Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1419
Peer-review started: November 30, 2022
First decision: January 17, 2023
Revised: January 17, 2023
Accepted: February 3, 2023
Article in press: February 3, 2023
Published online: February 26, 2023
Processing time: 86 Days and 2.9 Hours
Transverse myelitis (TM) is characterized by sudden lower extremity progressive weakness and sensory impairment, and most patients have a history of advanced viral infection symptoms. A variety of disorders can cause TM in association with viral or nonviral infection, vascular, neoplasia, collagen vascular, and iatrogenic, such as vaccination. Vaccination has become common through the global implementation against coronavirus disease 2019 (COVID-19) and reported complications like herpes zoster (HZ) activation has increased.
This is a 68-year-old woman who developed multiple pustules and scabs at the T6-T9 dermatome site 1 wk after vaccination with the COVID-19 vaccine (Oxford/ AstraZeneca ([ChAdOx1S{recombinant}]). The patient had a paraplegia aggrava
HZ developed after COVID-19 vaccination, which may lead to more severe complications. Therefore, HZ treatment itself should not be delayed. If neurolo
Core Tip: Reports are increasing that transverse myelitis (TM) is a rare herpes zoster (HZ) complication and is linked to coronavirus disease 2019 (COVID-19) vaccines. HZ following the COVID-19 vaccination may lead to more severe complications due to the vaccine immunocompromising the patient. Typically, complications like TM from HZ may be rare, and difficult to diagnose. If there is diagnosis confusion, TM will progress rapidly, delaying the appropriate treatment. A critical point is to implement appropriate HZ therapy without delay simultaneously with neurological examination to evaluate TM progress and treatment and HZ care with pain control to protect against complications like postherpetic neuralgia.
- Citation: Cho SY, Jang BH, Seo JW, Kim SW, Lim KJ, Lee HY, Kim DJ. Transverse myelitis caused by herpes zoster following COVID-19 vaccination: A case report. World J Clin Cases 2023; 11(6): 1419-1425
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1419.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1419
Herpes zoster (HZ) disease is caused by the varicella-zoster virus (VZV), another herpes virus member. The virus becomes dormant in the cranial nerve and dorsal root ganglia in the spine alongside the spine. It is later frequently reactivated, bringing about zoster and postherpetic neuralgia[1]. In immunocompromised patients, even in immunocompetent elderly, VZV can cause central nervous system diseases including encephalopathy, myelitis, and neuralgia[2]. Commonly, it begins to recover within a week of the outbreak. Still, it may last for weeks or even months, and it is a disease that can leave complications, such as urinary dysfunction and lower extremity motor or sensory weakness.
There are increasing reports regarding HZ activation after the coronavirus disease 2019 (COVID-19) vaccination administration, which is ongoing during the pandemic[3]. HZ is presumed to be caused by certain immunomodulation that allows VZV to arise from latency due to the vaccination, but its exact mechanism is unknown. It has been reported that transverse myelitis (TM) rarely occurs as an HZ complication. Nonetheless, reports that TM has occurred after COVID-19 vaccination is also increasing as vaccination has become widespread[4].
This is a transverse myelitis case, an unusual complication caused by VZV reactivation or COVID-19 vaccination. This case was diagnosed via spinal magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) examination. The patient experienced almost complete recovery with only minor muscle strength and sensory impairment after treatment.
A 68-year-old woman presented with 3 d of both lower limb weakness, associated with both lower limb numbness and multiple skin lesions in the left thoracic area.
4 wk before the visit, the patient received the AstraZeneca COVID-19 vaccination. A week later, multiple skin lesions developed on her back and buttock. Thoracic pain developed with hyperesthesia, for which she consulted the pain clinic. T-spine epidural nerve block two times was performed to control the pain. Worsening pain spread to the back 3 days after the procedure. Afterward, weakness and numbness were felt in both lower legs, and the patient developed a gait disorder.
After a slip and fall accident at the age of 7, the patient felt lower body weakness but could still ambulate. Gait instability became very severe 5 years ago ambulation could only be performed with a walker. No other specific findings were found in the patient's history.
The patient had no family history or genetic disease.
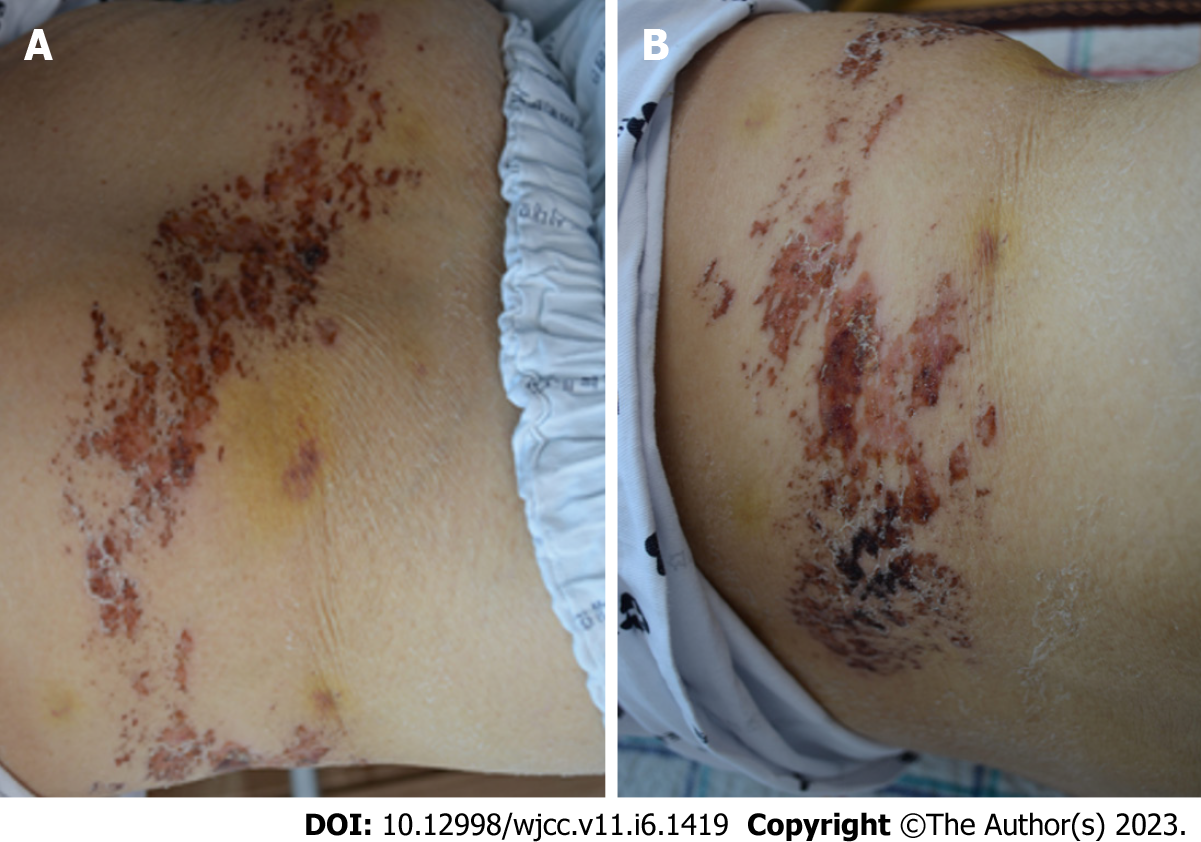
On physical examination, the patient’s mental status was alert with stable vital signs; body temperature was 36.8 °C. Multiple rashes and crusts were observed from the back to the chest, corresponding to the T6-T9 unilateral dermatome (Figure 1).
Motor and sensory examination showed decreased sensation (including vibratory and thermal) below the T7 Level by 50%-60% compared to the right side. The upper limb power average is grade 5 with grade 1 power in both lower limbs. Deep tendon reflexes were 2+ in the ankle, and 4+ in the knee, activated. Babinski’s sign was positive bilaterally with clonus at the right ankle. Along with the lower extremity weakness, there were limb numbness and urinary retention symptoms.
Based on the above symptoms, nerve damage by physical compression was considered, such as epidural hematoma; central nervous system diseases, such as TM by zoster and demyelinating diseases, such as multiple sclerosis, as differential diagnosis.
Investigations done were as follows: White blood count 9.7 × 10³/mL, hemoglobin 12.2 g/dL, platelet count 231 × 10³/mL, and ESR of 3.5 mm/h and CRP of 2.3 mg/dL. VZV DNA was detected via polymerase chain reaction (PCR) amplification in blood. The blood sample results for herpes simplex virus, cytomegalovirus, and Epstein–Barr virus were negative.
Lumbar CSF study showed the following: Intracranial pressure (ICP) 170 mmH2O, red blood cell count 100 cells/uL, white blood cell count 402 cells/uL (99% monocytes, 1% lymphocyte), protein 133 mg/dL, and glucose 66.7 mg/dL. VZV DNA was not detected via PCR amplification in CSF. AFB stain, Gram stain, cytology for malignant cells, and cultures were all negative.
Demyelinating workup was negative for AQP4-Ab, oligoclonal band, and IgG index.
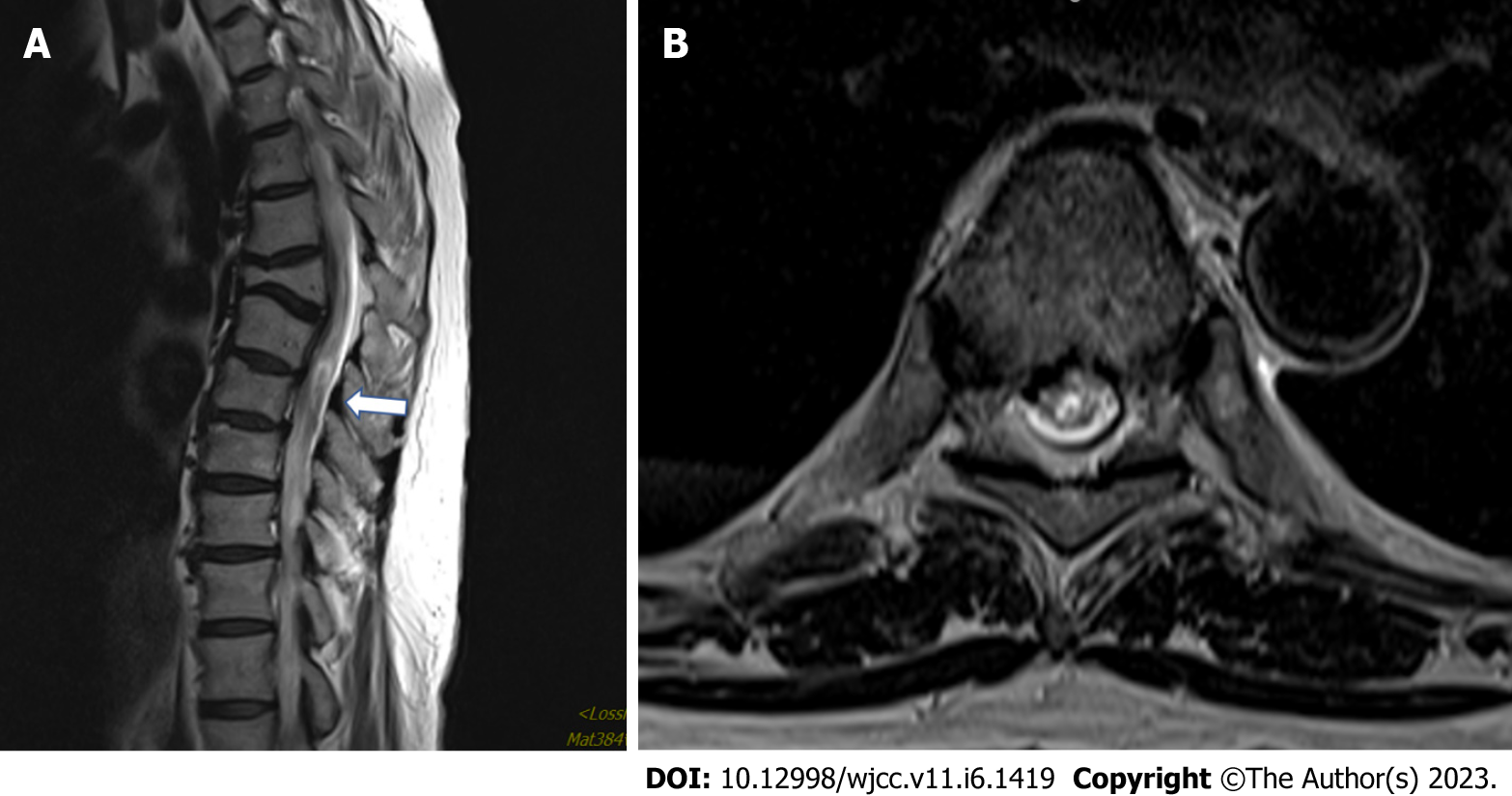
Brain computed tomography (CT) & magnetic resonance imaging (MRI) finding is nonspecific. Spine MRI showed old T5 compression fractures with some kyphotic deformity at the upper T-spine and subtle intramedullary T2 signal hyperintensity at the T6–T9 Levels, which suggested TM (Figure 2).
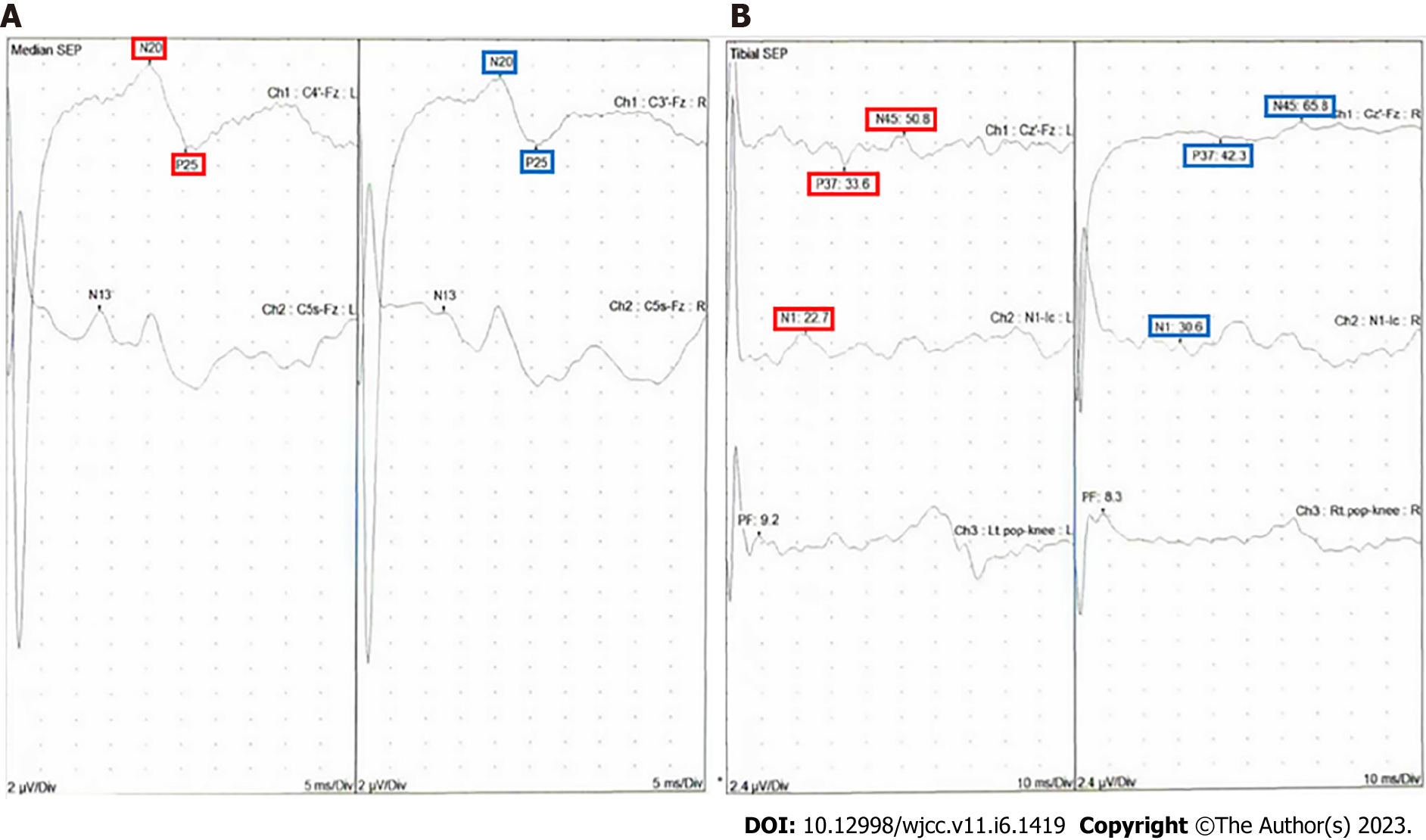
Somatosensory-evoked potential (SSEP) was performed. Median SSEP showed normal conduction, whereas tibial SSEP was delayed in the iliac crest and cerebral cortex level, right dominantly (Figure 3).
The diagnosis was TM with HZ.
With the HZ diagnosis, the patient was started on intravenous acyclovir for 14 days with methylprednisolone 1 g pulse therapy for 3 days. Because the symptom slowly improved, HZ treatment was decided to be continued with physical therapy and oral prednisolone for TM treatment. Because of continued pain from HZ, intravenous (IV) Ketorolac was administered as well as oral gabapentin.
On admission on Day 13, both lower limb distal motor power improved from grades I to grades III. On Day 17, standing and gait disturbance improved to partial impairment.
Subsequently, neurosurgery evaluation determined that it was not a surgical indication. Lower extremity muscle strength and sensation improved to some extent, 3 mo later, similar to before the HZ.
HZ is a disease that occurs when the VZV exists in a dormant state in the body and is reactivated. Usually, rashes and characteristic blister-type lesions appear on the skin within a few days, with accompanying pain in the area. Zoster is rare in young people and usually occurs in adults > 60 years old with poor immunity[5]. Zoster is not limited to the skin areas and sensory nerve distribution in immunocompromised patients. Still, it may appear with various neurological complications like encephalopathy, cerebral infarction, neuralgia, and myelitis[6].
There are numerous TM causes, including bacterial, fungi, parasitic, and viral infections. Autoimmune system disorders like lupus attack the body's tissues, and multiple sclerosis can be connected to TM as the first sign. Other myelin disorders, such as multiple sclerosis and neurosarcoidosis could also cause it[7]. Other conditions, such as a spinal cord stroke, are often confused with TM, requiring different treatment approaches[8]. TM diagnosis can be found in immunological and virological examinations, CSF examination results, MRI findings, and clinical manifestations[9]. MRI efficacy is unclear, but the typical MRI appearance in TM was iso or hypointense on T1 weighted imaging (T1WI) and poorly delineated hyperintense signal on T2WI in the central spinal cord lesion extending up to three to four spinal segments, involving more than two-thirds of the cord’s cross-sectional area[10]. CSF laboratory findings describe pleocytosis with moderate lymphocytosis and a slightly elevated protein level (100–120 mg/dL). Glucose levels are normal. Likewise, the CSF analysis, in this case, revealed an increased white blood cell count of 402/µL, a protein level of 133 mg/dL, and average glucose of 66.7 mg/dL, which is suggestive of inflammation.
In parainfectious TM, the injury may be associated with the systemic response to VZV reactivation, especially in immunocompromised patients, with inflammation on either side of the spinal cord. This neurological disorder often damages nerve myelin and interferes with nerve pathways transmitted throughout the body[11]. The most common first symptoms were sensory change, weakness, and pain. It can cause bowel and bladder dysfunction[12]. Because of its rarity, definitive treatment has not yet been established, but it is reported that symptoms improve when treated with antiviral drugs and steroids, which are zoster treatments[13]. VZV DNA and VZV antibody are detected in CSF to confirm the TM diagnosis by varicella zoster. However, in most cases, PCR tests in the CSF are confirmed negative, and there are only a small number of cases in which DNA was detected in PCR tests. Therefore, this disease cannot be completely excluded even if CSF PCR is negative[14,15].
This patient’s MRI findings and CSF examination suggested TM. After the skin symptoms of zoster appeared, neurological symptoms appeared consecutively. Accompanying neurological symptoms improved after antiviral and steroid therapy. The timing of recovery of the neurological symptoms and the time of negative zoster in the blood test PCR results are consistent to some extent.
This patient underwent a thoracic epidural block treatment before paraplegia occurred. There may be a suspicion of nerve damage due to direct trauma to the spinal cord from the epidural block needle or hematoma by bleeding in the epidural area, causing pressure on the spinal cord. In the event of paralysis due to mass effects caused by hematoma or drug, immediate decompression is required and permanent nerve damage may remain[10,16]. However, based on the imaging findings, it may be considered that the possibility is not high because cord damage and hematoma were not observed in the MRI image, and the symptom progression after the treatment continued to improve but could not be completely excluded.
In this case, paraplegia occurred in zoster patients, which was thought to be caused by a mass effect due to hematoma secondary to an epidural block but was diagnosed with TM, which does not indicate emergency surgery, after the MRI finding. Many patients with zoster proceeded with epidural blocks due to pain. Still, it is essential to explain the possibility of neurologic symptoms by transverse myelitis and hematoma by nerve compression. The fluoroscopy-guided method is better than the Loss of resistance (LOR)-a blind technique in reducing complications. Supposed neurological symptoms, such as muscle weakness can occur in zoster patients. In that case, additional tests, such as CSF and MRI imaging, are required to consider paraneoplastic and neurologic complications, such as TM. Active antiviral treatment may help the patient's prognosis[17].
As side effects caused by the COVID-19 vaccine are gradually reported, case reports are also increasing. Unlike ordinary cases, HZ by COVID-19 vaccine may have occurred in healthy men (46 and 42 years old) without any comorbid diseases[3]. Vaccination of both mRNA and adenovirus protein methods will cause complications, such as HZ and TM.
In this case, a diagnostic method is essential for differential diagnoses, such as early detection, multiple sclerosis, and neurosarcoidosis. However, it would have been challenging to suspect myelitis caused by the COVID-19 vaccine because it occurred close to the vaccination date. Therefore, hematoma caused by spinal cord damage was constantly suspected. If TM was suspected, there was also a report that symptoms improved 5 d after plasma exchange treatment[4]. The muscle weakness in this patient's lower extremities has also progressed. The newly developed neurologic symptoms confused with the diagnosis because of the history of leg weakness caused by an accident in childhood. The pain persisted due to HZ at the lesion site, making the differential diagnosis more difficult, especially in rare myelitis caused by the COVID-19 vaccine.
This case shows the association between the COVID-19 vaccination and the occurrence of HZ, as well as the association with TM occurrence, a secondary complication of HZ, and a complication of the COVID-19 vaccine. It rapidly progressed during the confusion with the diagnosis and delayed the appropriate treatment. The critical point is to implement appropriate treatment without delay while checking improvements in the neurological examination and HZ wound care with pain control to protect from postherpetic neuralgia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Guo XW, China; Liu D, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Kleinschmidt-DeMasters BK, Gilden DH. The expanding spectrum of herpesvirus infections of the nervous system. Brain Pathol. 2001;11:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Amlie-Lefond C, Jubelt B. Neurologic manifestations of varicella zoster virus infections. Curr Neurol Neurosci Rep. 2009;9:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Chiu HH, Wei KC, Chen A, Wang WH. Herpes zoster following COVID-19 vaccine: a report of three cases. QJM. 2021;114:531-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Notghi AA, Atley J, Silva M. Lessons of the month 1: Longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin Med (Lond). 2021;21:e535-e538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Gilden D, Nagel MA, Cohrs RJ, Mahalingam R. The variegate neurological manifestations of varicella zoster virus infection. Curr Neurol Neurosci Rep. 2013;13:374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Baldwin KJ, Cummings CL. Herpesvirus Infections of the Nervous System. Continuum (Minneap Minn). 2018;24:1349-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Cicia A, Nociti V, Bianco A, De Fino C, Carlomagno V, Mirabella M, Lucchini M. Neurosarcoidosis presenting as longitudinally extensive myelitis: Diagnostic assessment, differential diagnosis, and therapeutic approach. Transl Neurosci. 2022;13:191-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Singer H, Kossoff EH, Hartman AL, Crawford TO. Treatment of pediatric neurologic disorders: CRC Press; 2005. |
| 9. | Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002;59:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 580] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 10. | Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008;28:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 535] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 12. | Kerr D. The history of TM: The origins of the name and the identification of the disease. 2010. |
| 13. | Hung CH, Chang KH, Kuo HC, Huang CC, Liao MF, Tsai YT, Ro LS. Features of varicella zoster virus myelitis and dependence on immune status. J Neurol Sci. 2012;318:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Lee CC, Wu JC, Huang WC, Shih YH, Cheng H. Herpes zoster cervical myelitis in a young adult. J Chin Med Assoc. 2010;73:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Lee JE, Lee S, Kim KH, Jang HR, Park YJ, Kang JS, Han SY, Lee SH. A Case of Transverse Myelitis Caused by Varicella Zoster Virus in an Immunocompetent Older Patient. Infect Chemother. 2016;48:334-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Li SL, Wang DX, Ma D. Epidural hematoma after neuraxial blockade: a retrospective report from China. Anesth Analg. 2010;111:1322-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Zhou J, Li J, Ma L, Cao S. Zoster sine herpete: a review. Korean J Pain. 2020;33:208-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |