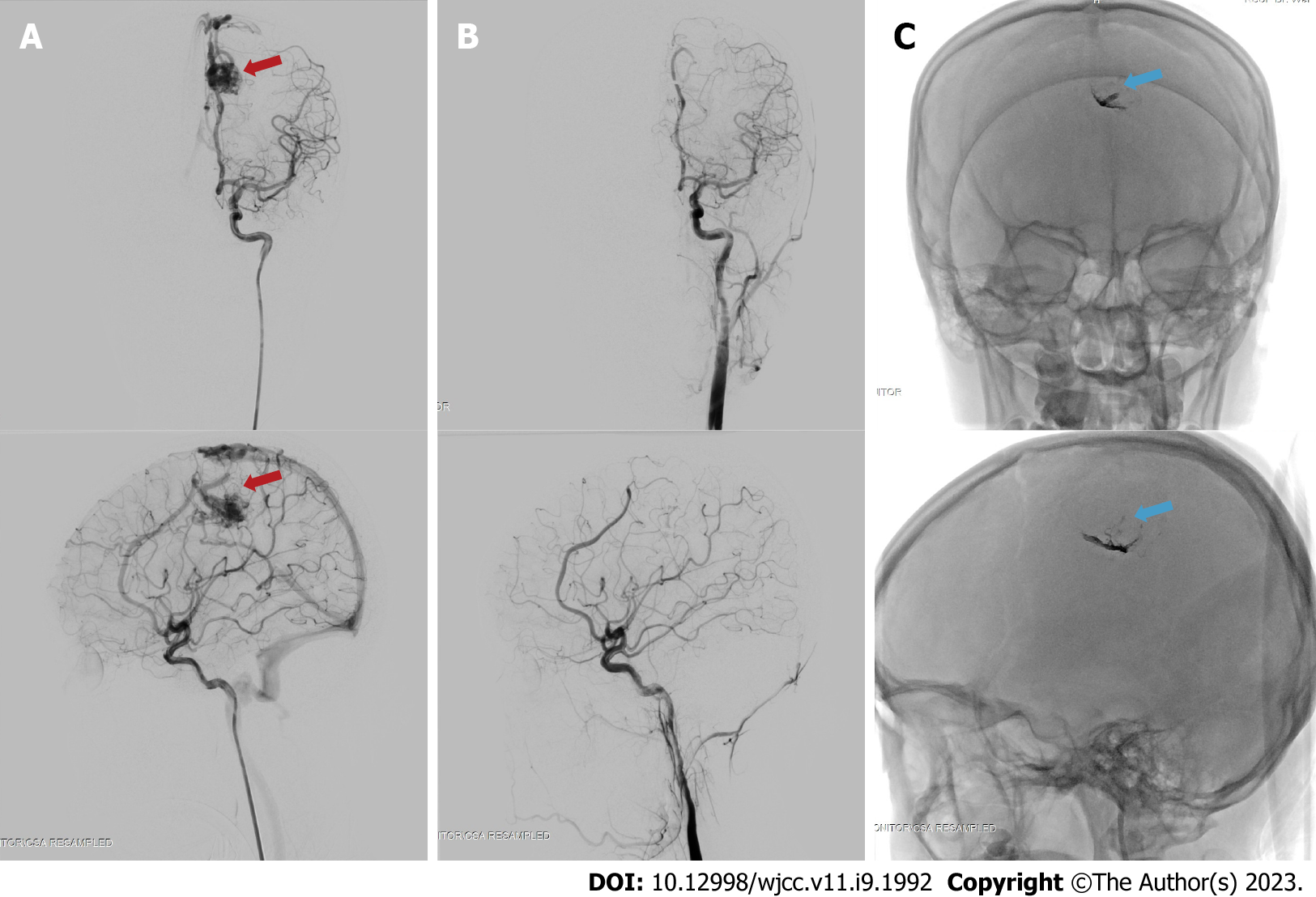
The patient was subjected to a definitive therapy of embolization on the left pericallosal artery using the Onyx 18 until the nidus and draining vein can no longer be visualized (Figure 4). The patient was tolerant of the whole procedure, and no additional deficits were experienced throughout her care, and was discharged from the hospital a few days following embolization.
The patient returned 3 mo after the procedure for a follow-up visit to the Neurology Outpatient Clinic of Dr. Wahidin Sudirohusodo General Hospital. Her follow-up CT-scan revealed encephalomalacia of the parasagittal region at the left frontal and parietal lobes with mild dilatation of the left lateral ventricle (Figure 5). And to date, approximately 1 year after the procedure, the patient has experienced no rebleeding. She currently lives with minor motor deficit resulting in abnormal gait, and experiences occasional headaches which are manageable with over-the-counter therapy (Glasgow Outcome Scale Score = 2).
The case we presented is one of the many ruptured bAVM cases encountered in our center that was successfully managed using delayed definitive treatment. The 21-year-old female experienced an intracerebral hematoma due to the bAVM rupture, leading to neurological deficits in the form of right-sided weakness and hypoesthesia. The bAVM was visualized on CT-angiography, then gold-standard DSA and definitive endovascular treatment was performed 3 mo after the rupture. The bAVM was treated successfully and the patient was able to recommence her daily activities.
In this instance, the delay to definitive investigations and therapy was coincidental, due to the high wave of the COVID-19 pandemic. But on many occasions, the delay is more deliberate, with interventionists arguing against immediate definitive intervention due to difficulties associated with vascular visualization during acute stages of hematoma. Despite the ongoing approach of either delayed or immediate treatment, there still is insufficient evidence to provide definite recommendations.
In this section, we present the current evidence regarding immediate and delayed treatment of bAVMs. Whilst there is currently a lack of studies comparing the two paradigm approaches, we have summarized individual studies looking at either of the two approaches when feasible, with results summarized in Table 1[6-15]. Traditionally, indications for emergency or immediate intervention of ruptured bAVMs include patients with life-threatening hematoma and its associated mass effects, deteriorating neurological status, and the presence of aneurysms[5,8]. In a state of emergency described above, there is no ambiguity, and the decision for immediate evacuation or decompressive craniectomy is clear. In conjunction with the emergency evacuation, extirpation of small superficial bAVMs is typically performed. The question that remains is whether immediate resection or obliteration of the bAVM is more beneficial in patients that do not have any life-threatening indications. Some experts have stated that acute definitive management is required for a good outcome, arguing that treatment delay require patients to recover twice, both from the hemorrhage and from the subsequent definitive intervention[16]. Some centers have argued that early surgery can achieve better rebleeding protection, reduce length-of-stay, and allow earlier initiation of rehabilitation.
| Ref. | Time from ictus (rupture) | bAVM type and SMG | Modality of definitive treatments | Number of subjects | Age | Outcome | Follow-up period | Result |
| Bartolomeo et al[6], 2021 | 48 h; > 48 h | I-IV | Surgery; multimodal evacuation of hematoma | 25 | Mean age 38 and 45.55 | mRS score | 3 mo and 1 yr | Surgery after rest period is better, but early surgery seems reasonable for young patients |
| Mansur et al[7], 2021 | 48 h; > 48 h | I-IV | Targeted embolization; surgery; combination of delayed therapy; observation | 32 acute; 284 non-acute | Mean age of acute: 32; non acute: 284 | Change in mRS score and clinical outcome/re-rupture | Mean 3.2 ± 1.4 yr | Delayed curative treatment preferred. Acute targeted embolization warranted for weak points |
| Beecher et al[8], 2017 | Delayed only: Minimal 4 wk post-hemorrhage | N/A | Radiation; surgery; embolization | 102 | N/A | Time to treatment failure (re-hemorrhage or neurological decline) | Median 248 d | 6 (5.8%) new hemorrhage. Authors in support of delaying treatment for at least weeks to allow for neurologic improvement prior to intervention |
| Bir et al[9], 2016 | < 24 h; 24-48 h; > 48 h | I-IV | Embolization; GK SRS; microsurgical resection | 78 | Median age 45 | Mean mRS grading | Mean mo 45.17 (1-223) | Significant difference in mean mRS: Group < 24h vs > 48h (P = 0.01); Group 24-48 h vs > 48 h (P = 0.03) |
| Todnem et al[10], 2019 | Immediate embolization (time not defined) followed by delayed GK-SRS | III-IV | Embolization and SRS combination | 16 | Mean age 46.63 yr | mRS score | 45.4 21.43 mo | Improvement of mRS score and no recurrent hemorrhage |
| van Rooij et al[11], 2012 | Acute phase: Within 10 d of ictus | N/A | Embolization with either coils or Onyx | 23 | Mean age 42 yr | Obliteration status; hemorrhage recurrence; mortality | 21 mo mean | 1 death (following surgical evacuation of frontal hematoma); 13 patients had complete obliteration; No new hemorrhage |
| Andreou et al[12], 2008 | Median 2 wk after ictus (range 1-28 wk) | Micro-AVM; SMG N/A | Embolization | 25 | N/A | Procedure-related complications; obliteration status; re-hemorrhage | 6 mo | 3 complications; 22 complete obliterations; 2 recurrences |
| Stemer et al[13], 2013 | Acute phaseMedian 4 d after ictus(Range 8-75) | I-VI | Embolization (Onyx only) | 21 | Mean age 38 yr | Procedure-related complications; obliteration status; re-hemorrhage; GOS | 7.5 mo mean | 2 asymptomatic complications; 0 new hemorrhage; 7 complete obliterations after the first procedure; mean GOS improvement from 4.0 on presentation to 4.4 after first procedure |
| Pavesi et al[14], 2009 | Acute phase (within 6 d of ictus) | I-II | Radical AVM surgical removal + hematoma evacuation | 27 | Mean age 41.3 yr | GOS; rehemorrhage; mortality | 22 mo mean | 85% favorable functional outcome (GOS: good recovery – moderate disability); 2 re-hemorrhages; 7.4% mortality |
| Kuhmonen et al[[15], 2005 | Acute phase (within 4 d of ictus) | 1-V | Craniotomy and extirpation; ventricular drainage; embolization | 49 | Mean age 32.8 | GOS; mortality | 2-3 mo | More than half of the patients had a good functional outcome; Mortality 12.2 % |
On the other hand, arguments supporting delayed definitive treatment state that surgical management of bAVMs should be performed following recovery of the brain from injury and to wait for stabilization of the patient’s general condition. A highly variable rest period of 1-6 wk between hemorrhage and definitive treatment is generally advocated in several literatures[4,15,17]. There are several other issues associated with acute interventions such as secondary edema and challenging visualization of compressed vessels, and therefore, delaying the intervention allows edema reduction that is most pronounced in the early days post hemorrhage[5]. There are also concerning observations regarding the hazardous consequences of early intervention, that have led to preventable neurological deficits[5]. And finally, several unique conditions may place the patient as a candidate for delayed definitive treatment, such as bAVMs in pregnancy that typically require delay of intervention until post-gestation[18].
Despite the ongoing debate of early vs delayed definitive bAVM management, there remains a lack of studies comparing the two approaches and its outcome, especially those performed in a single cohort. The spontaneous supratentorial lobar intracerebral hematomas trial has demonstrated that relative to early surgery, delayed hematoma surgery leads to deterioration. However, its sample population did not include those with hematoma due to AVM associated bleeding[19].
In order to pave the way for more definitive recommendations on the management of AVM ruptures, we propose that several urgent matters need to be addressed. Firstly, a clear consensus on the definition of immediate and delayed definitive treatment in bAVM management is urgently needed. In other words, when is a procedure considered immediate and when is it considered delayed? Most of the included studies in this report had varying time frames of patient inclusion. Additionally, a systematic approach to this topic is still hindered by the complexity of the management of bAVMs. Until today, intervention of ruptured bAVM is highly multidisciplinary, varies on a case-by-case basis, and is highly dependent on the clinical status, the size and location of the bAVM and the feeding-drainage pattern. This leads to the large heterogeneity in the literature of bAVM investigations. Current clinical trials on bAVM is highly limited, with the largest known trial only focusing on unruptured bAVM (A Randomized Trial of Unruptured Brain AVMs or the ARUBA trial)[20]. Even so, the trial continues to be criticized due to methodological deficiences[21], only further emphasizing the complexity of bAVM management and investigations into the management.
Outcomes of treatment success also needs to be more strictly defined. Commonly measured outcomes include nidus obliteration (which requires additional radiographic evidence in the follow-up period), hemorrhage recurrence, functional status or neurological sequelae, and mortality. A consensus on defined parameters, in addition to a pre-defined length of follow-up will greatly assist in the acquirement of homogenous data that is urgently needed to answer the current questions in regard to which time approach is more superior, with an end goal of a clear treatment paradigm for ruptured bAVM.
Recurrent hemorrhage itself is an important issue in bAVM management, and was shown to occur at an annual rate of 33% for males and 10% for females[22]. Beecher et al[8] identified obesity as the main predictor of AVM re-bleeding (P = 0.048). Meanwhile studies focusing on early intervention has identified age as a factor that significantly affects outcome[15]. While it remains unclear whether late or immediate approaches differ in re-hemorrhage rates, early intervention studies have claimed that the approach minimizes complications from delayed venous occlusion and is more efficient as it does not require multiple anesthetics or hospital admissions.
The decision to delay treatment may be supported by the difficulties associated with performing gold-standard angiography in the hyperacute phase. To illustrate, in a cohort described by Andreou et al[12], of all 25 patients with acute presentation of micro-bAVM ruputure, only 17 was diagnostic of the AVM. In the acute phase, cerebral lumens undergo compression or destruction due to the surrounding hematoma. Additionally, patients may also experience vascular thrombosis or spasm, further complicating clear visualization of the lesion. Some other reasons include delayed cross-institutional referral, need for additional diagnostic imaging, patient preference, or pregnancy[8].
A recent retrospective study has shown that delayed conclusive treatment yields more favorable functional outcome[6], and that both approaches are influenced by different factors, wherein early intervention is influenced by the ICH score, while the delayed intervention is influenced by the bAVM microarchitecture as defined by the Spetzler-Martin grades. In said study, the mortality rate between the early and delayed group was also significantly different, although this should be interpreted with caution, due to the higher likelihood of high-risk emergency comorbidities accompanying cases of early treatment. Another recent study comparing delayed vs acute approaches showed that delayed treatment had significantly higher nidus obliteration rates and were less likely to require multiple treatments[7]. No significant difference was observed in the re-rupture rate or mortality. Interestingly, the study stated that in patients with the acute targeted approach, it’s not the acute timing itself rather than the targeted approach that provided a protective effect on the acutely managed patients.
Although this review has focused mainly on the timing of treatment, there has been some interesting development of molecular investigations into bAVM pathomechanism using high-throughput next-generation sequencing and pathway analysis, which may have future implications in the future personalized management of bAVM[23,24]. An example is the recent discovery on the importance of TGF- β signaling in bAVM pathogenesis[24,25]. In the future, as more exploration into bAVM mechanism will be needed to improve personalized management of bAVM.
To our knowledge, this is the first review to summarize the current literature on the topic of delayed vs immediate definitive intervention for ruptured bAVMs. Clearly, unless a consensus is reached on the questions raised above, heterogeneity will continue to exist, and a clear paradigm on the timing of definitive treatment of bAVM will continue to be delayed.
In this case report and review, we illustrate a common scenario of a delayed approach to bAVM definitive treatment using an endovascular approach. The current treatment paradigm of ruptured bAVM remains elusive, with many interventionists favoring either a delayed or acute treatment approach. There is substantial heterogeneity in the current literature, rendering it highly difficult to determine which of the following time-based approach is more superior. We believe that a consensus on the definition of acute vs delayed, the goal of management in ruptured bAVM, the follow-up length and outcome parameters need to be determined in order to acquire homogenous data that can support the formation of a clear treatment paradigm.
We would like to thank the patient who has participated in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: Indonesia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar S, India; Sidoti A S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, Pile-Spellman J, Mohr JP. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 2. | Derdeyn CP, Zipfel GJ, Albuquerque FC, Cooke DL, Feldmann E, Sheehan JP, Torner JC; American Heart Association Stroke Council. Management of Brain Arteriovenous Malformations: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017;48:e200-e224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 3. | Kim H, Al-Shahi Salman R, McCulloch CE, Stapf C, Young WL; MARS Coinvestigators. Untreated brain arteriovenous malformation: patient-level meta-analysis of hemorrhage predictors. Neurology. 2014;83:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 4. | Zacharia BE, Vaughan KA, Jacoby A, Hickman ZL, Bodmer D, Connolly ES Jr. Management of ruptured brain arteriovenous malformations. Curr Atheroscler Rep. 2012;14:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 5. | Aoun SG, Bendok BR, Batjer HH. Acute management of ruptured arteriovenous malformations and dural arteriovenous fistulas. Neurosurg Clin N Am. 2012;23:87-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Di Bartolomeo A, Scafa AK, Giugliano M, Dugoni DE, Ruggeri AG, Delfini R. Ruptured Brain Arteriovenous Malformations: Surgical Timing and Outcomes-A Retrospective Study of 25 Cases. J Neurosci Rural Pract. 2021;12:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Mansur A, Kostynskyy A, Krings T, Agid R, Radovanovic I, Mendes Pereira V. The safety profile and angioarchitectural changes after acute targeted embolization of ruptured arteriovenous malformations. J Neurosurg. 2021;1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Beecher JS, Lyon K, Ban VS, Vance A, McDougall CM, Whitworth LA, White JA, Samson D, Batjer HH, Welch BG. Delayed treatment of ruptured brain AVMs: is it ok to wait? J Neurosurg. 2018;128:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Bir SC, Maiti TK, Konar S, Nanda A. Overall outcomes following early interventions for intracranial arteriovenous malformations with hematomas. J Clin Neurosci. 2016;23:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Todnem N, Ward A, Nahhas M, Vender JR, Alleyne CH, Rahimi SY. A Retrospective Cohort Analysis of Hemorrhagic Arteriovenous Malformations Treated with Combined Endovascular Embolization and Gamma Knife Stereotactic Radiosurgery. World Neurosurg. 2019;122:e713-e722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | van Rooij WJ, Jacobs S, Sluzewski M, Beute GN, van der Pol B. Endovascular treatment of ruptured brain AVMs in the acute phase of hemorrhage. AJNR Am J Neuroradiol. 2012;33:1162-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Andreou A, Ioannidis I, Lalloo S, Nickolaos N, Byrne JV. Endovascular treatment of intracranial microarteriovenous malformations. J Neurosurg. 2008;109:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Stemer AB, Bank WO, Armonda RA, Liu AH, Herzig DW, Bell RS. Acute embolization of ruptured brain arteriovenous malformations. J Neurointerv Surg. 2013;5:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Pavesi G, Rustemi O, Berlucchi S, Frigo AC, Gerunda V, Scienza R. Acute surgical removal of low-grade (Spetzler-Martin I-II) bleeding arteriovenous malformations. Surg Neurol. 2009;72:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Kuhmonen J, Piippo A, Väärt K, Karatas A, Ishii K, Winkler P, Niemelä M, Porras M, Hernesniemi J. Early surgery for ruptured cerebral arteriovenous malformations. Acta Neurochir Suppl. 2005;94:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Hafez A, Oulasvirta E, Koroknay-Pál P, Niemelä M, Hernesniemi J, Laakso A. Timing of surgery for ruptured supratentorial arteriovenous malformations. Acta Neurochir (Wien). 2017;159:2103-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ogilvy CS, Stieg PE, Awad I, Brown RD Jr, Kondziolka D, Rosenwasser R, Young WL, Hademenos G; Stroke Council, American Stroke Association. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation. 2001;103:2644-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Salvati A, Ferrari C, Chiumarulo L, Medicamento N, Dicuonzo F, De Blasi R. Endovascular treatment of brain arteriovenous malformations ruptured during pregnancy--a report of two cases. J Neurol Sci. 2011;308:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH; STICH investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 650] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 20. | Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, Al-Shahi Salman R, Vicaut E, Young WL, Houdart E, Cordonnier C, Stefani MA, Hartmann A, von Kummer R, Biondi A, Berkefeld J, Klijn CJ, Harkness K, Libman R, Barreau X, Moskowitz AJ; international ARUBA investigators. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 806] [Cited by in RCA: 788] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 21. | Meling TR, Proust F, Gruber A, Niemela M, Regli L, Roche PH, Vajkoczy P. On apples, oranges, and ARUBA. Acta Neurochir (Wien). 2014;156:1775-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Mast H, Young WL, Koennecke HC, Sciacca RR, Osipov A, Pile-Spellman J, Hacein-Bey L, Duong H, Stein BM, Mohr JP. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 314] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Scimone C, Donato L, Marino S, Alafaci C, D'Angelo R, Sidoti A. Vis-à-vis: a focus on genetic features of cerebral cavernous malformations and brain arteriovenous malformations pathogenesis. Neurol Sci. 2019;40:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Scimone C, Donato L, Alafaci C, Granata F, Rinaldi C, Longo M, D'Angelo R, Sidoti A. High-Throughput Sequencing to Detect Novel Likely Gene-Disrupting Variants in Pathogenesis of Sporadic Brain Arteriovenous Malformations. Front Genet. 2020;11:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Scimone C, Granata F, Longo M, Mormina E, Turiaco C, Caragliano AA, Donato L, Sidoti A, D'Angelo R. Germline Mutation Enrichment in Pathways Controlling Endothelial Cell Homeostasis in Patients with Brain Arteriovenous Malformation: Implication for Molecular Diagnosis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |