Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1869
Peer-review started: January 7, 2023
First decision: January 30, 2023
Revised: February 7, 2023
Accepted: February 21, 2023
Article in press: February 21, 2023
Published online: March 16, 2023
Processing time: 58 Days and 19.5 Hours
Intraoperative hyperlactatemia often affects circulatory stability, vital organ function, and postoperative recovery, poses a serious prognostic risk, and requires considerable attention from anesthesiologists. Here, we describe a case of hyperlactatemia during the postoperative resection of liver metastases after chemotherapy for sigmoid colon cancer. This did not affect the patient's circulatory stability or quality of awakening, which is rarely reported in clinical practice. We present our management experience with the aim of providing a reference for future studies and clinical practice.
A 70-year-old female patient was diagnosed with postoperative liver metastasis following chemotherapy for sigmoid colon cancer. Laparoscopic right hemicolectomy and cholecystectomy under general anesthesia were required. Metabolic disorders, primarily hyperlactatemia, often occur intraoperatively. After treatment, other indices quickly returned to normal, lactate levels decreased slowly, and hyperlactatemia persisted during the awakening period. However, this did not affect the patient's circulatory stability or awakening quality. This condition has rarely been clinically reported. Therefore, we report our manage
Active intraoperative rehydration avoided serious harm to the organism caused by hyperlactatemia. Strengthening body temperature protection could improve lactate circulation.
Core Tip: Intraoperative hyperlactatemia often affects circulatory stability and vital organ function, posing serious prognostic risks. Cases wherein circulatory stability and recovery quality are unaffected are rarely reported. We consider that aggressive intraoperative rehydration avoids serious harm to the organism caused by hyperlactatemia due to insufficient tissue perfusion. Enhanced thermoprotection also improved lactate circulation. There may be individual differences in metabolism and lactate may have a slow clearance profile. The impact on circulation and quality of awakening was mild. Awakening extubation can be performed to reduce the duration of mechanical ventilation and risk of delayed extubation, and to shorten the hospital stay.
- Citation: Meng Y, Pei HS, Yu JJ. Hyperlactemia associated with secondary hepatocellular carcinoma resection in relation to circulation stability and quality of recovery: A case report. World J Clin Cases 2023; 11(8): 1869-1877
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1869.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1869
The management of intraoperative hyperlactatemia poses a serious challenge to anesthesiologists. To date, it is well recognized that hyperlactatemia poses a serious risk to the organism, requiring a great deal of attention from anesthesiologists. The common causes of increased lactate are increased production due to inadequate tissue perfusion, decreased clearance due to decreased kidney function, and disturbed circulation of lactate due to decreased liver function.
Here, we describe a case of hyperlactatemia during postoperative resection of liver metastases following chemotherapy for sigmoid colon cancer that did not affect the patient's circulatory stability and quality of recovery, which has been rarely reported in clinical practice.
Based on our management experience, we found that this condition was not due to hyperlactatemia caused by inadequate tissue perfusion and did not cause irreversible damage to vital organs. There was minimal impact on the patient's circulation and quality of recovery. Therefore, we decided to remove the tracheal tube, reduce any unnecessary mechanical ventilation time, reduce the risk of delayed extubation, and shorten the length of hospital stay. We report our management experience with the aim of providing a reference for clinical anesthesia.
We report the case of a 70-year-old elderly female patient (weight, 60 kg; height, 156 cm; body mass index 24.7 kg/m2). Laparoscopic right hemicolectomy and cholecystectomy were performed under general anesthesia. The patient’s chief complaint was sigmoid colon cancer with a duration of 10 mo, as well as liver metastasis (with a duration of four months) following postoperative chemotherapy.
The patient was admitted to a local hospital 10 mo prior due to abdominal pain, mucus, and bloody stools. Colonoscopy revealed sigmoid colon cancer and pathological results showed hypofractionated adenocarcinoma. After completing the relevant examinations, the patient received three cycles of neoadjuvant chemotherapy. Later, the patient developed colic abdominal pain focused in left lower abdomen that was accompanied by nausea and vomiting; intestinal obstruction was considered. Under general anesthesia, the patient underwent laparoscopic-assisted radical resection of the sigmoid colon cancer, left adnexal resection, double-lumen fistula of the terminal ileum, and chemotherapy with membrane infusion and release of intestinal adhesions.
Postoperative pathology of the sigmoid colon reported moderate-to-low differentiated adenocarcinoma with necrosis, small foci with phosphorylation, invasion of the muscular layer to the peri-intestinal fat, and visible nerve invasion. A pathological diagnosis of sigmoid colon cancer was made. After four cycles of postoperative chemotherapy, the patient was treated with three cycles of intravenous targeted drug infusion (four months prior), and the lesion was reduced on review.
The patient had no previous history of hypertension, diabetes, or coronary heart disease. She had a history of cerebral infarction for more than six months and had not undergone any special treatment.
The patient denied any relevant family history of cancer or chronic disease.
The patient had a temperature of 36.3 °C, a heart rate of 85 beats/min, a respiratory rate of 19 breaths/min, and a blood pressure (BP) of 139/97 mm Hg. Her height and weight were 156 cm and 60 kg, respectively. The patient showed normal development, a positive nutritional status, and clear consciousness. Difficult airway (Mallampati class II) was not detected during the preoperative evaluation. The nail-chin spacing was > 6 cm, mouth opening was > 6 cm, and head extension was > 35°. Cardiopulmonary examination findings were unremarkable, and the patient was classified as American Society of Anesthesiology (ASA) class II.
The patient’s blood counts were as follows: Erythrocyte count 3.07 × 1012/L, hemoglobin 103.0 g/L; erythrocyte pressure volume, 31.7%; liver function: Alanine transferase 11.2 U/L, aspartate aminotransferase 27.9 U/L, total protein 56.2 U/L, albumin 34.7 U/L, albumin:globulin ratio 1.61, total bilirubin 6.3 μmol/L. Other laboratory tests, such as routine urine and renal function tests, were within normal limits.
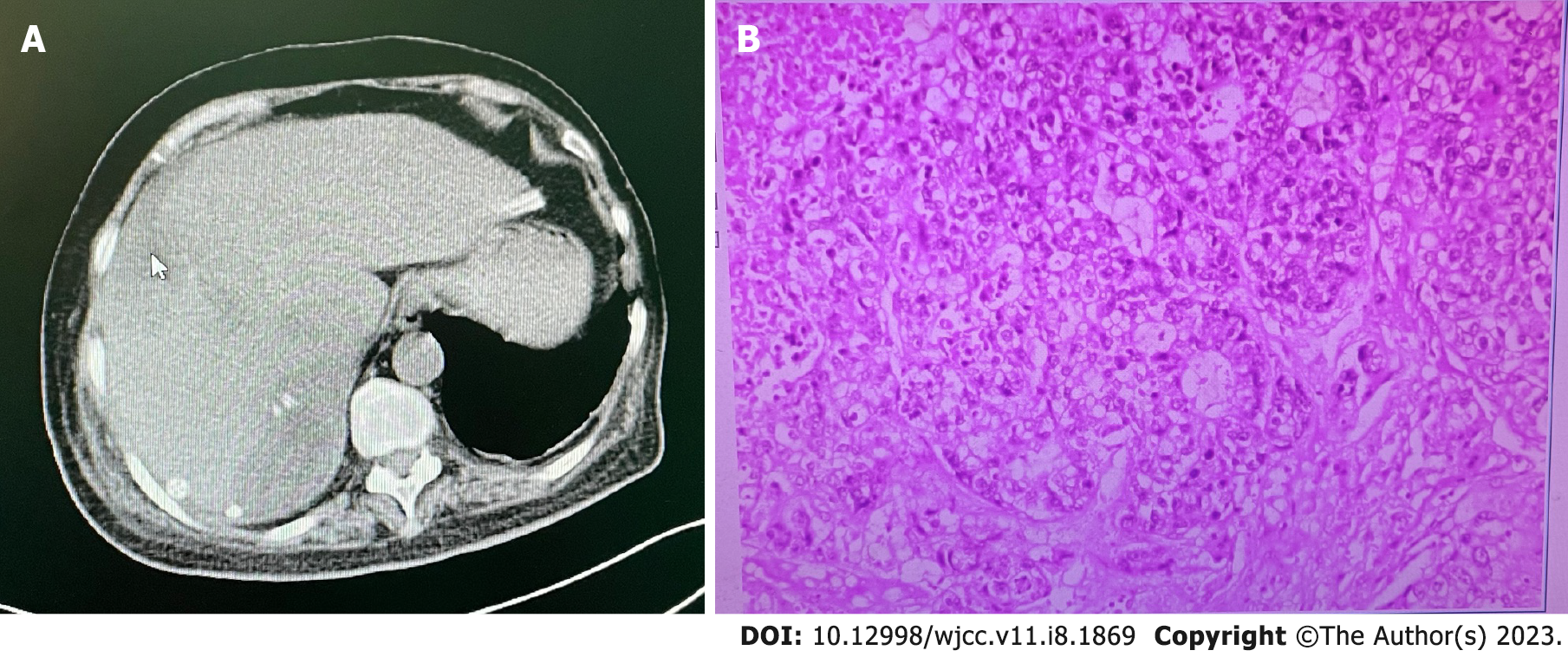
Abdominal computed tomography suggested liver metastasis (Figure 1A). All other test results were unremarkable.
Postoperative liver metastasis following chemotherapy for sigmoid colon cancer and cerebral infarction.
Hypofractionated adenocarcinoma (Figure 1B).
The patient underwent laparoscopic resection of multiple metastatic carcinomas in the right lobe of the liver and ileostomy with a double-lumen fistula return under general anesthesia. The patients fasted and abstained from food and drink prior to surgery. The patient was admitted to the room where cuff BP, electrocardiography, partial pressure of oxygen, and the bispectral index were routinely monitored. Venous access to the left upper limb was established.
Invasive arterial BP monitoring was performed by radial artery puncture after local anesthesia, and arterial blood gas testing was performed. The patient’s BP on admission was 188/75 mmHg, her heart rate was 67 beats/min, and her pulse oximetry was 92%.
After the anesthesia plan was finalized, we administered a routine induction of anesthesia, as follows: Oxygen denitrogenation by face mask for 3 min, intravenous sufentanil 50 μg, remazolam 14 mg, rocuronium 50 mg. Two minutes later, a 7.5 single-lumen tracheal tube was successfully inserted via the mouth. After successful tracheal intubation, a single-lumen central venous catheter was inserted through the right internal jugular vein. An ultrasonography-guided transverse abdominal muscular nerve block was performed.
Anesthesia was maintained with continuous intravenous pumping of 0.05-0.20 μg/kg/min remifentanil, inhalation of 2%-3% sevoflurane, and intermittent sedation of cisatracurium. In this patient presenting with multiple hepatic metastases, we implemented the Pringle method as a blocking technique to reduce intraoperative bleeding. Intermittent intraoperative arterial blood gas testing was performed (Table 1).
| Measure | Pre-anesthesia | Intraoperative 1 | Intraoperative 2 | Intraoperative 3 | Recovery room 1 | Recovery room 2 | Recovery room 3 |
| pH | 7.4199 | 7.2946 | 7.2964 | 7.4551 | 7.3541 | 7.2965 | 7.3666 |
| Potassium (mmol/L) | 3.6836 | 3.8524 | 3.9770 | 3.5277 | 3.3718 | 3.6577 | 3.0349 |
| Glucose (mmol/L) | 6.2544 | 10.6121 | 15.5280 | 14.4482 | 11.2476 | 10.3345 | 10.1226 |
| Lactic acid (mmol/L) | 3.1100 | 2.3355 | 4.7321 | 6.3023 | 8.1477 | 9.3126 | 9.8924 |
| Base surplus (mmol/L) | -1.53 | -3.53 | -6.24 | -0.56 | -5.10 | -7.82 | -4.87 |
| Hct | 33.382 | 31.879 | 29.717 | 24.776 | 27.867 | 25.012 | 22.024 |
| PaO2 (mmHg) | 72.2 | 207.4 | 253.8 | 185.9 | 226.9 | 86.0 | 101.5 |
| FiO2 | 0.21 | 0.60 | 0.60 | 0.60 | 0.60 | 0.21 | 0.21 |
For metabolic disturbances that appeared intraoperatively, we provided timely treatment, including rehydration, potassium supplementation, and acid correction. Laparoscopic exploration revealed a non-sclerotic liver with multiple hepatic metastatic carcinomas. The tumors were located in segment V of the liver, with a tumor size of approximately 2 cm × 3 cm, adjacent to the gallbladder, and in segment VI of the liver, with a tumor size of approximately 4 cm × 5 cm.
Due to the large number of tumors, an intraoperative portal block was performed several times using the Pringle blocking technique. A hepatobiliary surgeon performed laparoscopic resection of multiple metastatic carcinomas in the right lobe of the liver and ileal double-lumen fistula reduction under general anesthesia. The patient was administered 5 mg dizocine for analgesia 0.5 h before the end of the operation as well as intravenous self-administered analgesia after the operation. The operation lasted 320 min, anesthesia lasted 375 min, 1990 mL of crystalloid fluid was infused, intraoperative blood loss was 400 mL, and the urine volume was 1000 mL.
After surgery, the patient was transferred to a recovery room for tracheal intubation. While the patient was in the recovery room, we continued to monitor her arterial blood gas and found that metabolic disorders such as hyperlactatemia were still present (Table 1). We actively treated her with rehydration, potassium supplementation, acid correction and temperature protection. After 40 min, the patient's arterial blood gas normalized, with the exception of hyperlactatemia. At this point, we found that the patient's vital signs were stable and she did not show signs of hyperlactatemia inhibiting circulation, which is extremely rare in clinical practice.
After 0.5 h, the patient's vital signs remained stable, and we decided to wake the patient up again without referring to the lactate level. The patient was then intravenously injected with neostigmine 1.5 mg and atropine 0.4 mg for inotropic antagonism, followed by intravenous flumazenil 0.4 mg five minutes later. After the patient became conscious, muscle tone was restored, spontaneous breathing and the cough reflex were restored, respiratory secretions were cleared, and the tracheal tube was removed. The patient was observed for 0.5 h.
After it was determined that the patient had no complaints of discomfort and had stable vital signs, the was transferred back to the ward. The patient remained in the recovery room for 120 min, during which hyperlactatemia did not affect the patient's circulatory stability and quality of awakening, which is a rarely reported phenomenon. We consider that delayed extubation can shorten the length of hospital stay.
The patient returned to the ward with clear consciousness and a cooperative mindset. Her BP was 135/75 mmHg, her heart rate was 67 beats/min, and her pulse oximetry reading was 100%. On the first day after surgery, comprehensive treatment, including blood transfusion and oxygen, was administered. The patient's vital signs were stable.
On the second day after surgery, the patient's vital signs were stable. Lactate was rechecked at 1.98 mmol/L, liver function was rechecked at 383.3 U/L and 531.5 U/L. On the fourth postoperative day, the patient’s liver function normalized. She recovered well and was successfully discharged from the hospital. On the postoperative pathology report, multifocal hypofractionated carcinoma was observed in the liver. The patient's vital signs were stable at the two-month postoperative follow-up visit.
Approximately 57% of secondary liver cancers are caused by hematogenous metastasis of pancreatic, gastric, and colorectal cancers, whereas lung, cervical, breast, and ovarian cancers commonly metastasize to the liver via hematogenous metastasis. Secondary liver cancer is insensitive to radiotherapy and chemotherapy. Therefore, early surgical resection is the single most effective treatment.
Since the liver is rich in blood flow and liver tissue is fragile, the risk of intraoperative hemorrhage is high. Therefore, a key goal of surgery is to effectively control liver bleeding. To reduce bleeding, resection is often performed after hilar block. Both Pringle's method and the hemihilar block are common clinical methods for blocking hepatic blood flow. The Pringle method is advantageous in reducing bleeding. At the same time, the anesthesiologist needs to pay close attention to the bleeding volume and rate during tumor resection, the status of the operative field, and the dynamic changes of the patient's BP and pulse rate as well as other indicators. While actively administering fluids and transfusing blood, it is necessary to pay extra attention to changes in electrolytes and metabolic indices to avoid serious metabolic disorders such as hyperlactatemia, which may affect the function of vital organs.
In this case, the patient had multiple hepatic metastases after undergoing chemotherapy for sigmoid colon cancer, which was secondary to hepatocellular carcinoma. The preoperative examination showed no significant abnormalities in cardiopulmonary, liver, or kidney function, and the routine blood tests were within the acceptable range for elective surgery. The proposed surgery was laparoscopic right hemicolectomy + cholecystectomy, and the overall assessment of this case was ASA grade II. The surgery was medium risk, such that elective surgery could be performed under general anesthesia.
Considering the proposed resection of multiple hepatic metastases in this case, bleeding control was the core consideration. Therefore, surgery was performed using the Pringle method with a hepatic portal block. The main points of perioperative anesthesia management were to ensure the appropriate depth of anesthesia; to pay extra attention to the extent of liver surgical resection, bleeding volume, and bleeding rate; to strengthen invasive hemodynamic monitoring; to emphasize enhanced volume and urine volume management; to dynamically monitor changes in the internal environment; and to administer effective temperature protection and other treatments. In summary, we used remifentanil + sevoflurane inhalation compound general anesthesia combined with a transversus abdominis nerve block, invasive arterial monitoring, and strengthened volume management, while paying attention to urinary volume, closely monitoring arterial blood gas changes, providing timely adjustment, and striving to achieve individualized and refined anesthetic management.
Normal lactate blood concentrations are in the approximate range of 1.3 mmol/L. Clinically, a blood lactate concentration of > 2.25 mmol/L is usually indicative of hyperlactatemia. A lactate concentration of > 5 mmol/L indicates lactic acidosis. In this case, the patient developed severe intraoperative metabolic disorder (Table 1). After aggressive rehydration, potassium supplementation, and acid correction, all indices except hyperlactatemia quickly returned to the normal range.
It is well known that hyperlactatemia poses a serious risk to the body. Numerous studies have shown that blood lactate levels are closely related to the criticality and prognosis of serious diseases, with higher blood lactate levels leading to more serious conditions and worse prognoses. Hyperlactatemia leads to reduced myocardial contractility and cardiac output, resulting in tissue and organ hypoperfusion, arrhythmia, and reduced cardiovascular responsiveness to catecholamines[1].
The relationship between severe hyperlactatemia and prognosis is divided into two main categories: (1) Severe hyperlactatemia is associated with the failure of various vital organs, such as the liver, kidney, heart, sepsis, as well as other diseases whose primary conditions are difficult to reverse; and (2) Severe hyperlactatemia is associated with cardiac surgery, epilepsy, and other diseases whose primary conditions can be reversed. It was found that the second condition is associated with a significantly lower mortality rate and a mild effect on the organism[2].
In the present case, hyperlactatemia occurred intraoperatively. We actively searched for the cause and administered various treatments in a timely manner to reduce the harm caused by hyperlactatemia to the organism, with a special focus on avoiding aggravating vital organ failure due to hyperlactatemia caused by insufficient tissue perfusion.
The occurrence of hyperlactatemia is due to excessive lactate production on the one hand, and to reduced lactate clearance on the other[3]. Excessive lactate production is associated with various causes such as inadequate tissue perfusion due to volume deficit caused by the intraoperative controlled hypocentral venous pressure technique, a large extent of liver resection or use of the intraoperative Pringle method block technique, long duration of hepatic portal block, traumatic injury resulting in interruption of blood flow to the injured tissue, ischemia-reperfusion injury, hypothermia, and other causes of inadequate tissue perfusion leading to lactic acidosis. In a study by Theodoraki et al[4], it was found that postoperative lactic acid was significantly increased in patients undergoing Pringle's method of blockade compared to those without this method. The authors concluded that blocking hepatic blood flow not only decreased the hepatic oxygen supply and increased anaerobic metabolism, but also significantly decreased the ability of the liver to remove lactic acid.
Impaired liver function associated with surgical resection, which affects the circulation and clearance of lactic acid, can also cause lactic acid accumulation and acidosis. In addition, the kidney can use lactic acid to supply energy and synthesize glycogen via the pyruvate pathway, and it can also secrete lactic acid for excretion[5]. The kidney's ability to remove lactic acid increases with increased blood lactate concentrations.
In this case, hyperlactatemia did not affect the patient’s circulatory stability and quality of recovery during awakening, and we did not give vasoactive drugs for supportive treatment. This suggests that hyperlactatemia in this case did not cause serious harm to the vital organs of the body, which is rarely reported.
In regard to the possible causes of the hyperlactatemia in this case, our analysis is as follows. First, in comparing the patients’ preoperative and postoperative liver functions (Table 2), we found that the patients’ preoperative liver function was normal, whereas her postoperative liver function changed significantly. Moreover, combined with the fact that this patient had multiple liver metastases, intraoperative bleeding was relatively high. To reduce bleeding, the surgeon performed multiple hepatic portal blocks using Pringle's method, showed a greater impact on the patient's liver function. Combined with the surgery, we actively carried out rehydration, potassium supplementation, acid correction and other treatments to avoid the increase of lactic acid due to insufficient tissue perfusion and ensure the stability of the patient's circulation. On comparing the patient's preoperative and postoperative renal function, we found no significant difference. Combined with the total intraoperative crystalloid infusion of 1990 mL and a urinary volume of 1000 mL, this suggests that hyperlactatemia did not affect the patient's circulation and renal function. Comprehensive analysis showed that the patient had stable circulation and good tissue perfusion, which excluded hyperlactatemia caused by insufficient tissue perfusion and also suggested that lactate clearance in the kidneys was not affected. We consider that the extent of liver resection, intraoperative hepatic portal block time, traumatic liver injury and ischemia-reperfusion injury affected the patient's liver function to some extent, resulting in a decrease in the liver's ability to metabolize lactate, while impaired liver function affecting lactate circulation and leading to lactate accumulation may be the main reason for hyperlactatemia in this case. We note that hypothermia may affect lactate circulation, and that the observed findings may also be due to individual differences in lactate metabolism. In addition, lactate is characterized by relatively slow clearance. In sum, we analyzed the hyperlactatemia in this case as arising from decreased lactate clearance due to impaired liver function associated with surgical resection, presenting a reversible hyperlactatemia with minimal impact on the patient (Table 3).
| Measure | Preoperative | Postoperative day 1 | Postoperative day 2 | Postoperative day 5 | Postoperative day 6 |
| ALT (U/L) | 11.2 | 405.4 | 383.3 | 151.1 | 109.9 |
| AST (U/L) | 27.9 | 745.8 | 531.3 | 54.2 | 35.1 |
| TP (g/L) | 56.2 | 48.1 | 41.6 | 49.1 | 48.8 |
| ALB (g/L) | 34.7 | 30.4 | 27.4 | 35.8 | 36.4 |
| A:G | 1.61 | 1.72 | 1.93 | 2.69 | 2.94 |
| TBIL (μmol/L) | 6.3 | 6.7 | 12.3 | 11.9 | 15.4 |
| Causes of disease | Treatment | Prognosis | |
| Excessive lactic acid production | Insufficient volume leading to inadequate tissue perfusion | Aggressive fluid replacement to improve tissue perfusion | Severe hyperlactatemia is associated with failure of all vital organs and is difficult to reverse with a poor prognosis |
| Longer hepatic portal block, traumatic injury leading to ischemic reperfusion injury | Rehydration, potassium replacement, correction of acidosis | ||
| Hypothermia | Insulation therapy | ||
| Decreased lactate clearance | Blockage of blood flow to the liver leading to a decrease in the liver's ability to remove lactic acid | Minimize hepatic portal block time, temperature protection | Severe hyperlactatemia is associated with cardiac surgery, epilepsy and other conditions where the primary condition can be reversed, and has a mild impact on patients with a good prognosis |
| Impaired liver function associated with surgical resection | Hepatoprotective therapy | ||
| Impaired kidney function | Maintain circulatory stability and rehydration | ||
The treatment of hyperlactatemia presents a management challenge for anesthesiologists. Identification of the cause of hyperlactatemia is the key to aggressive treatment. The first priority of treatment is to improve tissue perfusion, and patients should be treated and managed individually in consideration of their condition and in accordance with treatment principles. Blood lactate levels are an important indicator for taking therapeutic measures, and treatment should be initiated when serum lactate levels are > 4.0 mmol/L[6]. Studies have shown that aggressive rehydration can improve lactate production due to insufficient tissue perfusion while reducing the serious harm caused by hyperlactatemia to the organism.
Hypothermia affects the lactate cycle. Hypothermia is often triggered in patients undergoing hepatectomy due to several factors[7]. The results of a study by Li et al[8] showed that hypothermia may lead to further compromise of the patient's liver function as well as long-term ischemia and hypoxia of liver tissue, which can further affect blood lactate and cause metabolic disorders. General nursing measures cannot ensure a normal body temperature in these patients, which leads to increased hypoxia and affects lactate metabolism. It is reported in the literature that body temperature protection can improve lactate metabolic function by maintaining a normal body temperature[8,9]. The Pringle block can completely block the portal vein, which can reduce bleeding during hepatectomy; however, it can also cause visceral and inferior vena cava system stasis. Studies have shown that visceral and inferior vena cava system stasis and tissue hypoxia due to hypothermia can increase lactate concentrations. Maintaining the patient's body surface temperature can improve the degree of hypoxia and significantly reduce lactate concentrations[10].
In this patient, to avoid hyperlactatemia due to inadequate tissue perfusion that could in turn affect vital organ function, we actively treated the patient with volume expansion, acid correction, and arterial blood gas testing. The patient's severe hyperlactatemia did not affect circulatory stability and quality of awakening, and the patient's good postoperative recovery showed that our analysis of the causes of hyperlactatemia, comprehensive treatment, and timely decision to awaken and extubate were correct (Table 4).
| Item | Timeline | |
| Preoperative | 1 | Ten months after diagnosis of sigmoid colon cancer, 4 months after postoperative chemotherapy for liver metastasis |
| 2 | History of “cerebral infarction” for more than six months without special treatment | |
| 3 | Abdominal CT scan suggestive of liver metastasis | |
| 4 | The operation was performed under general anesthesia | |
| Perioperative | 5 | Invasive blood pressure was monitored and arterial blood gas analysis was conducted |
| 6 | BIS was monitored | |
| 7 | Induction of conventional anesthesia with tracheal intubation | |
| 8 | CVC after general anesthesia. Ultrasound-guided transversus abdominis block was performed | |
| 9 | Maintenance of anesthesia was performed using static inhalation compound general anesthesia | |
| 10 | The operation was performed using Pringle's method to block the hepatic metastases, and arterial blood gases were monitored dynamically intraoperatively. Hyperlactatemia was detected and treated aggressively with fluid replacement and other treatments. However, the patient's vital signs were stable | |
| 11 | The surgery was successfully completed | |
| Postoperative | 12 | After the operation, the patient was transferred to the PACU. There was still hyperlactatemia detected. However, the patient's vital signs were stable |
| 13 | Treatments such as temperature protection as well as arterial blood gas testing were implemented, and although hyperlactatemia was present, vital signs were stable and awakening was satisfactory. The patient returned to the ward after surgery | |
| 14 | The patient was discharged six days after surgery | |
| 15 | The patient was followed up two months after the operation | |
This case suggests that anesthesiologists should pay close attention to intraoperative hyperlactatemia, strengthen their knowledge about the causes of lactic acid increase and lactic acid metabolism, and gain a deep understanding of the varying effects of different causes of hyperlactatemia on the body, so that anesthesiologists can make correct analyses, perform timely management, and enact effective decisions on follow-up treatment when intraoperative hyperlactatemia occurs. At the same time, the surgical team should also pay closer attention to patients’ preoperative liver function, and evaluate and actively treat said patients.
For patients with normal preoperative liver function, clinicians should attempt to control the inflammatory response during the perioperative period, maintain stable hemodynamics during surgery, reasonably control low central venous pressure while ensuring tissue perfusion, and minimize operative and hepatic portal block times to reduce the incidence of hyperlactatemia by reducing the degree of liver function impairment associated with surgery. In elective surgery patients with preoperative hepatitis or cirrhosis resulting in hepatic insufficiency, the incidence of intraoperative hyperlactatemia is greatly increased, which can seriously affect the function of important organs and increase the difficulty of anesthesia management. Therefore, preoperative hepatoprotective therapy should be actively administered, and surgical treatment should be performed when liver function is restored to that required for elective surgery. Intraoperative attention should be paid to strengthening prevention and treatment and actively maintaining fragile liver function to reduce the incidence of hyperlactatemia, improve patient prognosis, and increase postoperative survival and quality of life.
In conclusion, our patient presented with multiple hepatic metastatic carcinomas secondary to sigmoid colon cancer. Intraoperative hyperlactatemia associated with surgical resection occurred, but the patient's circulatory stability and quality of recovery were not affected by hyperlactatemia during the awakening period, which had specific and somewhat unique reasons in this case. The quality of recovery during the awakening period and the patient’s good postoperative outcome suggest that the analysis of the causes of hyperlactatemia, comprehensive management, and the decision to extubate were correct.
In this case, we found that hyperlactatemia did not affect the patient’s circulatory stability or the quality of awakening. We considered that active intraoperative rehydration avoided serious harm to the organism caused by hyperlactatemia due to insufficient tissue perfusion, while hyperlactatemia caused by decreased lactate clearance due to impaired liver function associated with surgical resection had a mild effect on the function of important organs. Additionally, strengthening body temperature protection could improve lactate circulation. Future clinical trials are needed for further confirmation of the preliminary findings of this case study, which is likewise intended to provide a reference for general anesthesiologists in their clinical work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ji X, China; Salimi M, Iran S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371:2309-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 553] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 2. | Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, Kluge S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 2016;42:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | Bian LY, Yu K, Long C. Hyperlactatemia in cardiac surgery. Yixue Zongshu. 2010;16:3281-3283. |
| 4. | Theodoraki K, Arkadopoulos N, Fragulidis G, Voros D, Karapanos K, Markatou M, Kostopanagiotou G, Smyrniotis V. Transhepatic lactate gradient in relation to liver ischemia/reperfusion injury during major hepatectomies. Liver Transpl. 2006;12:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Su WL, Zhu TH. Research progress on the etiology and treatment of hyperlactatemia. Yinanbing Zazhi. 2017;8:848-851. |
| 6. | Hu BJ, Bo LL, Deng S, Li XM, Duan HW. Advances in research related to lactic acidosis. Zhongguo Yiyao Daobao. 2018;15:22-25. |
| 7. | Liu Y, Liu YL, Cheng W, Yin XM, Jiang B. The expression of SIRT3 in primary hepatocellular carcinoma and the mechanism of its tumor suppressing effects. Eur Rev Med Pharmacol Sci. 2017;21:978-998. [PubMed] |
| 8. | Li CX, Zhang JQ, Ma LB, Li L, Du XH, Meng FM. Effect of multimodal insulation on postoperative regression and fee-for-service in patients undergoing radical hepatocellular carcinoma surgery. Zhonghua Mazuixue Zazhi. 2017;37:1304-1307. |
| 9. | Zhou H, Jia WD, Qiao XF, Liu FP, Chen L, Hu CL. [Clinical values of multimodal preventive analgesia in patients with partial hepatectomy for liver cancer]. Zhonghua Wai Ke Za Zhi. 2017;55:141-145. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Cheung TT, Chok KS, Chan AC, Tsang SH, Dai WC, Yau TC, Kwong A, Lo CM. Survival analysis of breast cancer liver metastasis treated by hepatectomy: A propensity score analysis for Chinese women in Hong Kong. Hepatobiliary Pancreat Dis Int. 2019;18:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |