Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1403
Peer-review started: November 10, 2022
First decision: December 13, 2022
Revised: December 26, 2022
Accepted: February 7, 2023
Article in press: February 7, 2023
Published online: February 26, 2023
Processing time: 105 Days and 19.6 Hours
We report on a large family of Chinese Han individuals with hidrotic ectodermal dysplasia (HED) with a variation in GJB6 (c.31G>A). The patients in the family had a triad of clinical manifestations of varying degrees. Although the same variation locus have been reported, the clinical manifestations of this family were difficult to distinguish from those of congenital thick nail disorder, palmoplantar keratosis, and congenital hypotrichosis.
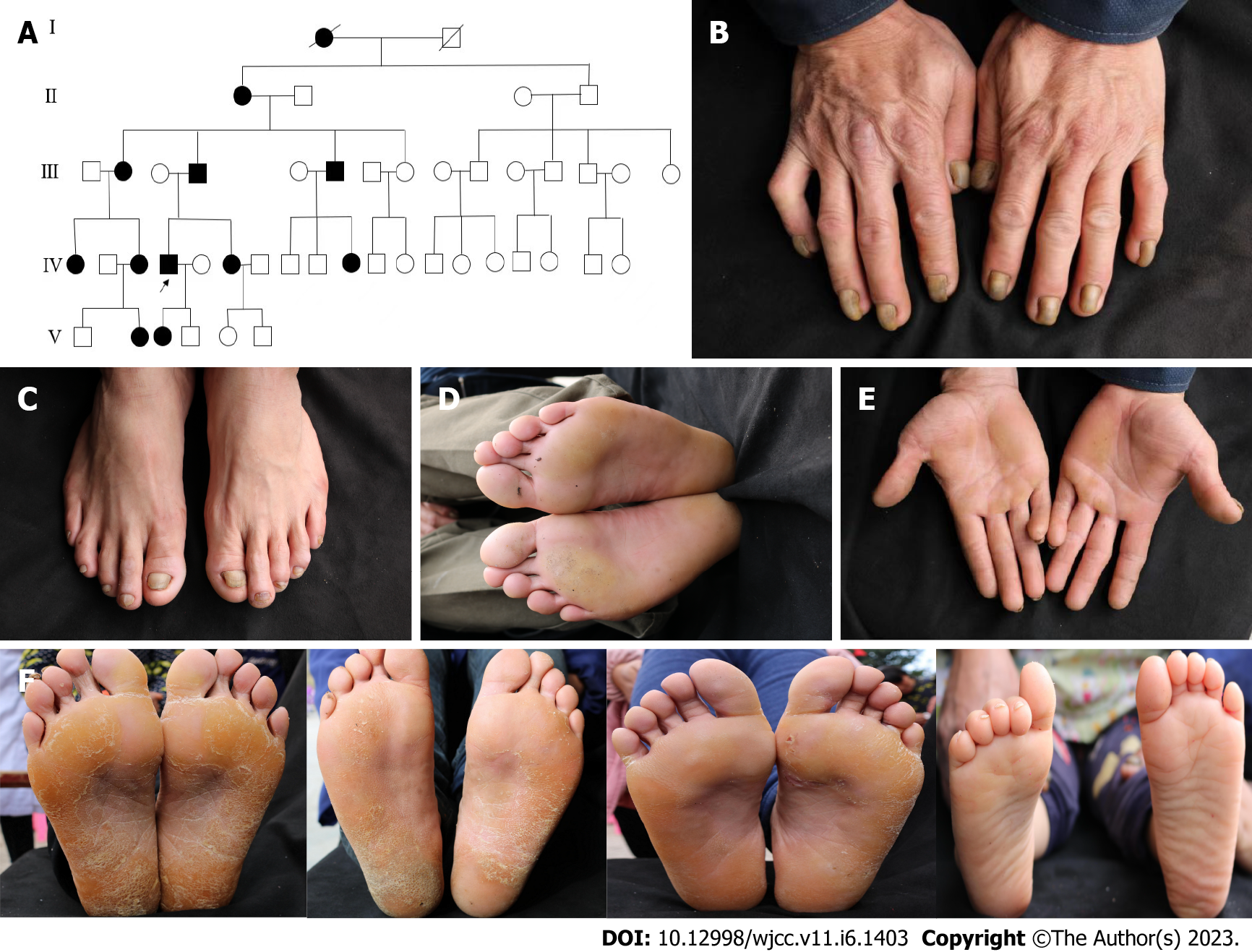
This investigation involved a large Chinese family of 46 members across five generations and included 12 patients with HED. The proband (IV4) was a male patient with normal sweat gland function and dental development, no skeletal dysplasia, no cognitive disability, and no hearing impairments. His parents were not consanguineously married. Physical examination of the proband revealed thinning hair and thickened grayish-yellow nails and toenails with some longitudinal ridges, in addition to mild bilateral palmoplantar hyperkeratosis. GJB6, GJB2, and GJA1 have been reported to be the causative genes of HED; therefore, we subjected the patient’s samples to Sanger sequencing of these three genes. In this family, the variation locus was at GJB6 (c.31G>A, p.Gly11Arg). Overexpression vectors of wild-type GJB6 and its variants were established and transfected into HaCaT cell models, and the related mRNA and protein expression changes were determined using real-time reverse transcriptase-polymerase chain reaction and Western blot, respectively.
We report another HED phenotype associated with GJB6 variations, which can help clinicians to diagnose HED despite its varying presentations.
Core Tip: We report on a Chinese family with hidrotic ectodermal dysplasia (HED), with patients in the family presenting varying degrees of hair dysplasia, nail dysplasia, and palmoplantar hyperkeratosis. In addition, we performed a literature review of other reported HED genotypes and their corresponding phenotypes, which lays a foundation for subsequent studies on these associations. Overexpression vectors of the GJB6 gene and its variants (variation sites: c.31G>A, c.263C>T, c.110T>A) were established and transfected into a HaCaT cell model. The expression changes of related mRNA and proteins before and after gene editing were obtained by real-time reverse transcriptase-polymerase chain reaction and Western blot, respectively, to provide clues for subsequent pathogenesis studies.
- Citation: Liao MY, Peng H, Li LN, Yang T, Xiong SY, Ye XY. Hidrotic ectodermal dysplasia in a Chinese pedigree: A case report. World J Clin Cases 2023; 11(6): 1403-1409
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1403.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1403
To date, four variations in GJB6 (G11R, A88V, V37E, and D50N), a GJA1 (V41L) variation combined with a GJB2 (R127H) variation, and an independent GJB2 (V27I) variation have been found to trigger hidrotic ectodermal dysplasia (HED)[1]. The typical clinical presentation of HED is a triad of symptoms: Hair development disorders, palmoplantar hyperkeratosis, and finger/toenail dysplasia[2]. The variant locus of the investigated case has been reported previously; however, the clinical presentation characteristics of the investigated family differed from those previously reported. Each patient in the family exhibited a triad of symptoms, with varying degrees of severity; when a patient is characterized by one of the clinical manifestations of hyperkeratosis of the palm and toes, sparse hair, or hypoplasia of the finger (toe) nails, it is difficult to distinguish the disease from congenital thick nail disease, palmoplantar keratosis, or congenital oligodactyly based on clinical symptoms.
A 32-year-old Chinese man presented with sparse hair, grayish-yellow thickened nails, and hyperkeratosis of the palmoplantar from birth.
The patient had normal sweat gland function and dental development, no cognitive disability, no hearing impairments, and no skeletal dysplasia. His parents were not consanguineously married.
In the five generations of the 46 members of the proband’s family, 12 HED patients (nine males and three females) were included (Figure 1A). The proband (IV4) and his affected family members had varying degrees of hair dysplasia, nail dysplasia, and palmoplantar hyperkeratosis from birth (Table 1).
| No. | Age | Sex | Nail lesion | Alopecia | Keratoderma |
| Ⅱ1 | 72 | F | +++ | ++ | +++ |
| Ⅲ2 | 52 | F | ++ | ++ | +++ |
| Ⅲ4 | 56 | M | +++ | +++ | +++ |
| Ⅲ6 | 48 | M | +++ | + | ++ |
| Ⅳ1 | 28 | F | ++ | + | ++ |
| Ⅳ3 | 23 | F | ++ | + | ++ |
| Ⅳ4 | 32 | F | +++ | + | ++ |
| Ⅳ6 | 29 | F | +++ | + | +++ |
| Ⅳ10 | 27 | F | ++ | +++ | ++ |
| Ⅴ2 | 4 | F | ++ | + | + |
| Ⅴ3 | 2 | F | + | +++ | + |
Physical examination revealed thinning hair, thickened grayish-yellow nails and toenails with some longitudinal ridges visible (Figure 1B and C), and mild bilateral palmoplantar hyperkeratosis (Figure 1D and E). The 11 surviving patients from the family had hair deficiency of varying severity and presentation, including sparse hair, slow growth, and/or easy breakage (Table 1). All 11 patients had thickened and brittle finger/toenails, with some patients having grayish-yellow finger/toenails and slow growth. All patients had varying degrees of palmoplantar keratinization, which decreased in severity in later generations (Figure 1F).
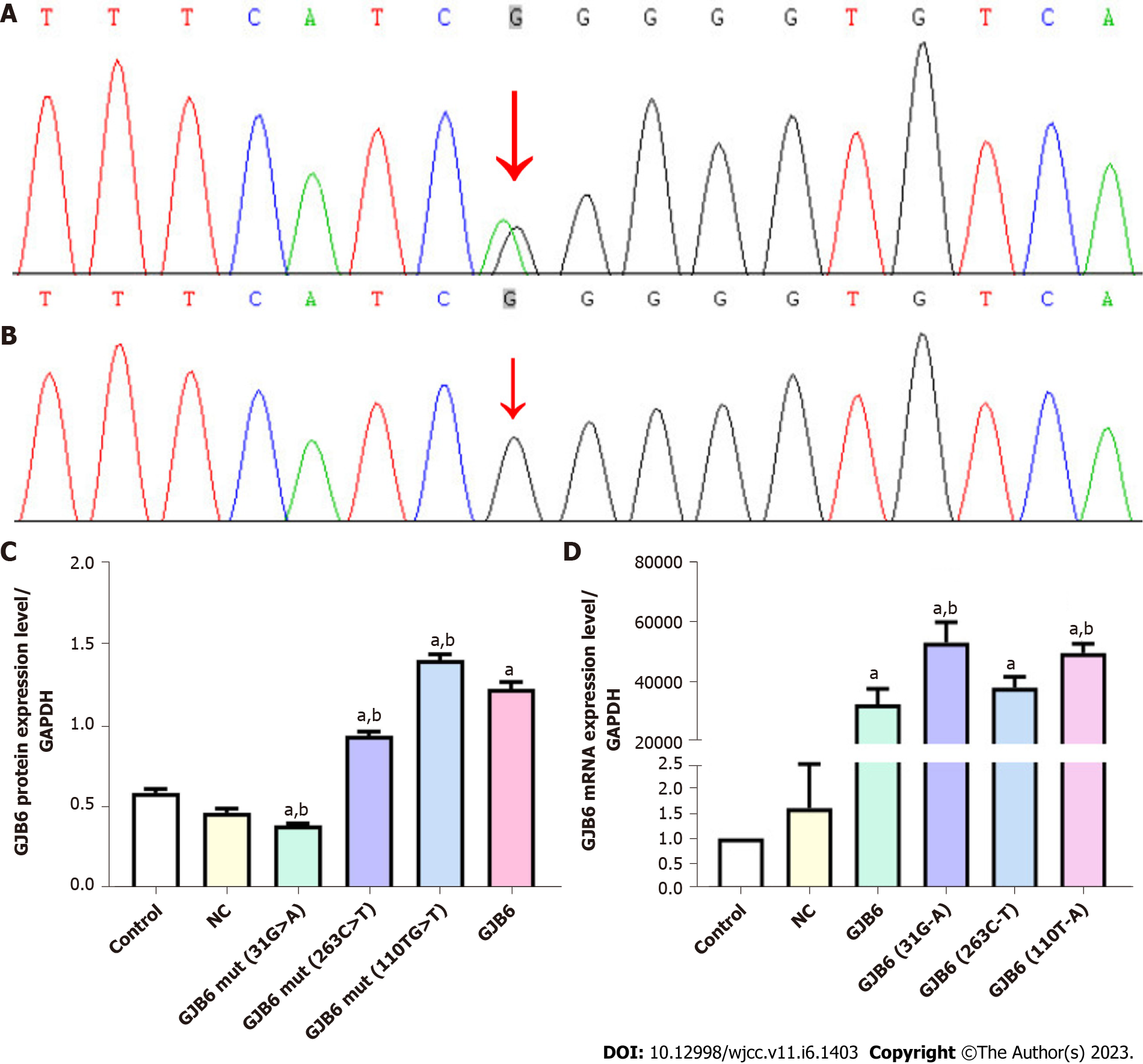
Target genes were extracted using a DNA Blood Mini Kit (CWBIO, Beijing, China) and amplified via polymerase chain reaction, and mutated genes were detected via Sanger sequencing. A known heterozygous variation (c.31G>A) in GJB6 was found in all 11 patients with HED (Figure 2A and B) but not detected in any of the nine healthy individuals of the family.
Based on the current findings combined with the patient’s medical history, the final diagnosis was HED.
Because there is no effective treatment for this disease, the pedigree of patients was not treated after diagnosis.
Overexpression vectors of GJB6 and its variants (c.31G>A, c.263C>T, and c.110T>A) were constructed. The empty vector was used as a normal control (NC) group, and cells grown in normal culture were used as a blank control group. Overexpression vectors of GJB6 and its variants were transfected into HaCaT cells. After 24 h of cell transfection, the expression levels of GJB6 mRNA and protein were detected via real-time reverse transcriptase-polymerase chain reaction and Western blot, respectively. The data were statistically analyzed using SPSS Statistics 19, GraphPad Prism 7, and Quantityone software. Significant differences between groups were analyzed via one-way ANOVA, and statistical significance was set at P < 0.05. The mRNA and protein expression levels of GJB6 and its variant loci (c.31G>A, c.263C>T, and c.110T>A) are shown in Figure 2C and D.
Six months later, 11 patients from this pedigree were still alive.
In the reported family, the variation locus was at c.31G>A in GJB6, and although this variation locus has been reported previously, the clinical presentations of the patients of this family differed from those of other cases (Table 2). Table 2 shows that different variant loci lead to different clinical phenotypes and that the same variant locus can correspond to different clinical phenotypes, even in the same family. No one-to-one correspondence could be formed between the genotype and clinical phenotype of patients with HED.
| Location (GRCh38) | NM accession number | Mutation locus | Ethnic group | Sex | Hair loss/sparse hair | Nail dysplasia | Palmoplantar hyperkeratosis | Supplementary | Ref. |
| chr13:20223450 | NM_001110219.3 | c.31G>A | American | 2M/4F | 4/6 | 6/6 | 1/6 | Precedent with eccrine syringofibroadenoma | Poonawalla et al[3], 2009 |
| c.31G>A | Chinese | 4M/4F | 8/8 | 8/8 | 0/8 | / | Chen et al[4], 2010 | ||
| c.31G>A | Polish | 4M/1F | 5/5 | 5/5 | 5/5 | Inherent immune deficiency combined with skeletal abnormalities | Pietrzak et al[5], 2016 | ||
| c.31G>A | Polish | 4M/3F | 7/7 | 7/7 | 7/7 | Some patients had hypotonia and delayed motor development | Kutkowska et al[6], 2015 | ||
| c.31G>A | Taiwan, Chinese | 10M/9F | 2/19 | 19/19 | / | Patients presented with rolled nails without nail thickening | Hu et al[7], 2015 | ||
| c.31G>A | Chinese | 3M/9F | 12/12 | 12/12 | 12/12 | / | Present case, 2022 | ||
| chr13:20223218 | NM_001110219.3 | c.263C>T | French | 2M/3F | 5/5 | 5/5 | 5/5 | / | Lamartine et al[8], 2000 |
| c.263C>T | Russians | 2M/2F | 4/4 | 4/4 | 4/4 | Precedent with progressive corneal dystrophy | Marakhonov et al[9], 2012 | ||
| c.263C>T | Chinese | 26M/19F | 44/45 | 42/45 | 33/45 | / | Yang et al[10], 2016 | ||
| c.263C>T | Chinese | 3M/2F | 5/5 | 5/5 | 4/5 | Two patients with GJB2 (c. 109G>A) mutations | Shi et al[1], 2019 | ||
| c.263C>T | Chinese | 16M/17F | 33/33 | 33/33 | 33/33 | Proband and her father had hearing impairment | Zhan et al[11], 2020 | ||
| chr13:20223371 | NM_001110219.3 | c.110T>A | Scottish | 1F | 1/1 | 1/1 | / | / | Smith et al[12], 2002 |
Connexin 30 (Cx30) is the protein product of GJB6 and is primarily utilized in the human cochlea and skin; therefore, deafness and skin problems may occur when there is a variation in GJB6. In this study, the conversion of guanine (G) at position 31 of GJB6 to adenine (A) in patients of the family was detected using Sanger sequencing. This nucleotide base change results in the replacement of a normal glycine (Gly) with arginine (Arg), leading to an altered Cx30 product. This change affects the conformational and structural flexibility of the N-terminus of Cx30, which regulates the selectivity and gating polarity of the linker protein[3]. This leads to an abnormal transport activity through the skin gap junctions, which causes the phenotypic characteristics of HED. In addition, we performed a literature review of other reported genotypes of HED and their corresponding phenotypes, which may help clinicians diagnose the disease despite its varied presentations (Table 2). However, further study is required to determine how the pathogenesis of HED is affected by aberrant mRNA and protein expression because of GJB6 variation.
There is no standard, effective treatment for HED, which can only be treated palliatively by wearing wigs, applying topical moisturizers, and following special nail care. Although HED is generally not life-threatening, it has serious physical and psychological effects on patients because of its effect on appearance. Sensorineural deafness, cataracts, oral mucosal leukoplakia, mental retardation, impaired immune system, skeletal malformations, and pestle finger have also been reported in HED patients. Therefore, prenatal genetic counseling and genetic testing remain effective methods to reduce the transmission of this hereditary disease.
We thank the patient and his family members for their ongoing participation in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bains L, India; Naz S, Pakistan S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Shi X, Li D, Chen M, Liu Y, Yan Q, Yu X, Zhu Y, Li Y. GJB6 mutation A88V for hidrotic ectodermal dysplasia in a Chinese family. Int J Dermatol. 2019;58:1462-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Cammarata-Scalisi F, Rinelli M, Pisaneschi E, Diociaiuti A, Willoughby CE, Avendaño A, Digilio MC, Novelli A, Callea M. Novel clinical features associated with Clouston syndrome. Int J Dermatol. 2019;58:e143-e146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Poonawalla T, Xia L, Patten S, Stratman EJ. Clouston syndrome and eccrine syringofibroadenomas. Am J Dermatopathol. 2009;31:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 4. | Chen N, Xu C, Han B, Wang ZY, Song YL, Li S, Zhang RL, Pan CM, Zhang L. G11R mutation in GJB6 gene causes hidrotic ectodermal dysplasia involving only hair and nails in a Chinese family. J Dermatol. 2010;37:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Pietrzak A, Grywalska E, Gerkowicz A, Krasowska D, Chodorowska G, Michalska-Jakubus M, Roliński J, Wawrzycki B, Radej S, Dybiec E, Wroński J, Sobczyńska-Tomaszewska A, Rudzki M, Hadj-Rabia S. Immune system disturbances in Clouston syndrome. Int J Dermatol. 2016;55:e241-e249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Kutkowska-Kaźmierczak A, Niepokój K, Wertheim-Tysarowska K, Giza A, Mordasewicz-Goliszewska M, Bal J, Obersztyn E. Phenotypic variability in gap junction syndromic skin disorders: experience from KID and Clouston syndromes' clinical diagnostics. J Appl Genet. 2015;56:329-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Hu YH, Lin YC, Hwu WL, Lee YM. Pincer nail deformity as the main manifestation of Clouston syndrome. Br J Dermatol. 2015;173:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaître G, Hand C, Hayflick SJ, Zonana J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der Kaloustian V, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G. Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet. 2000;26:142-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Marakhonov A, Skoblov M, Galkina V, Zinchenko R. Clouston syndrome: first case in Russia. Balkan J Med Genet. 2012;15:51-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yang R, Hu Z, Kong Q, Li W, Zhang L, Du X, Huang S, Xia X, Sang H. A known mutation in GJB6 in a large Chinese family with hidrotic ectodermal dysplasia. J Eur Acad Dermatol Venereol. 2016;30:1362-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Zhan Y, Luo S, Pi Z, Zhang G. A recurrent mutation of GJB6 in a big Chinese family with Hidrotic ectodermal dysplasia. Hereditas. 2020;157:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Smith FJ, Morley SM, McLean WH. A novel connexin 30 mutation in Clouston syndrome. J Invest Dermatol. 2002;118:530-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |