Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1410
Peer-review started: November 11, 2022
First decision: January 12, 2023
Revised: January 14, 2023
Accepted: February 8, 2023
Article in press: February 8, 2023
Published online: February 26, 2023
Processing time: 101 Days and 4.8 Hours
Acute acalculous cholecystitis (AAC) is inflammation of the gallbladder without evidence of calculi. Although rarely reported, its etiologies include hepatitis virus infection (e.g., hepatitis A virus, HAV) and adult-onset Still’s disease (AOSD). There are no reports of HAV-associated AAC in an AOSD patient.
Here we report a rare case of HAV infection-associated AAC in a 39-year-old woman who had a history of AOSD. The patient presented with an acute abdomen and hypotension. Elevated hepatobiliary enzymes and a thickened and distended gallbladder without gallstones on ultrasonography suggested AAC, but there were no signs of anemia nor thrombocytopenia. Serological screening revealed anti-HAV IgM antibodies. Steroid treatment did not alleviate her symptoms, and she was referred for laparoscopic cholecystectomy. The resected gallbladder was hydropic without perforation, and her clinical signs gradually improved after surgery.
AAC can be caused by HAV in AOSD patients. It is crucial to search for the underlying etiology for AAC, especially uncommon viral causes.
Core Tip: Acute acalculous cholecystitis (AAC) can be caused by hepatitis A virus (HAV) infection or adult-onset Still’s disease (AOSD). Cholestasis is more likely to occur in HAV-associated AAC, whereas hematological complications are more common in AOSD-associated AAC. When AAC cannot be explained by AOSD, it is necessary to find other causes of AAC. An acute abdomen caused by HAV-related AAC requires careful consideration of the surgical necessity, since most cases are self-limiting and gallbladder perforation is rare.
- Citation: Chang CH, Wang YY, Jiao Y. Hepatitis A virus-associated acute acalculous cholecystitis in an adult-onset Still’s disease patient: A case report and review of the literature. World J Clin Cases 2023; 11(6): 1410-1418
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1410.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1410
Acute acalculous cholecystitis (AAC) accounts for 2%-15% of all acute cholecystitis cases[1]. In contrast to acute calculous cholecystitis (ACC), no gallstones can be identified in the gallbladder in AAC, and its pathogenesis is thought to be related to ischemia-reperfusion injury after surgery or trauma, cholestasis caused by long-term fasting or intestinal obstruction, bacterial infection, or abnormal biliary tract anatomy[2-4]. Although rarely reported, hepatitis A virus (HAV) infection or adult-onset Still’s disease (AOSD) can also cause AAC. HAV-associated AAC has mostly been reported in children and teenagers in developing countries, and it is often accompanied by fever, vomiting, transient liver dysfunction, and cholestasis[5,6]. On the other hand, AOSD is a chronic, systematic inflammatory disease characterized by recurrent fever, arthralgia, rash, and anemia or thrombocytopenia[7]. Patients with AOSD also may have high ferritin levels and sometimes concurrent AAC[8].
However, there are no reports of co-morbid AAC, HAV infection, and AOSD, which would represent a diagnostic and management challenge. Here we describe the case of a 39-year-old woman with AAC complicated by AOSD who was found to be anti-HAV IgM positive. We also searched the PubMed database with the keywords “[(hepatitis A virus) OR (adult-onset Still’s disease)] AND acalculous cholecystitis” and found 14 HAV-associated AAC cases and three AOSD-associated AAC cases. Our case and review allow us to identify diagnostic clues that might help favor a particular diagnosis and discuss the necessity for surgical intervention to treat the AAC under these circumstances.
A 39-year-old woman presented to our hospital with a one-week history of fever (39-40 °C) and headache, chest tightness, and a sharp right upper quadrant pain.
The woman had a five-month history of AOSD, for which she had been taking oral methylprednisolone (26 mg/d) as maintenance therapy. She had suddenly developed a cough and sore throat two weeks previously, for which she was prescribed amoxicillin and clavulanate potassium (3.75 g/d), which was ineffective. In the week preceding admission, she had a fever of 39 °C accompanied by malaise, cutaneous icterus, and loss of appetite. Her methylprednisolone dose was increased to 48 mg/d and moxifloxacin hydrochloride (0.4 g iv, once) was administered, but this failed to control the symptoms.
The patient had ovarian endometriosis ten years previously and underwent laparoscopic ovarian cystectomy. She also had a history of a skin rash after taking a cephalosporin antibiotic.
There was no personal nor family history of cholecystitis, nor was there a family history of AOSD or other auto-immune diseases.
On admission, she had cutaneous icterus and her temperature was 40 °C. Her blood pressure dropped to 83/45 mmHg and her heart rate increased to 100-110 bpm. Her liver was palpable under the costal margin, and she had abdominal distension and tenderness in the right upper quadrant and a positive Murphy’s sign.
Laboratory tests (Table 1) showed elevated lactate (1.9 mmol/L), white blood cell (WBC) count (17.34 × 109/L), and inflammatory markers [high-sensitivity C-reactive protein (hsCRP) 15.35 mg/L]. Moreover, total/direct bilirubin (TBil/DBil) (12.2/10.5 mg/dL) and hepatobiliary enzymes [aspartate aminotransferase (AST) 724 U/L, alanine aminotransferase (ALT) 223 U/L, gamma-glutamyl transferase (GGT) 735 U/L, alkaline phosphatase (ALP) 336 U/L] were elevated, and the prothrombin time (PT) was prolonged at 15.1 s. Her hemoglobin 14.7 g/dL and platelets 292 × 109/L were within normal limits. Further serological screening demonstrated anti-HAV IgM antibodies.
| Laboratory test | Laboratory value | Reference range |
| White blood cell count (WBC) | 17.34 | 3.5-9.5 (× 109/L) |
| Hemoglobin (Hb) | 14.70 | 11-15 (g/dL) |
| Platelets (PLT) | 292.00 | 125-350 (× 109/L) |
| High-sensitivity C-reactive protein (hsCRP) | 15.35 | < 8.2 (mg/L) |
| Erythrocyte sedimentation rate (ESR) | 7.00 | 0-20 (mm/h) |
| Aspartate aminotransferase (AST) | 724.00 | 10-40 (U/L) |
| Alanine aminotransferase (ALT) | 223.00 | 9-50 (U/L) |
| Gamma-glutamyl transferase (GGT) | 735.00 | 8-55 (U/L) |
| Alkaline phosphatase (ALP) | 336.00 | 40-100 (U/L) |
| Total bilirubin (TBil) | 12.20 | 0.2-1.2 (mg/dL) |
| Direct bilirubin (DBil) | 10.50 | 0-0.3 (mg/dL) |
| Creatinine (Cr) | 58.00 | 45-84 (μmol/L) |
| Lactate | 1.90 | 0.5-1.0 (mmol/L) |
| Prothrombin time (PT) | 15.10 | 11-13 (s) |
| Activated partial thromboplastin time (APTT) | 40.20 | 20-25 (s) |
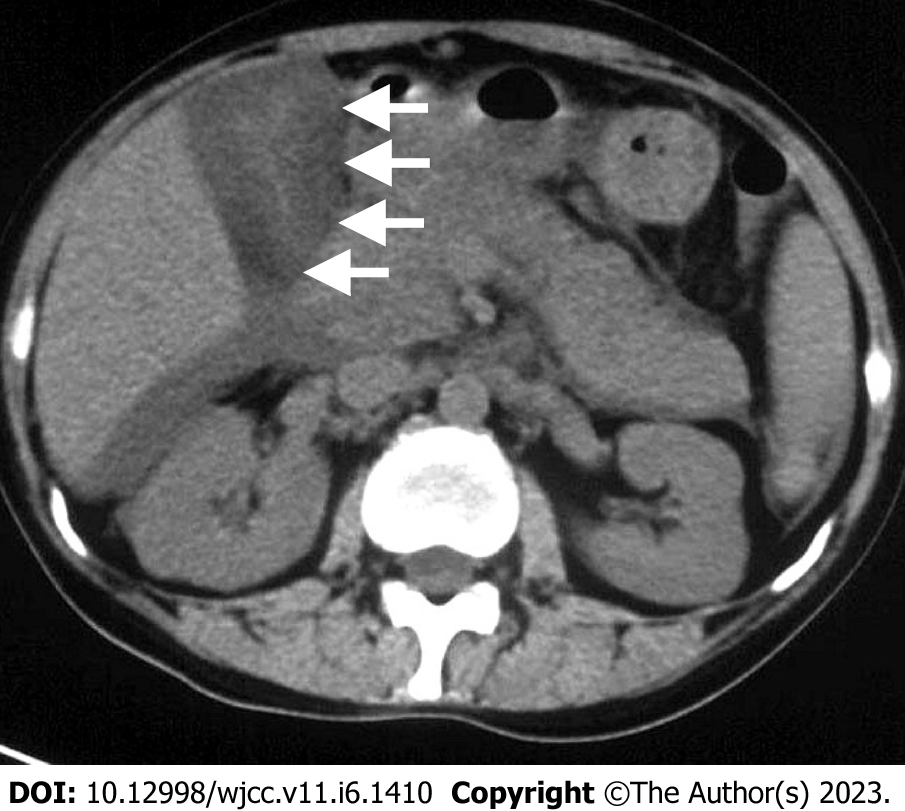
Ultrasonography revealed a dilated gallbladder (9.1 cm × 4.1 cm) with an evenly thickened wall (approximately 1.8 cm) and hepatosplenomegaly, but the intrahepatic bile duct was not dilated. Computed tomography (CT) suggested pericholecystic and hepatic fluid collection, a thickened gallbladder wall, a right pleural effusion, and ascites (Figure 1). No calculi were present.
The evidence suggested that the patient had HAV-associated AAC. The AAC could not be explained by the active state of AOSD, since steroid treatment did not alleviate the symptoms.
Hypovolemia and septic shock were considered, and she was given supportive intravenous fluid treatment and norepinephrine as a vasoactive agent (0.2 µg/kg/min). However, the acute abdominal pain, chest tightness, and acute abdomen signs such as positive Murphy’s sign and epigastric guarding continued. Gallbladder perforation was suspected, and she was referred for emergent laparoscopic cholecystectomy. During the surgery, the gallbladder was found to be hydropic without perforation with no evidence of calculi. Prednisone was maintained at the same dose (48 mg/d) after cholecy
Her clinical signs gradually improved after surgery. Microscopic examination of the gallbladder revealed normal epithelial architecture with mild lymphocyte infiltration. There was no perforation nor necrosis. During follow-up, her liver function returned to normal and the cutaneous icterus resolved.
Here we present a case of AAC complicated by HAV infection and AOSD. We searched the PubMed database for published articles on the topic using the search terms “acalculous cholecystitis”, “hepatitis A virus”, and “adult-onset Still’s disease”. Fourteen patients have been reported in the literature with AAC due to acute HAV infection and three due to AOSD, and we compared these with our case.
Previous studies reporting HAV-associated AAC are summarized in Table 2. These patients commonly presented with fever (11/14), fatigue (7/14), nausea (5/14), vomiting (9/14), and abdominal pain (12/14). On physical examination, icterus (12/14), right upper abdominal tenderness (12/14), and an enlarged liver or spleen (6/12) were common. Ultrasonography and CT revealed thickened gallbladders accompanied by pericholecystic fluid and hepatosplenomegaly. Laboratory tests showed that all patients had elevated TBil/DBil, ALT, and AST. Anemia (2/8) and thrombocytopenia (3/10) occurred in several cases. However, despite advanced imaging and laboratory techniques, the diagnosis of complicated HAV-associated AAC as a cause of an acute abdomen seems to be challenging. Ciftci et al[10] presented a case of HAV-associated AAC in a child whose initial diagnosis was an acute abdomen due to blunt abdominal trauma. After physical examination, laboratory testing, and CT scanning, the patient was suspected to have gangrenous cholecystitis, but the exploratory laparotomy revealed no gallbladder necrosis nor perforation.
| Ref. | Age/sex | Symptoms | Physical examination | Vital signs | Ultrasound/CT findings | Bilirubin (total/direct, mg/dL) (0.2-1.2/0-0.3) | AST/ALT (U/I) (10-40/9-50) | WBC (109/L) (3.5-9.5) | Hb (g/dL) (11-15) | Platelets (109/L) (125-350) |
| Mourani et al[9], 1994 | 68/M | Fever, chills, nausea, vomiting | Icterus, diaphoretic, hypotensive | / | Thickened gallbladder wall, acalculous | 4.8/ | 5629/8670 | / | / | / |
| Ciftci et al[10], 2001 | 7/M | Fever, fatigue, abdominal pain, mild respiratory distress | Icterus, abdominal distention, right upper quadrant tenderness | T 37.8 °C, HR 100 bpm, BP 100/70 mmHg | Subhepatic fluid, thickened gallbladder wall, acalculous | 7.6/4.8 | 221/1288 | 8.8 | 13.9 | / |
| Ozaras et al[11], 2003 | 28/M | Fatigue, abdominal pain, dark urine, anorexia, pale stool | Icterus, palpable liver with tenderness, murphy’s sign (+) | / | Perihepatic fluid, thickened gallbladder wall, pericholecystic | 8.4/3.9 | 370/1386 | 3.7 | / | 199 |
| 20/F | Nausea, vomiting, fatigue, pruritus, and anorexia | Icterus, right abdominal tenderness, enlarged liver and spleen | / | Hepatosplenomegaly, hydropic gallbladder, acalculous | 6.58/2.90 | 400/815 | 5.9 | / | 287 | |
| Basar et al[12], 2005 | 19/F | Fever, right upper abdominal pain | Icterus, right upper quadrant tenderness Murphy’s sign (+) | / | Hepatomegaly, thickened gallbladder wall, acalculous, pericholecystic, intraabdominal fluid | 11.6/5.7 | 984/1213 | 4.1 | / | 215 |
| Bouyahia et al[13], 2008 | 14/M | Fever, vomiting, abdominal pain, myalgia | Right hypochondrium tenderness, enlarged liver and spleen | / | Thickened gallbladder wall, acalculous, pericholecystic fluid collection | 4.97/3.33 | 1327/1112 | 5.2 | 13 | 130 |
| Arroud et al[14], 2009 | 11/M | Fever, fatigue, vomiting, abdominal pain. myalgia, dark urine, pale stool | Icterus, enlarged liver and spleen | T 38.8 °C | Thickened gallbladder wall, acalculous, pericholecystic fluid collection | 4.8/2.7 | 2953/1918 | 6.3 | 11.4 | / |
| Suresh et al[15], 2009 | 2.5/F | Fever, fatigue nausea, vomiting, abdominal pain, loss of appetite, dark urine, pale stool | Icterus, tenderness in right side abdomen, Murphy’s sign (+), enlarged liver and spleen | T 38.5 °C, HR 86 bpm, RR 22, BP 100/70 mmHg | Hepatosplenomegaly, thickened gallbladder wall, acalculous, pericholecystic fluid | 2.8/0.9 | 20.6/23.4 | 6.1 | 13.6 | 186 |
| Souza et al[6], 2009 | 16/M | Fever, fatigue, nausea, vomiting, abdominal pain, cephalalgia | Diffuse abdominal pain to superficial and deep palpation | / | Hepatomegaly, thickened gallbladder wall, acalculous | 5.01/3.69 | 1265/1046 | / | 14.2 | 112 |
| Al-Amir et al[16], 2015 | 13/F | Fever, fatigue, vomiting, abdominal pain, dark urine, pale stool | Icterus, epigastric and right upper quadrant tenderness, Murphy’s sign (+) | T 38.8 °C, other vital signs were normal | Thickened gallbladder wall, acalculous, pericholecystic fluid collection | 16.1/12.3 | 3242/4298 | 4.5 | 15.4 | / |
| Herek et al[5], 2011 | 9/M | Fever, nausea, vomiting, abdominal pain | Icterus, tenderness in the right upper quadrant, enlarged liver | T 37.9 °C, HR 84 bpm, BP 100/55 mmHg | Thickened gallbladder wall, acalculous, pericholecystic-free fluid | 4.3/3.0 | 2261/2586 | 8.1 | / | 254 |
| Prashanth et al[17], 2012 | 12/F | Abdominal pain and vomiting | Icterus, tenderness in the right hypochondrium | T 36.9 °C, HR 102 bpm, RR 18, BP 110/80 mmHg | Thickened gallbladder wall, acalculous | 3.5/1.05 | 2150/2580 | 9 | 10 | 180 |
| Kaya et al[18], 2013 | 31/F | Fever, nausea, abdominal pain, loss of appetite, back and joint pain, darkening of urine | Icterus, tenderness in the right side of the abdomen, Murphy’s sign (+), enlarged liver | 37.5 °C, HR 92 bpm, BP 110/60 mmHg | Hepatosplenomegaly, thickened gallbladder wall, acalculous, ascites | 2.11/1.92 | 559/618 | 3.3 | 9.5 | 139 |
| Aldaghi et al[19], 2015 | 5/M | Fever, abdominal pain | Icterus, mass in the right upper quadrant with tenderness | 38 °C, HR 100 bpm, RR 30, BP 100/60 mmHg | Distended gallbladder, normal thickness, acalculous | 5.3/3.9 | 516/722 | 8 | / | 426 |
With respect to treatment and prognosis, most patients received conservative treatment (12/14) and only two patients underwent surgery. All patients had good outcomes. Most HAV-associated AAC cases were self-limiting, and the thickened, hydropic gallbladder decompressed within two weeks following conservative treatment. These findings were consistent with those of Kaya et al[18].
Cases of AAC in patients with AOSD (three cases) are summarized in Table 3. AOSD-associated AAC patients, all female, had recurrent fever, rash, and arthritis. On physical examination, two presented with a palpable liver and spleen, which was further confirmed by CT. All cases showed gallbladder enlargement or wall thickening but no calculi by ultrasonography or CT. Laboratory findings showed liver dysfunction (elevated TBil and hepatobiliary enzymes), anemia, and thrombocytopenia. Moreover, hyperferritinemia was presented in these patients, which might reflect the inflammatory state in autoimmune disease.
| Ref. | Age/Sex | Symptoms | Physical examination | Vital signs | Ultrasound/CT findings | Bilirubin (total/direct, mg/dL) (0.2-1.2/0-0.3) | AST/ALT (U/I) (10-40/9-50) | WBC (109/L) (3.5-9.5) | Hb (g/dL) (11-15) | Platelets (109/L) (125-350) |
| Park et al[20], 2004 | 49/F | Recurrent fever, rash, and polyarthritis. Confused mental status on admission | Icterus, Murphy’s sign (+), enlarged liver and spleen, facial rash, dehydrated tongue, equivocal neck stiffness, purpuras over the limbs, scabs of zoster | T 39 °C, BP 90/60 mmHg, HR 100 bpm, RR 30 | Hepatosplenomegaly, thickened gallbladder wall, pericholecystic fluid collection, ileocolitis, right pleural effusion and ascites | 3.7/2.8 | 453/154 | 7.1 | 9.5 | 17 |
| Vallianou et al[21], 2014 | 28/F | Fever, vomiting, confused mental status, abdominal pain | Severe and diffuse abdominal tenderness | T 40 °C, no peripheral pulse, systolic BP 50 mmHg | Gallbladder enlargement with edema | / | / | / | / | / |
| Arai et al[8], 2021 | 21/F | Recurrent fever, rash, polyarthritis, nausea, vomiting, right hypochondriac pain | Rash, enlarged liver and spleen, tenderness in both shoulder and knee joints | T 40 °C | Hepatosplenomegaly, gallbladder enlargement and wall thickening, acalculous, cervical lymphadenopathy | 2.15/1.56 | 763/469 | 2.3 | 11 | 79 |
Arai et al[8] found several shared characteristics in AOSD patients. Two cases were complicated by macrophage activation syndrome (MAS) based on the findings of splenomegaly, cytopenia, and pathological changes in myeloid cells revealed by bone marrow biopsy. In addition, two cases were complicated by disseminated intravascular coagulation (DIC). The hypercytokinemia caused by MAS and widespread hypercoagulable state might aggravate multi-organ failure and severe illness. Furthermore, they showed that by inhibiting cytokine production and immune activation with glucocorticoids and cyclosporin A, both AAC and AOSD-related MAS or DIC could be resolved, suggesting that MAS and DIC might be secondary to their primary AOSD and that prompt and correct management of the primary disease can also slow or halt the progression of MAS and DIC.
It is worth considering whether surgery is needed in patients with AOSD-associated AAC. From the three previously reported cases, two patients received conservative treatment and one had cholecystectomy. The patient who underwent surgery[21] experienced hypovolemic shock, including no peripheral pulse and a systolic blood pressure of 50 mmHg. However, the surgery did not fully ameliorate the disease, since the patient experienced a rise in temperature after surgery and was later successfully treated with prednisone and naproxen. This leads us to consider whether surgical intervention is necessary in these cases. However, Arai et al[8] claimed that surgery should remain a treatment option for AOSD-associated AAC due to the possibility of gallbladder perforation as a complication. All three patients survived and had good outcomes. Overall, given the rarity of the condition, further reporting of individual cases would be helpful for guiding evidence-based treatment of AOSD-associated AAC.
AOSD-associated AAC and HAV-associated AAC share several common characteristics. They both present with acute abdomen symptoms, elevated bilirubin and hepatobiliary enzymes, and imaging findings of hepatosplenomegaly and a thickened gallbladder without gallbladder calculi. However, cholestasis is often more severe in HAV-associated AAC, resulting in higher bilirubin levels and cutaneous icterus[22]. Meanwhile, hematological abnormalities are more obvious in AOSD-associated AAC: Anemia and thrombocytopenia were more frequent in AOSD-associated AAC, as was MAS or DIC[20]. In this patient, a differential diagnosis of MAS was considered. However, anemia, thrombocytopenia, and DIC were not present. Steroid treatment did not alleviate the patient’s symptom, disfavoring AOSD as the cause of AAC in this case. Thus, based on serology and other laboratory findings, our final diagnosis for the patient was HAV-associated AAC.
Viral infections other than HAV may also lead to AAC. Hepatitis B virus[23,24] and hepatitis C virus[25,26] have both been reported as causes of AAC. Other viruses such as Epstein-Barr virus (EBV)[27,28], dengue virus[29,30], and human immunodeficiency virus[31,32] have also been implicated in AAC and have presented with an acute abdomen. More recently, coronavirus (COVID-19) has been reported in AAC cases[33], even leading to gangrenous cholecystitis[34]. Therefore, viral serology is an important diagnostic modality to search for a possible underlying etiology when a patient presents with AAC of unknown cause.
Due to the complexity of the case, our patient received intravenous fluid support, a vasoactive agent, steroid treatment, antibiotic management, and surgical intervention. Surprisingly, no perforation nor necrosis was found in the gallbladder after cholecystectomy. Due to the limitations of current imaging modalities and laboratory testing, it can be difficult to accurately determine the actual condition of the gallbladder prior to operation. However, as summarized previously, most HAV-associated AAC cases are self-limiting, and conservative management of AAC may be adequate[18]. Thus, cholecystectomy may be an option when faced with AAC but requires careful consideration and evaluation of the surgical necessity, not least given the positive outcomes of most patients with HAV-associated AAC with conservative therapy alone.
This study has several limitations. First, we only showed the association between hepatitis virus infection and AAC, and the cause-effect relationship between them is still debatable. Further validation of the cause of HAV-associated AAC requires evidence from animal experiments or cohort studies. Second, we did not examine whether the patient had hyperferritinemia, which is often present in active AOSD. However, our patient did not have anemia, thrombocytopenia, and did not develop DIC, and steroid treatment did not control the clinical course. These findings strongly disfavor active AOSD causing the AAC. This study is also limited by the availability of only three cases of AOSD-associated AAC, so we cannot be certain that these cases are representative. More cases of AOSD-associated AAC need to be described to verify our conclusions.
In conclusion, although AAC caused by HAV or AOSD is rare, it is possible that these conditions can overlap and complicate the diagnosis and management of AAC. When AAC cannot be explained by AOSD, it is important to search for other primary causes of AAC, and viral serology should form part of the diagnostic work-up. HAV-associated AAC is mostly self-limiting, and conservative therapy is usually adequate management for these patients unless gallbladder perforation is likely. Overall, however, the prognosis of AAC caused by HAV is very good, with conservative management the cornerstone of treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt; Zarębska-Michaluk D, Poland S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Ganpathi IS, Diddapur RK, Eugene H, Karim M. Acute acalculous cholecystitis: challenging the myths. HPB (Oxford). 2007;9:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Fu Y, Pang L, Dai W, Wu S, Kong J. Advances in the Study of Acute Acalculous Cholecystitis: A Comprehensive Review. Dig Dis. 2022;40:468-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Gallaher JR, Charles A. Acute Cholecystitis: A Review. JAMA. 2022;327:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 182] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 4. | Owen CC, Jain R. Acute Acalculous Cholecystitis. Curr Treat Options Gastroenterol. 2005;8:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Herek O, Cördük N, Herek D, Bagci S. Acute acalculous cholecystitis due to hepatitis A infection in a child: a rare cause of acute abdomen. Ann Afr Med. 2011;10:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Souza LJ, Braga LC, Rocha Nde S, Tavares RR. Acute acalculous cholecystitis in a teenager with hepatitis a virus infection: a case report. Braz J Infect Dis. 2009;13:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, Kashiwazaki S, Tanimoto K, Matsumoto Y, Ota T. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-430. [PubMed] |
| 8. | Arai Y, Ishikawa Y, Abe K, Kato Y, Abe D, Fujiwara M, Kita Y. A Recurrent Case of Adult-onset Still’s Disease with Concurrent Acalculous Cholecystitis and Macrophage Activation Syndrome/Hemophagocytic Lymphohistiocytosis Successfully Treated with Combination Immunosuppressive Therapy. Intern Med. 2021;60:1955-1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Mourani S, Dobbs SM, Genta RM, Tandon AK, Yoffe B. Hepatitis A virus-associated cholecystitis. Ann Intern Med. 1994;120:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Ciftci AO, Karnak I, Tanyel FC. The association of hepatitis A virus infection, acalculous cholecystitis, and blunt abdominal trauma: a diagnostic challenge. J Pediatr Gastroenterol Nutr. 2001;32:92-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Ozaras R, Mert A, Yilmaz MH, Celik AD, Tabak F, Bilir M, Ozturk R. Acute viral cholecystitis due to hepatitis A virus infection. J Clin Gastroenterol. 2003;37:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Başar O, Kisacik B, Bozdogan E, Yolcu OF, Ertugrul I, Köklü S. An unusual cause of acalculous cholecystitis during pregnancy: hepatitis A virus. Dig Dis Sci. 2005;50:1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Bouyahia O, Khelifi I, Bouafif F, Mazigh Mrad S, Gharsallah L, Boukthir S, Sammoud El Gharbi A. Hepatitis A: a rare cause of acalculous cholecystitis in children. Med Mal Infect. 2008;38:34-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Arroud M, Benmiloud S, Oudghiri B, Afifi MA, Hida M, Bouabdallah Y. Acute acalculous cholecystitis revealing hepatitis A virus infection in children. Saudi J Gastroenterol. 2009;15:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Suresh DR, Srikrishna R, Nanda SK, Annam V, Sunil K, Arjun B. Acalculous gallbladder distension in a young child due to HAV infection: Diagnostic dilemma. Indian J Clin Biochem. 2009;24:316-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Al-Amir S, Ghandourah H, Althobaiti K, Hasosah M. Acute hepatitis a virus (HAV) infection associated with acalculous cholecystitis. J Pediat Inf Dis. 2015;6:079-081. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Prashanth GP, Angadi BH, Joshi SN, Bagalkot PS, Maralihalli MB. Unusual cause of abdominal pain in pediatric emergency medicine. Pediatr Emerg Care. 2012;28:560-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kaya S, Eskazan AE, Ay N, Baysal B, Bahadir MV, Onur A, Duymus R. Acute Acalculous Cholecystitis due to Viral Hepatitis A. Case Rep Infect Dis. 2013;2013:407182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Aldaghi M, Haghighat M, Dehghani SM. Gallbladder hydrops due to viral hepatitis a infection: a case report. Jundishapur J Microbiol. 2015;8:e15779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Park JH, Bae JH, Choi YS, Lee HS, Jun JB, Jung S, Yoo DH, Bae SC, Kim TH. Adult-onset Still’s disease with disseminated intravascular coagulation and multiple organ dysfunctions dramatically treated with cyclosporine A. J Korean Med Sci. 2004;19:137-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Vallianou NG, Kouvidou C, Naxaki A, Aristodimou A. Acalculous cholecystitis with multiple organ failure and disseminated intravascular coagulation in a patient with adult onset Still’s disease. Ann Gastroenterol. 2014;27:289-290. [PubMed] |
| 22. | Cortellazzo Wiel L, Spezzacatene A, Gortani G, Saccari A, Taddio A, Barbi E. Acute Acalculous Cholecystitis: Think of Hepatitis A Infection and Do Not Underestimate Pain. Pediatr Emerg Care. 2022;38:304-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Unal H, Korkmaz M, Kirbas I, Selcuk H, Yilmaz U. Acute acalculous cholecystitis associated with acute hepatitis B virus infection. Int J Infect Dis. 2009;13:e310-e312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Mohammed RA, Ghadban W, Mohammed O. Acute acalculous cholecystitis induced by acute hepatitis B virus infection. Case Reports Hepatol. 2012;2012:132345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Wright WF, Palisoc K, Pinto CN, Lease JA, Baghli S. Hepatitis C Virus-Associated Acalculous Cholecystitis and Review of the Literature. Clin Med Res. 2020;18:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Omar A, Osman M, Bonnet G, Ghamri N. Acute acalculous cholecystitis caused by Hepatitis C: A rare case report. Int J Surg Case Rep. 2016;19:78-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Avcu G. Acute Acalculous Cholecystitis due to EBV Infection Presenting as Acute Abdomen. J Coll Physicians Surg Pak. 2022;32:662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Boninsegna S, Storato S, Riccardi N, Soprana M, Oliboni E, Tamarozzi F, Bocus P, Martini M, Floreani A. Epstein-Barr Virus (EBV) acute acalculous cholecystitis in an immunocompromised adult patient: a case report and a literature review of a neglected clinical presentation. J Prev Med Hyg. 2021;62:E237-E242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Sood A, Midha V, Sood N, Kaushal V. Acalculous cholecystitis as an atypical presentation of dengue fever. Am J Gastroenterol. 2000;95:3316-3317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Goh BK, Tan SG. Case of dengue virus infection presenting with acute acalculous cholecystitis. J Gastroenterol Hepatol. 2006;21:923-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Liu WD, Cheng CN, Lin YT, Kuo CH, Ho SY, Hung CC. Acute HIV infection with presentations mimicking acalculous cholecystitis: A case report. Medicine (Baltimore). 2021;100:e26653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Shinha T, Zabarsky G. Acalculous Cholecystitis Due to Histoplasma capsulatum in a Patient With HIV Infection. ACG Case Rep J. 2015;2:245-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Berdugo Hurtado F, Guirao Arrabal E, Barrientos Delgado A, Ruiz Rodríguez AJ. SARS-CoV-2 infection presenting as acute acalculous cholecystitis. Rev Esp Quimioter. 2022;35:87-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Hajebi R, Habibi P, Maroufi SF, Bahreini M, Miratashi Yazdi SA. COVID-19 patients presenting with gangrenous acalculous cholecystitis: Report of two cases. Ann Med Surg (Lond). 2022;76:103534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |