Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1753
Peer-review started: December 13, 2022
First decision: January 19, 2023
Revised: January 20, 2023
Accepted: February 21, 2023
Article in press: February 21, 2023
Published online: March 16, 2023
Processing time: 83 Days and 21.9 Hours
Fas ligand (FasL) is one ligand that activates extrinsic apoptosis pathway. High expression in lymphocytes of FasL have been found in patients with acute rejection of liver transplantation (LT). No high blood concentrations of soluble FasL (sFasL) have been found in patients with acute LT rejection; however, the samples size of those studies was small.
To determine whether patients with hepatocellular carcinoma (HCC) that dead during the first year of LT have higher blood sFasL concentrations previously to LT that those who that remain alive in a study of higher sample size.
Patients underwent LT due to HCC were included in this retrospective study. Serum sFasL levels prior to LT were measured and one-year LT mortality was registered.
Non-surviving patients (n = 14) showed higher serum sFasL levels [477 (269-496) vs 85 (44-382) pg/mL; P < 0.001] than surviving patients (n = 113). Serum sFasL levels (pg/mL) were associated with mortality (OR = 1.006; 95%CI = 1.003-1.010; P = 0.001) independently of age of LT donor in the logistic regression analysis.
We report for the first time that HCC patients who die within the first year of HT have higher blood sFasL concentrations prior to HT than those who remain alive.
Core Tip: Fas ligand (FasL) is one of the main ligands that activate apoptosis via the extrinsic pathway. Elevated blood concentrations of soluble FasL (sFasL) have not been found in patients with acute liver transplant (LT) rejection; however, the sample sizes of those studies were small. We found in this retrospective study of 127 patients with hepatocellular carcinoma underwent to LT that patients that die during the first year of LT have higher blood sFasL concentrations previously to LT than those who that remain alive. The beneficial results of blockade of the Fas system in animal models could motivate its investigation in these patients.
- Citation: Lorente L, Rodriguez ST, Sanz P, González-Rivero AF, Pérez-Cejas A, Padilla J, Díaz D, González A, Martín MM, Jiménez A, Cerro P, Portero J, Barrera MA. Patients with hepatocellular carcinoma that die during the first year of liver transplantation have high blood sFasL concentrations . World J Clin Cases 2023; 11(8): 1753-1760
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1753.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1753
Hepatocellular carcinoma (HCC) is one of the most common malignancies with the highest attributable mortality[1-4]. Liver transplantation (LT) in some HCC patients is the treatment of choice, as it removes the liver tumour and treats liver failure[5-8].
Apoptotic cell death occurs in healthy subjects (for morphogenesis and tissue remodelling) and in different diseases such as liver diseases[9-13]. Apoptosis can be activated by so-called extrinsic and intrinsic pathways. Extrinsic apoptosis pathway is activated when binding a protein of ligands superfamily of the tumour necrosis factor (TNFSF) to its receptor of membrane receptors superfamily of the tumour necrosis factor (TNFRSF). Fas ligand (FasL) is a major ligand for TNFSF, and Fas is a major receptor for TNFRSF. Upon binding of FasL to Fas, a death signal appears that will activate caspase 8. Subsequently, caspase 8 will produce caspase-3 activation, leading to cell death[9-13].
Little data has been reported on the FasL/Fas system in LT patients. Higher lymphocyte expression of Fas[14,15] and FasL[15] has been found in patients with acute LT rejection than in patients without rejection. In addition, higher blood sFas levels have been found in patients with acute LT rejection than in patients without rejection[16-18]; however, two of these studies found no difference in blood sFasL levels between patients with and without LT rejection[16,17] and one study did not report blood sFasL levels[18]. Finally, another study found no differences in sFas and sFasL blood levels between patients with and without LT rejection[19]. However, the sample size in all these studies was small (less than 70 patients undergoing LT). Therefore, we aimed to determine whether HCC patients who die during the first year of LT have higher pre-LT blood sFasL concentrations than those who remain alive in a larger sample size study.
Inclusion criteria were the following: Patients undergoing donor LT in brain death by HCC. Patients were recluted at the Hospital Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain). Recruitment period was from January 1996 to May 2017 by HCC. No exclusion criteria were considered. This retrospective study was performed with Institutional Ethic Review Board approval and with informed (and written) consent of patients (or a legal guardian). Formently, we determined DNA and RNA oxidative damage[20] in some of those LT patients. Now, in this research, we have measured serum levels of sFasL concentrations.
Variables recorded for each patient were serum alpha-fetoprotein (AFP) levels, Child-Pugh score[21], nodule size, within Milan criteria[22] pre- and post-LT, degree of tumour differentiation, age of liver donor, model for end-stage liver disease (MELD) score[23] by liver function, liver recipient age, sex, infiltration, macrovascular and microvascular invasion, LT technique, portal hypertension, multinodular tumour, pre-LT treatment and 1-year survival from LT (our endpoint study).
Analyses to measure serum FasL concentrations were performed at the Laboratory Department of the Hospital Universitario de Canarias (Tenerife, Spain) by enzyme-linked immunosorbent assay. The human FasL ELISA kit (Elabscience, Houston, TX, United States) with intra-assay and inter-assay coefficients of variation of less than 6% and a limit of detection of 15.6 pg/mL was used.
We employed χ2 test to compare categorical variables (reported as frequencies and percentages) and Mann-Whitney test to compare continuous variables (reported as medians and interquartile ranges). Areceiver operating characteristic (ROC) curve was employed to test the predictive capability of LT 1-year mortality with pre-LT serum sFasL levels. Kaplan-Meier survival curves were constructed using 1-year LT survival and pre-LT serum sFasL levels below/above 190 pg/mL (this cut-off point was selected based on Youden-Jindex). We employed logistic regression analysis to determine a possible association between pre-LT serum sFasL levels and mortality at 1-year post-LT, controlling for LT donor age. Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, United States) and MedCal 15.2.1 (Ostend, Belgium).
A total of 127 patients were included in the study, 113 (89.0%) of them remain alive at one year post-LT and 14 (11.0%) dead during the first year of LT. Their mean age was 51 ± 17 years, 109 (85.8%) were males and 18 (14.2%) were females. Of them, 121 (95.3%) were inside Milan criteria previously to LT and 107 (84.3%) after LT, 41 had (32.3%) infiltration, 29 (22.8%) microvascular invasion, 87 (68.5%) portal hypertension, 44 (34.6%) multinodular tumor, and 7 (5.5%) macrovascular invasion.
We found that 1-year LT survivors revealed younger LT donors (P = 0.049) and lower serum sFasL levels (P < 0.001) than non-surviving ones (Table 1). However, serum AFP levels, Child-Pugh score, nodule size, fulfilment of Milan criteria before and after HT, degree of tumour differentiation, MELD score by liver function, age of the liver recipient, sex, infiltration, macrovascular invasion, microvascular invasion, HT technique, portal hypertension, multinodular tumour and pre-HT treatment showed no statistical difference in the comparison between surviving and non-surviving patients (Table 1).
| 1 yr survivor patients, (n = 113) | 1 yr non-survivor patients, (n = 14) | P value | |
| Serum sFasL (pg/mL) - median (p 25-75) | 85 (44-382) | 477 (269-496) | < 0.001 |
| Age of liver donor (yr) - median (p 25-75) | 52 (35-64) | 62 (49-73) | 0.049 |
| Age of liver recipient (yr) - median (p 25-75) | 59 (52-62) | 56 (53-62) | 0.88 |
| Serum alpha-fetoprotein (ng/dL) - median (p 25-75) | 8 (4-37) | 13 (5-180) | 0.36 |
| MELD score - median (p 25-75) | 15 (12-18) | 16 (14-18) | 0.78 |
| Nodules size (cm) - median (p 25-75) | 3.0 (2.0-3.5) | 2.8 (1.6-4.3) | 0.94 |
| Protein (g/dL) - median (p 25-75) | 6.7 (6.0-7.1) | 6.7 (5.7-7.7) | 0.83 |
| Leukocytes (count/mm3) - median (p 25-75) | 4800 (3590-6200) | 4940 (3490-7920) | 0.60 |
| Albumin (g/dL) - median (p 25-75) | 3.3 (2.9-4.1) | 3.3 (2.9-4.2) | 0.91 |
| Creatinine (mg/dL) - median (p 25-75) | 0.9 (0.8-1.1) | 1.0 (0.8-1.1) | 0.43 |
| BMI (kg/m2) - median (p 25-75) | 27.6 (24.5-30.0) | 28.1 (23.1-31.1) | 0.80 |
| Gender female - n (%) | 18 (15.9) | 0 | 0.11 |
| Portal hypertension - n (%) | 77 (68.1) | 10 (71.4) | 0.80 |
| Child-Pugh score - n (%) | 0.47 | ||
| A | 53 (46.9) | 9 (64.3) | |
| B | 34 (30.1) | 3 (21.4) | |
| C | 26 (23.0) | 2 (14.3) | |
| Multinodular tumor- n (%) | 33 (29.2) | 5 (35.7) | 0.62 |
| Macrovascular invasion - n (%) | 7 (6.2) | 0 | 0.34 |
| Microvascular invasion - n (%) | 26 (23.0) | 3 (21.4) | 0.89 |
| Infiltration - n (%) | 38 (33.6) | 3 (21.4) | 0.36 |
| Inside Milan criteria previously to LT - n (%) | 108 (95.6) | 13 (92.9) | 0.65 |
| Inside Milan criteria after LT - n (%) | 97 (85.8) | 10 (71.4) | 0.16 |
| Treatment previously to LT - n (%) | 62 (54.9) | 10 (71.4) | 0.24 |
| Transplantation technique – n (%) | 0.63 | ||
| By-pass | 41 (36.3) | 6 (42.9) | |
| Piggy back | 72 (63.7) | 8 (57.1) | |
| Degree of tumor differentiation – n (%) | 0.51 | ||
| Well | 83 (73.5) | 11 (78.6) | |
| Moderate | 27 (23.9) | 2 (14.3) | |
| Poor | 3 (2.7) | 1 (7.1) |
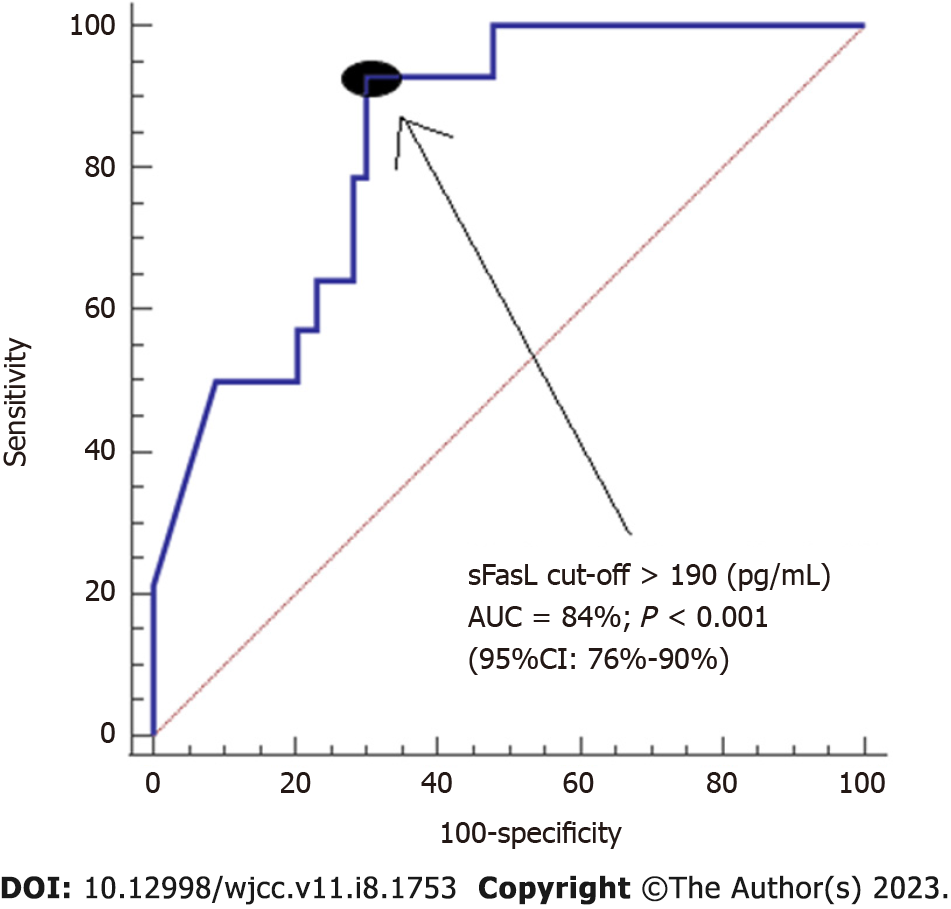
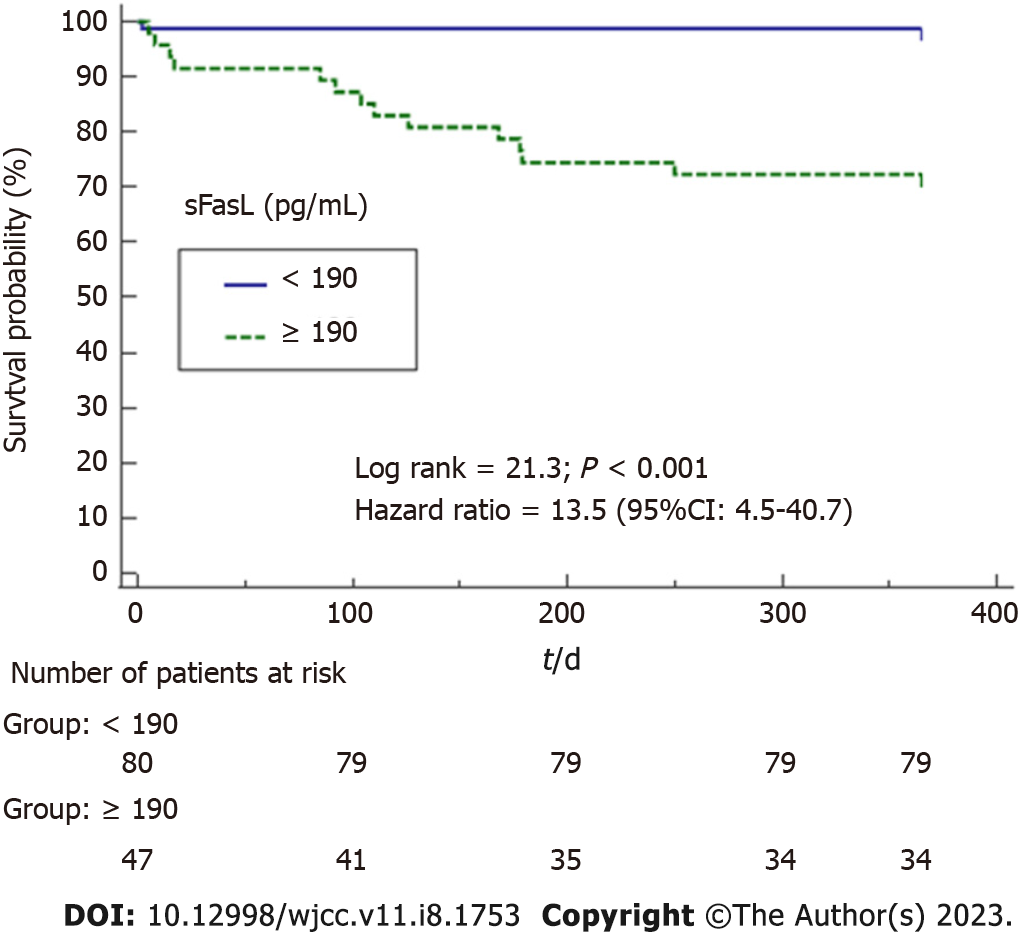
Serum sFasL levels (pg/mL) were associated mortality (Odds Ratio = 1.006; 95%CI = 1.003-1.010; P = 0.001) independently of LT donor age in logistic regression analysis (Table 2). We found an area under the curve of 84% (95%CI = 75%-93%; P < 0.001) for prediction of mortality by serum sFasL levels (Figure 1). Kaplan-Meier analysis showed that patients with serum sFasL concentrations higher than 190 pg/mL had an increased risk of death (Hazard ratio = 13.5; 95%CI = 4.5-40.7); P < 0.001) (Figure 2).
| Odds ratio | 95% confidence interval | P value | |
| Age of liver donor (age) | 1.044 | 1.001-1.088 | 0.045 |
| Serum sFasL levels (pg/mL) | 1.006 | 1.003-1.010 | 0.001 |
We found a positive correlation between serum levels of sFasL and AFP (rho = 0.73; P < 0.001). However, we no found significant differences in serum sFasL levels related to LT recipient age (P = 0.36), sex (P = 0.27), MELD score (P = 0.12), Child–Pugh score (P = 0.48), portal hypertension (P = 0.82), multinodular tumor (P = 0.16), macrovascular invasion (P = 0.48), degree of tumor differentiation (P = 0.22), microvascular invasion (P = 0.19), serun protein concentration (P = 0.67), serum albumin concentration (P = 0.07) and serum creatinine concentration (P = 0.96).
The main novel finding that showed our study was that association between elevated serum sFasL levels prior to LT and increased risk of death during the first year after LT. The 1-year survival rate from LT in our series (89%) is similar to those reported in other series (75%-95%)[24-28]. Several factors associated with worse prognosis in HCC patients undergoing LT have been reported (tumour size, outside Milan criteria, hepatic microinvasion, tumour number, degree of differentiation, vascular infiltration, serum AFP levels, and invasion)[29]; however, only higher serum sFasL levels and age of the LT donor were factors associated with worse prognosis in our series.
Previously, higher lymphocyte expression of FasL[15] has been found in patients with acute LT rejection than in patients without rejection. Furthermore, three studies found no difference in blood sFasL levels between patients with and without LT rejection[16,17,19]. Therefore, the association between elevated serum sFasL levels before LT and death is a new finding of our study. Possibly the larger sample size (127 patients) of our study compared to that of previous studies (less than 70 patients) contributes to this new finding.
In addition, we also found a positive association between serum levels of sFasL and AFP, and that association is according to the findings of one previous study[30]; however, we did not found an association between serum sFasL levels and other variables.
FasL is one of TNFSF that activates apoptosis extrinsic pathway when binding to its receptor Fas. Due to this binding, a death signal is generated (which will activate caspase 8). Subsequently, caspase 8 will lead to the activation of caspase 3 (responsible for cell death)[9-13]. Therefore, we think that the findings of our study that non-surviving patients showed higher serum sFasL levels compared to surviving ones could be related to a higher activation of apoptosis. Although limitations of our study were the absence of data on apoptosis in liver tissue and data on blood sFasL levels during patient follow-up. Another limitation was the relatively low sample size of our study to include more variables in the regression model.
In rodent animal models of hepatic ischaemia-reperfusion injury, administration of Fas and/or FasL-blocking agents reduced hepatic caspase-3 activity and hepatocyte apoptosis, and increased animal survival[31-33]. We think that the findings from our study with patients undergoing HT due to HCC could encourage research to clarify the potential role of serum sFasL levels in estimating the prognosis of HT patients in a larger series. In addition, the findings from rodent animal models could encourage research to clarify the potential role of administration of Fas/FasL-blocking agents in improving the prognosis of these patients.
We report for the first time that HCC patients who die during the first year of LT have higher blood sFasL concentrations prior to LT than those who remain alive.
Fas ligand (FasL) is one ligands that activates extrinsic apoptosis pathway. High expression in lymphocytes of FasL have been found in patients with acute rejection of liver transplantation (LT).
No high blood concentrations of soluble FasL (sFasL) have been found in patients with acute LT rejection; however, the samples size of those studies was small.
To determine whether patients with hepatocellular carcinoma (HCC) that dead during the first year of LT have higher blood sFasL concentrations previously to LT that those who that remain alive in a study of higher sample size.
Patients underwent LT due to HCC were included in this retrospective study. Serum sFasL levels prior to LT were measured and one-year LT mortality was registered.
Non-surviving patients (n = 14) showed higher serum sFasL levels [477 (269-496) vs 85 (44-382) pg/mL; P < 0.001] than surviving patients (n = 113). Serum sFasL levels (pg/mL) were associated with mortality (OR = 1.006; 95%CI = 1.003-1.010; P = 0.001) independently of LT donor in the logistic regression analysis.
We report for the first time that HCC patients who die within the first year of HT have higher blood sFasL concentrations prior to HT than those who remain alive.
The beneficial results of blockade of the Fas system in animal models could motivate its investigation in these patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng HW, China; Li ZZ, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Tümen D, Heumann P, Gülow K, Demirci CN, Cosma LS, Müller M, Kandulski A. Pathogenesis and Current Treatment Strategies of Hepatocellular Carcinoma. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 2. | Ince V, Sahin TT, Akbulut S, Yilmaz S. Liver transplantation for hepatocellular carcinoma: Historical evolution of transplantation criteria. World J Clin Cases. 2022;10:10413-10427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Bzeizi KI, Abdullah M, Vidyasagar K, Alqahthani SA, Broering D. Hepatocellular Carcinoma Recurrence and Mortality Rate Post Liver Transplantation: Meta-Analysis and Systematic Review of Real-World Evidence. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 4. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1176] [Article Influence: 392.0] [Reference Citation Analysis (41)] |
| 5. | Bodzin AS, Busuttil RW. Hepatocellular carcinoma: Advances in diagnosis, management, and long term outcome. World J Hepatol. 2015;7:1157-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Guerrero-Misas M, Rodríguez-Perálvarez M, De la Mata M. Strategies to improve outcome of patients with hepatocellular carcinoma receiving a liver transplantation. World J Hepatol. 2015;7:649-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Slotta JE, Kollmar O, Ellenrieder V, Ghadimi BM, Homayounfar K. Hepatocellular carcinoma: Surgeon's view on latest findings and future perspectives. World J Hepatol. 2015;7:1168-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Shojaie L, Iorga A, Dara L. Cell Death in Liver Diseases: A Review. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 10. | Magusto J, Majdi A, Gautheron J. [Cell death mechanisms in non-alcoholic steatohepatitis]. Biol Aujourdhui. 2020;214:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | García-Pras E, Fernández-Iglesias A, Gracia-Sancho J, Pérez-Del-Pulgar S. Cell Death in Hepatocellular Carcinoma: Pathogenesis and Therapeutic Opportunities. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Chu Q, Gu X, Zheng Q, Wang J, Zhu H. Mitochondrial Mechanisms of Apoptosis and Necroptosis in Liver Diseases. Anal Cell Pathol (Amst). 2021;2021:8900122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Gruevska A, Moragrega ÁB, Cossarizza A, Esplugues JV, Blas-García A, Apostolova N. Apoptosis of Hepatocytes: Relevance for HIV-Infected Patients under Treatment. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Boix F, Millan O, San Segundo D, Mancebo E, Rimola A, Fabrega E, Fortuna V, Mrowiec A, Castro-Panete MJ, Peña Jde L, Llorente S, Minguela A, Bolarin JM, Paz-Artal E, Lopez-Hoyos M, Brunet M, Muro M. High expression of CD38, CD69, CD95 and CD154 biomarkers in cultured peripheral T lymphocytes correlates with an increased risk of acute rejection in liver allograft recipients. Immunobiology. 2016;221:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Yu LX, Han SL, Miao Y. [Detection of CD95 and CD95L mRNA expression after liver transplantation using SYBR real-time PCR during acute rejection]. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:185-188. [PubMed] |
| 16. | Seino K, Kayagaki N, Yamaguchi N, Takada Y, Uyama S, Kiuchi T, Tanaka K, Yagita H, Okumura K, Fukao K. Soluble forms of CD95 and CD95 ligand after living related liver transplantation. Transplantation. 1999;67:634-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Wang YL, Zhang YY, Li G, Tang ZQ, Zhou YL, Zhu ZJ, Yao Z. Correlation of CD95 and soluble CD95 expression with acute rejection status of liver transplantation. World J Gastroenterol. 2005;11:1700-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Rivero M, Crespo J, Mayorga M, Fábrega E, Casafont F, Pons-Romero F. Involvement of the Fas system in liver allograft rejection. Am J Gastroenterol. 2002;97:1501-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Marín LA, Moya-Quiles MR, Miras M, Minguela A, Bermejo J, Ramírez P, García-Alonso AM, Parrilla P, Alvarez-López MR, Muro M. Evolution of soluble forms of CD86, CD95 and CD95L molecules in liver transplant recipients. Transpl Immunol. 2012;26:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Lorente L, Rodriguez ST, Sanz P, González-Rivero AF, Pérez-Cejas A, Padilla J, Díaz D, González A, Martín MM, Jiménez A, Cerro P, Portero J, Barrera MA. DNA and RNA oxidative damage in hepatocellular carcinoma patients and mortality during the first year of liver transplantation. World J Hepatol. 2022;14:1182-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5721] [Article Influence: 110.0] [Reference Citation Analysis (2)] |
| 22. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5297] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 23. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3668] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 24. | Gelson W, Hoare M, Dawwas MF, Vowler S, Gibbs P, Alexander G. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation. 2011;91:1240-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Doyle MB, Vachharajani N, Maynard E, Shenoy S, Anderson C, Wellen JR, Lowell JA, Chapman WC. Liver transplantation for hepatocellular carcinoma: long-term results suggest excellent outcomes. J Am Coll Surg. 2012;215:19-28; discussion 28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Dumitra S, Alabbad SI, Barkun JS, Dumitra TC, Coutsinos D, Metrakos PP, Hassanain M, Paraskevas S, Chaudhury P, Tchervenkov JI. Hepatitis C infection and hepatocellular carcinoma in liver transplantation: a 20-year experience. HPB (Oxford). 2013;15:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Teng F, Wang GH, Tao YF, Guo WY, Wang ZX, Ding GS, Shi XM, Fu ZR. Criteria-specific long-term survival prediction model for hepatocellular carcinoma patients after liver transplantation. World J Gastroenterol. 2014;20:10900-10907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 28. | Ninomiya M, Shirabe K, Facciuto ME, Schwartz ME, Florman SS, Yoshizumi T, Harimoto N, Ikegami T, Uchiyama H, Maehara Y. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J Am Coll Surg. 2015;220:297-304.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Lorente L. New prognostic biomarkers of mortality in patients undergoing liver transplantation for hepatocellular carcinoma. World J Gastroenterol. 2018;24:4230-4242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Song le H, Binh VQ, Duy DN, Bock TC, Kremsner PG, Luty AJ, Mavoungou E. Variations in the serum concentrations of soluble Fas and soluble Fas ligand in Vietnamese patients infected with hepatitis B virus. J Med Virol. 2004;73:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Al-Saeedi M, Steinebrunner N, Kudsi H, Halama N, Mogler C, Büchler MW, Krammer PH, Schemmer P, Müller M. Neutralization of CD95 ligand protects the liver against ischemia-reperfusion injury and prevents acute liver failure. Cell Death Dis. 2018;9:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Nakajima H, Mizuta N, Fujiwara I, Sakaguchi K, Ogata H, Magae J, Yagita H, Koji T. Blockade of the Fas/Fas ligand interaction suppresses hepatocyte apoptosis in ischemia-reperfusion rat liver. Apoptosis. 2008;13:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Wang J, Li W, Min J, Ou Q, Chen J. Fas siRNA reduces apoptotic cell death of allogeneic-transplanted hepatocytes in mouse spleen. Transplant Proc. 2003;35:1594-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |