Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1862
Peer-review started: January 7, 2023
First decision: January 17, 2023
Revised: January 19, 2023
Accepted: February 21, 2023
Article in press: February 21, 2023
Published online: March 16, 2023
Processing time: 58 Days and 23 Hours
Video-assisted thoracic surgery (VATS) lobectomy is a common treatment for patients with early-stage lung cancer. Some patients can experience slight gastrointestinal discomfort after lobectomy for a moment. Gastroparesis is a gastrointestinal disorder that can be severe; it is associated with an increased risk of aspiration pneumonia and impaired postoperative recovery. Here, we report a rare case of gastroparesis after VATS lobectomy.
A 61-year-old man underwent VATS right lower lobectomy uneventfully but had an obstruction of the upper digestive tract 2 d after surgery. Acute gastroparesis was diagnosed after emergency computed tomography and oral iohexol X-ray imaging. After gastrointestinal decompression and administration of prokinetic drugs, the patient’s gastrointestinal symptoms improved. Since perioperative medication was applied according to the recommended dose and there was no evidence of electrolyte imbalance, intraoperative periesophageal vagal nerve injury was the most likely underlying cause of gastroparesis.
Although gastroparesis is a rare perioperative complication following VATS, clinicians should be on the alert when patients complain about gastrointestinal discomfort. When surgeons resect paraesophageal lymph nodes with electrocautery, excessive ambient heat and compression of paraesophageal hematoma might induce vagal nerve dysfunction.
Core Tip: While postoperative gastroparesis is quite common in patients undergoing vagotomy for peptic ulcers and pylorus-sparing pancreatoduodenectomy, there are few reports following lobectomy. We report a rare case of gastroparesis after video-assisted thoracoscopic surgery. Since there was no evidence of drug-induced or electrocyte disorder-related gastrointestinal dysfunction, intraoperative periesophageal vagal nerve injury was most likely to account for gastroparesis. Clinicians should keep in mind that there is a potential possibility of vagal nerve injury after thoracic surgery even without direct nerve operation. For patients suffering gastroparesis after video-assisted thoracic surgery, conservative treatment, including gastrointestinal decompression and prokinetic medicines, can help relieve symptoms.
- Citation: An H, Liu YC. Gastroparesis after video-assisted thoracic surgery: A case report. World J Clin Cases 2023; 11(8): 1862-1868
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1862.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1862
According to the Global Cancer Statistics 2020 report, lung cancer is one of most common cancers worldwide, with an estimated 2.2 million new cases in 2020 and making up 11.4% of all cancer cases[1]. Curative surgery is the preferred treatment approach for patients with early-stage lung cancer. Over the past two decades, video-assisted thoracic surgery (VATS) has become an alternative to open thoracotomy with the advantage of being minimally invasive[2]. Compared with open thoracotomy, patients receiving VATS tend to experience less postoperative pain and fewer complications, and VATS has been indicated to have comparable or superior oncologic outcomes to thoracotomy[2,3]. A retrospective study carried out in China showed that the VATS rate for lung cancer was 47.6% nationwide, and in some hospitals, the rate even reached 89.7%[4]. The most common complications following VATS are prolonged air leakage, bleeding, infection and postoperative pain[5]; few patients experience gastrointestinal disorders after surgery[6,7].
Gastroparesis is an annoying gastrointestinal disorder associated with symptoms such as epigastric discomfort, abdominal pain, nausea, vomiting, and bloating. While postoperative gastroparesis is quite common in patients undergoing vagotomy for peptic ulcers and pylorus-sparing pancreatoduodenectomy, there are few case reports demonstrating delayed gastrointestinal emptying following pulmonary lobectomy. Here, we report a case of gastroparesis after VATS lobectomy.
A 61-year-old man complained about early satiety and postprandial fullness 2 d after VATS right lower lobectomy.
The patient drank clear fluid 2 h after VATS and had his first meal the next morning after surgery, which was in line with routine care in our hospital. At first, he complained about early satiety and postprandial fullness after eating food. Two days after surgery, gastrointestinal symptoms were aggravated, as the patient appeared to have upper abdominal pain and vomited a large amount of yellow-green fluid. During the process of the disease, exhaust and defecation were not impeded.
He presented to our hospital with coughing for the past three months. He had a chest computed tomography (CT) one month before, which showed a 6.0 cm × 4.4 cm mass in the right lower lobe of the lung with hilar and mediastinal lymphadenopathy. The subsequent bronchoscopic pulmonary biopsy failed to confirm the pathological nature of this mass.
Since the onset of symptoms, he lost 6 kg of weight even though there was no significant change in his diet and appetite.
VATS right lower lobectomy was carried out under general anesthesia combined with a paravertebral block. We exposed the thoracic cavity using a horizontal incision through the muscle between the fourth and fifth intercostal spaces, and no malignant pleural nodules or pleural effusions were found during thoracoscopic exploration. As the tumor was closely adhered to the pleura, electrocautery was used for dissection, and the right lower lobe was sealed smoothly with an endoscope linear stapler (PSE60-GIA, Johnson & Johnson, New Brunswick, NJ, United States). Subcarinal, paraesophageal and bronchial lymph node stations were dissected using monopolar electrocautery. The surgery was performed with caution, and there was no direct evidence of iatrogenic nerve injury. During the surgery, an accumulated dose of 25 µg sufentanil was intermittently administered, and the target concentration of remifentanil at the effect site was maintained at 3 ng/mL. The total duration of this surgery was 3 h and 52 min, and the total blood loss was 300 mL. The patient used patient-control analgesia with sufentanil (1 µg/mL), which was programmed to deliver 2-mL boluses with a lockout interval of 8 min and a background infusion of 1 mL/h. According to paraffin pathological sections, the tumor was invasive adenocarcinoma with a maximum diameter of 4 cm, and no metastasis was observed in any of the examined lymph nodes (T2aN0M0 according to the eighth edition of TNM classification of lung cancer)[8].
The patient had no symptom-related medical history but had smoked for many years. He denied any family history of gastrointestinal dysfunction.
On physical examination, the vital signs were as follows: Body temperature, 36.8 ℃; blood pressure, 127/68 mmHg; heart rate, 79 beats per min; and respiratory rate, 19 breaths per min. Furthermore, abdominal examination revealed a distended abdomen, and the bowel sounds were diminished on auscultation. When we rocked him back and forth from the hip, a succussion splash was elicited.
The electrolyte levels were within the normal limits (Table 1). No abnormalities were found in routine blood and urine analyses.
| Before surgery | First day after surgery | Third day after surgery | At discharge from hospital | |
| Albumin (g/L) | 31.9 | 29.2 | 32.1 | 29.8 |
| Creatinine (µmol/L) | 60.1 | 65.08 | 82.38 | 50.85 |
| Natrium (mmol/L) | 132.46 | 137.61 | 137.42 | 136.54 |
| Potassium (mmol/L) | 4.06 | 4.78 | 4.29 | 4.43 |
| Magnesium (mmol/L) | 0.86 | 0.86 | 1.03 | 0.92 |
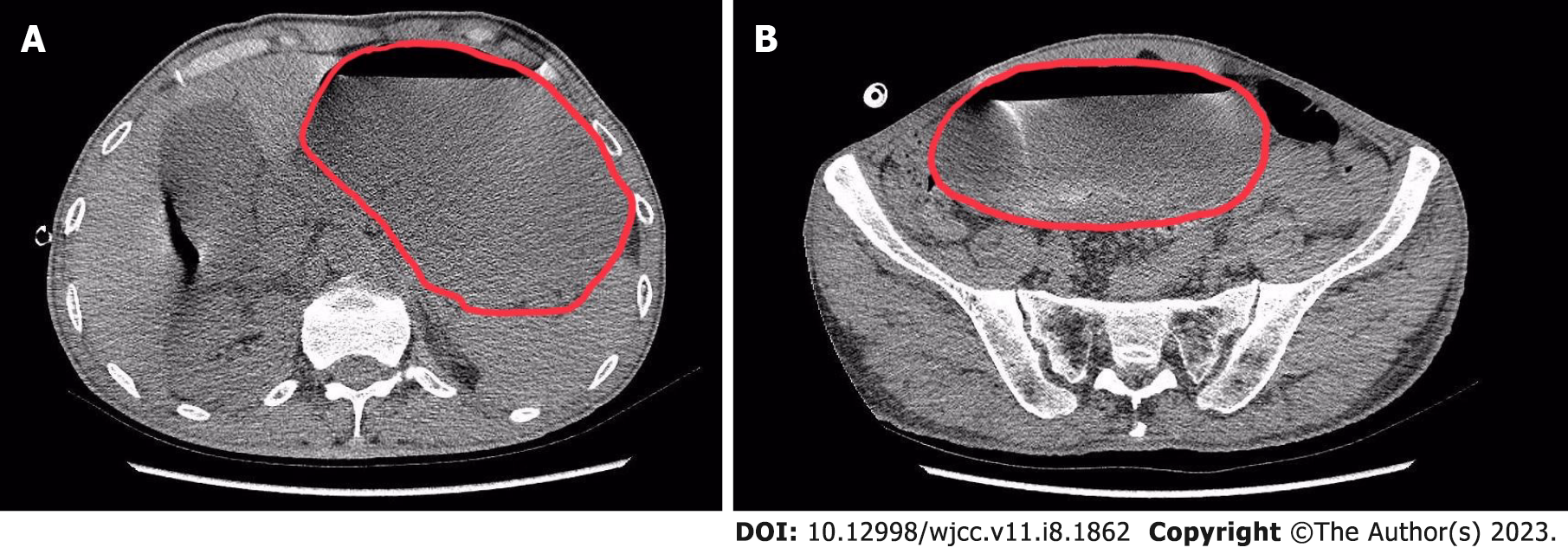
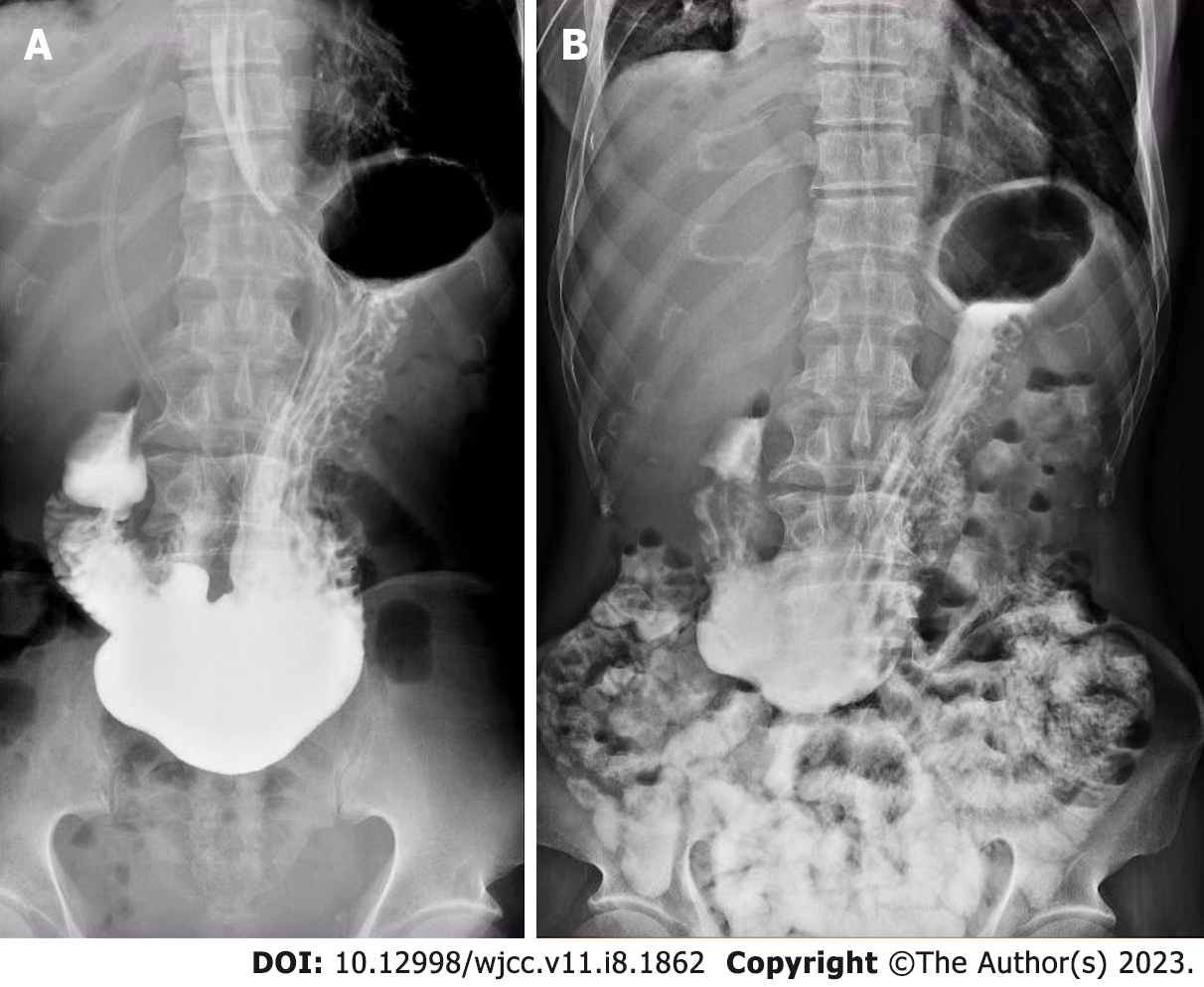
Emergency abdominal CT showed multiple effusions in the esophagus, while the massively dilated stomach and proximal duodenum contained a mass of fluid. The inferior edge of the distended stomach reached the pelvic cavity (Figure 1). Oral iohexol X-ray imaging was carried out after gastrointestinal decompression and demonstrated delayed gastric emptying (10 min after ingestion of iohexol, the first sign of passage into the duodenum was observed, and some contrast agent was retained in the stomach 4 h after administration) (Figure 2).
The patient’s presentation, physical examination, and laboratory and radiographic investigations narrowed the working diagnosis to digestive tract obstruction or acute gastroparesis. Since CT showed no obstructing mass or stenosis in the gastrointestinal tract, the diagnosis of acute gastroparesis was more likely. The only postoperative medication interfering with gastrointestinal function was sufentanil, which was infused at a low dose for analgesia, and we ceased the opioid immediately after the onset of gastrointestinal dysfunction. Drug-related gastrointestinal motility disorder was less likely to explain the marked nausea and vomiting, and the symptoms did not improve after drug withdrawal. Since there was no evidence of electrolyte imbalance, the most likely underlying cause of gastroparesis was vagal nerve injury during surgery.
Gastrointestinal decompression was implemented immediately to relieve gastrointestinal symptoms. After draining out 2600 mL of gastric contents, the patient’s symptoms improved significantly. Meanwhile, medication interfering with gastrointestinal function, such as sufentanil, was ceased immediately. We provided parecoxib at a dose of 40 mg twice daily as an alternative for analgesia since the patients had no contraindication. Prokinetic drugs such as metoclopramide and cisapride were administered according to medication instructions to enhance the patient’s recovery of gastrointestinal function. Gastroenterologists and nutritionists were consulted to formulate a specific therapy plan. As the patient could not tolerate enteral nutrition, parenteral support (structolipid and compound amino acid injection) was given to compensate for the need for nutritional supplementation. Antiemetics were used to relieve nausea and vomiting, and a proton-pump inhibitor was used to protect the mucosa from gastric acids.
Six days later, the patient was symptom-free and developed progressive feeding tolerance. He was discharged from the hospital 10 d after surgery and was able to tolerate a normal diet at the 30-d postoperative follow-up.
Surgery is the standard treatment for curable patients (clinical stage I and II non-small cell lung cancer) in whom there is no evidence of mediastinal involvement prior to surgical resection. VATS is a minimally invasive approach to the treatment of early-stage non-small cell lung cancer and has been reported to decrease postoperative complications, especially for those with significant medical comorbidities[2,3,9]. While the most common complication following thoracic surgery is pneumonia, there are few case reports on gastrointestinal disorders secondary to VATS lobectomy[5-7].
Gastroparesis is an annoying gastrointestinal disorder defined by delayed gastric emptying in the absence of a mechanical obstruction, with symptoms of nausea, vomiting, bloating, and abdominal pain. Diabetes with poor glycemic control is the most common etiology of gastroparesis[10]. The normal motor function of the gastrointestinal tract is a complex sequence of events mediated by an extrinsic nerve supply from the brain and spinal cord, complex neuronal plexus and other intrinsic or enteric pathways within the wall of the stomach and intestine (the enteric brain). They work together and alter the excitability of gastrointestinal smooth muscle. Abnormalities in any part of the sequence can result in delayed gastric emptying[11]. Previous studies have reported that some patients developed gastroparesis after surgery; among these patients suffering from gastroparesis, two-thirds had undergone therapeutic vagotomy for peptic ulcers[12]. Opioids used for postoperative analgesia can also aggravate gastrointestinal dysfunction[13]. However, the most common opioid-related gastrointestinal complication was constipation after long-term treatment with high-dose opioids[14,15]. In this case report, the patient received opioids at a low dose for a short duration and had no evidence of electrolyte imbalance, restricting the most likely underlying etiology to thoracic autonomic nervous system injury during the surgery. The sympathetic fibers innervating the stomach can cause contraction of the pylorus and reduce gastric blood flow, while the vagal nerves promote the secretion of gastrin and acid and relax the pyloric sphincter during gastric emptying[16]. Generally, they work together to coordinate gastrointestinal activity, and when the vagal nerves are injured, the activity of the sympathetic nerves will be enhanced.
The vagal nerves run behind the root of the lung and form the anterior and posterior esophageal plexuses, which are distributed along the front and back of the esophagus, respectively. Then, they merge into the anterior vagal trunk and the posterior vagal trunk, passing through the esophageal hiatus into the abdominal cavity and innervating the gastric glands and muscularis[17]. Gastroparesis has been reported as a complication after heart and lung transplantation because the vagal nerves are at high risk of injury when surgeons dissect the native lung[18]. The use of immunosuppressive medications and the progression of preexisting motility disorders aggravate gastrointestinal dysfunction in patients with end-stage lung disease as well[18]. In this case, the only medication disturbing gastrointestinal function was sufentanil, which was infused at a low dose and ceased immediately after the onset of gastrointestinal discomfort. There was no evidence of electrolyte imbalance or previous gastrointestinal disorder. The most likely underlying cause of gastroparesis was vagal nerve injury. However, no manipulation of the vagal nerve was performed during surgery, which excluded the possibility of direct neuronal damage. However, it is likely that the vagal nerve was exposed to edema caused by excessive ambient heat when we resected paraesophageal lymph nodes with electrocautery. Another possible reason accounting for the development of gastroparesis is that the thoracic vagal nerve might be compressed by a tiny paraesophageal hematoma, which would also influence the function of the vagal nerve for a while.
Although most patients suffering from gastroparesis after abdominal surgery can recover spontaneously without therapy, the alleviation of gastroparesis symptoms after thoracic surgery is still unknown. If the etiology of gastrointestinal dysfunction is reversible, conservative management (such as antacid use, raising the head of the bed, and frequent small meals) is effective for some patients with mild symptoms. Moreover, prokinetics such as metoclopramide and cisapride can be administered to improve gastric emptying and enhance the recovery of gastrointestinal function in most patients[12]. Since the most common etiology of gastroparesis after thoracic surgery is vagal nerve injury, surgical intervention can be considered if the symptoms are refractory and last for more than one year. Recently, surgical interventions, including pyloroplasty and gastrojejunostomy, have been indicated to be effective in treating refractory gastroparesis[12,19,20]. However, the proportion of patients receiving surgical treatment has yet to be studied. In this case, there was no direct damage to the patient’s vagal nerve, which allowed for gastrointestinal symptom relief after decompression with a nasogastric tube and medication with metoclopramide and cisapride. We also had some limitations regarding the management of this patient. First, the etiology of gastrointestinal dysfunction is putative, and it was impractical to assess the severity of nerve injury. After excluding other causes, intraoperative autonomic nervous system injury was the most likely etiology for the patient’s gastroparesis. Second, the gold standard for the diagnosis of gastroparesis is delayed gastric emptying on scintigraphy[21], but the patient refused to be examined due to financial reasons. We chose oral iohexol X-ray imaging as an alternative to evaluate gastrointestinal motility.
In this article, we report a rare case of gastroparesis after VATS lobectomy. The most likely etiology of the patient’s postoperative gastroparesis was indirect vagal nerve injury during the surgery. In this procedure, no direct manipulation of the vagal nerves was performed, but neuronal edema caused by electrocautery or compression of a tiny hematoma might account for transient gastrointestinal dysfunction. For patients suffering severe nausea and vomiting after thoracic surgery, gastroparesis should be considered after exhaustive inspection. Early detection and early treatment are vital to the recovery of patient gastrointestinal function.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Rosboch GL, Italy S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63990] [Article Influence: 15997.5] [Reference Citation Analysis (174)] |
| 2. | Yan TD, Cao C, D'Amico TA, Demmy TL, He J, Hansen H, Swanson SJ, Walker WS; International VATS Lobectomy Consensus Group. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardiothorac Surg. 2014;45:633-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Klapper J, D'Amico TA. VATS versus open surgery for lung cancer resection: moving toward a minimally invasive approach. J Natl Compr Canc Netw. 2015;13:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Cai J, Zhou J, Yang F, Wang J. Adoption Rate of Video-Assisted Thoracic Surgery for Lung Cancer Varies Widely in China. Chest. 2018;153:1073-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Imperatori A, Rotolo N, Gatti M, Nardecchia E, De Monte L, Conti V, Dominioni L. Peri-operative complications of video-assisted thoracoscopic surgery (VATS). Int J Surg. 2008;6 Suppl 1:S78-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Burel J, El Ayoubi M, Baste JM, Garnier M, Montagne F, Dacher JN, Demeyere M. Surgery for lung cancer: postoperative changes and complications-what the Radiologist needs to know. Insights Imaging. 2021;12:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Bédat B, Abdelnour-Berchtold E, Perneger T, Licker MJ, Stefani A, Krull M, Perentes JY, Krueger T, Triponez F, Karenovics W, Gonzalez M. Comparison of postoperative complications between segmentectomy and lobectomy by video-assisted thoracic surgery: a multicenter study. J Cardiothorac Surg. 2019;14:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:138-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 9. | Cheng AM, Wood DE. VATS versus open surgery for lung cancer resection: moving beyond the incision. J Natl Compr Canc Netw. 2015;13:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Sharayah AM, Hajjaj N, Osman R, Livornese D. Gastroparesis in a patient with diabetic ketoacidosis. Cleve Clin J Med. 2019;86:238-239. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Lacy BE, Parkman HP, Camilleri M. Chronic nausea and vomiting: evaluation and treatment. Am J Gastroenterol. 2018;113:647-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Shafi MA, Pasricha PJ. Post-surgical and obstructive gastroparesis. Curr Gastroenterol Rep. 2007;9:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Bianchi G, Ferretti P, Recchia M, Rocchetti M, Tavani A, Manara L. Morphine tissue levels and reduction of gastrointestinal transit in rats. Correlation supports primary action site in the gut. Gastroenterology. 1983;85:852-858. [PubMed] |
| 14. | Balbale SN, Cao L, Trivedi I, Stulberg JJ, Suda KJ, Gellad WF, Evans CT, Lambert BL, Jordan N, Keefer LA. High-Dose Opioid Use Among Veterans with Unexplained Gastrointestinal Symptoms Versus Structural Gastrointestinal Diagnoses. Dig Dis Sci. 2021;66:3938-3950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 15. | Bell TJ, Panchal SJ, Miaskowski C, Bolge SC, Milanova T, Williamson R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 2009;10:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 391] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 16. | Gillespie JS, Maxwell JD. Adrenergic innervation of sphincteric and nonsphincteric smooth muscle in the rat intestine. J Histochem Cytochem. 1971;19:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Jackson RG. Anatomy of the vagus nerves in the region of the lower esophagus and the stomach. Anat Rec. 1949;103:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Blackett JW, Benvenuto L, Leiva-Juarez MM, D'Ovidio F, Arcasoy S, Jodorkovsky D. Risk Factors and Outcomes for Gastroparesis After Lung Transplantation. Dig Dis Sci. 2022;67:2385-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Akindipe OA, Faul JL, Vierra MA, Triadafilopoulos G, Theodore J. The surgical management of severe gastroparesis in heart/lung transplant recipients. Chest. 2000;117:907-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 20. | Hibbard ML, Dunst CM, Swanström LL. Laparoscopic and endoscopic pyloroplasty for gastroparesis results in sustained symptom improvement. J Gastrointest Surg. 2011;15:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Vijayvargiya P, Jameie-Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2019;68:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |