Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1379
Peer-review started: October 30, 2022
First decision: December 13, 2022
Revised: December 19, 2022
Accepted: February 7, 2023
Article in press: February 7, 2023
Published online: February 26, 2023
Processing time: 116 Days and 20.4 Hours
It is not uncommon to develop viral encephalitis. Epidemic Japanese B encephalitis infection combined with contactin-associated protein-like 2 (CASPR-2) antibody-positive autoimmune encephalitis has not been reported at present. In clinical work, we need to consider more options.
A 32-year-old male worker presented with headache, fever and call-unresponsive presentation. Complete cranial magnetic resonance image showed symmetrical abnormal signals in bilateral medial temporal lobe, bilateral thalamus and basal ganglia. Improved lumbar puncture showed that cerebrospinal fluid protein and cell count increased significantly. Viral encephalitis was considered, and the patient's consciousness still increased rapidly after antiviral treatment. Further detection of Cerebrospinal fluid Japanese B encephalitis virus Polymerase Chain Reaction positive, serum autoimmune encephalitis antibody showed CASPR-2 antibody positive (1:320), the patient's condition gradually improved after plasma exchange treatment. 3 mo later, the serum CASPR-2 antibody was negative and the patient's condition was stable.
This article reports the world’s first case of Epidemic Japanese B encephalitis infection combined with CASPR-2 antibody-positive autoimmune encephalitis, with a view to raising awareness.
Core Tip: For viral encephalitis in summer, there is no significant improvement after antiviral treatment, so the possibility of Japanese B encephalitis and autoimmune encephalitis should be considered.
- Citation: Huang P. Epidemic Japanese B encephalitis combined with contactin-associated protein-like 2 antibody-positive autoimmune encephalitis: A case report. World J Clin Cases 2023; 11(6): 1379-1384
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1379.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1379
Epidemic Japanese B encephalitis is a disease of the central nervous system caused by Japanese encephalitis virus. Due to the birth of a vaccine, this disease is relatively rare. Autoimmune encephalitis is a type of encephalitis caused by an immune response directed against central nervous system antigens mediated by autoimmune mechanisms. contactin-associated protein-like 2 (CASPR-2) antibody-positive autoimmune encephalitis is currently clinically rare and only a few cases have been reported. This article reports the world’s first case of Epidemic Japanese B encephalitis infection combined with CASPR-2 antibody-positive autoimmune encephalitis, with a view to raising awareness.
A male patient, 32 years old, a worker, was admitted to the hospital due to headache, fever for 7 d and unconsciousness for 1 d.
7 d before admission, the patient developed headache and fever after cold (the patient was poorly conscious at the time of admission and could not describe the nature and location of the headache), with a maximum body temperature of 42 degree Celsius, accompanied by chills, no nausea and vomiting, abdominal pain, diarrhea, incontinence, and limb The convulsions were treated at the local hospital but no significant improvement was seen. The patient's condition worsened 1 d before admission, and began to grab clothes and sheets, and gradually developed to be unresponsive. The body temperature continued to exceed 39 degree Celsius. Considering the possibility of encephalitis, in order to further confirm the diagnosis "Infection" income department.
In the past, he suffered from asthma and improved after hormone therapy.
The patient had no relevant personal or family history.
Body temperature 38.8 degree Celsius, pulse 84 times/min, breathing 22 times/min, blood pressure 137/50 mmHg. Sleepy, uncooperative physical examination, no autonomous speech, advanced intelligent activity examination cannot be performed. Double pupils and other large round shapes, about 3mm in diameter, light reflection is dull, conjunctival congestion and edema. No facial tongue paralysis, pharyngeal reflex exists. The muscle tension of the extremities increased, and there was avoidance during pain stimulation. The tendon reflexes of the extremities were normal (++), and the bilateral pathological signs were negative. Neck resistance was positive, 5 transverse fingers, and Glasgow Coma Scale was 8 points.
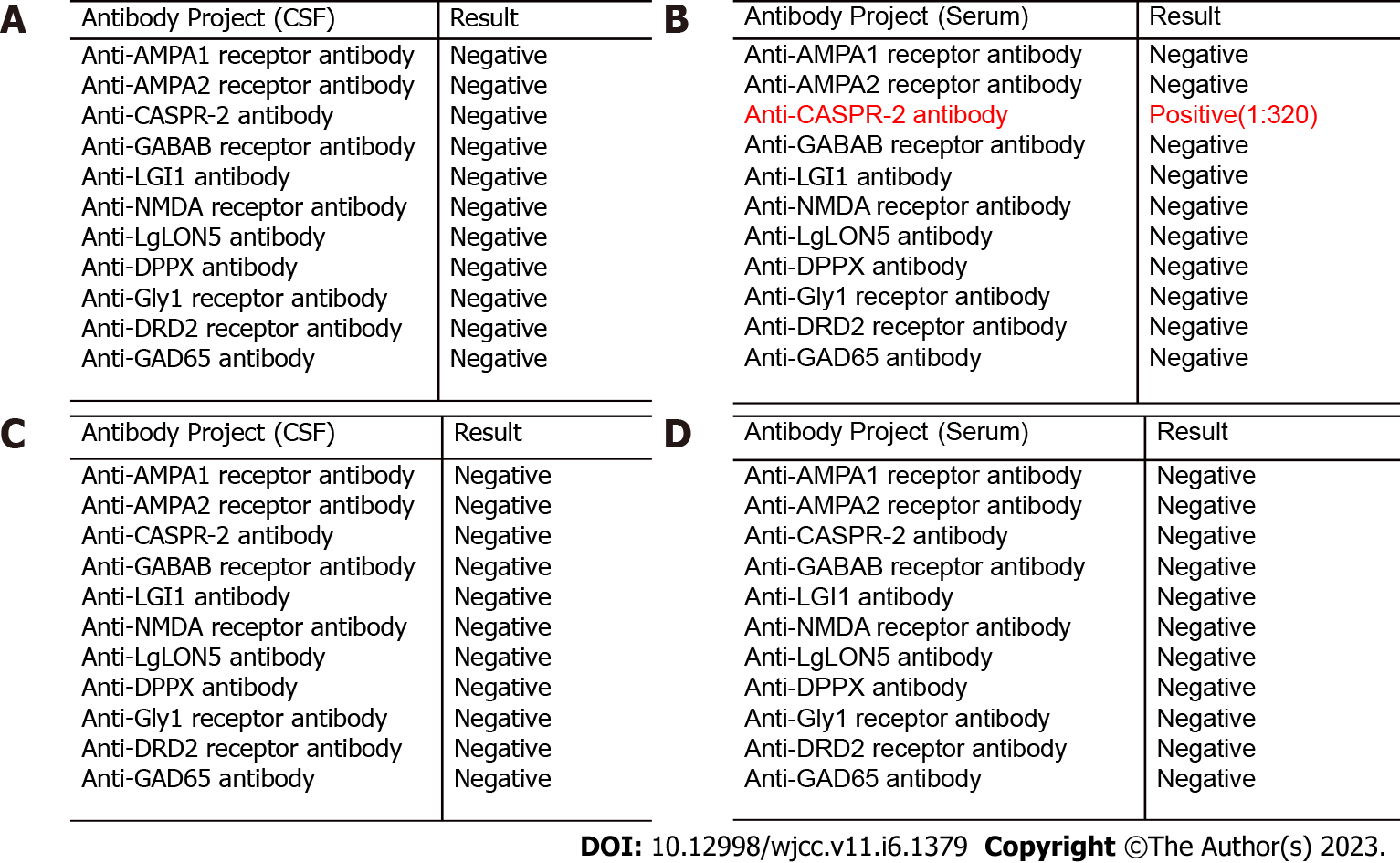
At admission, C reactive protein was 52.68 mg/L, and the white blood cells were normal. Admitted to consider viral encephalitis, given acyclovir antiviral, dehydration and intracranial pressure treatment, the next day after admission, oxygen saturation decreased, consciousness deepened to coma, weak spontaneous breathing, bilateral pupils 4mm, light reflection disappeared, limb The cyanosis is cold and immediately transferred to intensive care unit (ICU) for tracheal intubation. A complete lumbar puncture examination during ICU treatment revealed cerebrospinal fluid protein 0.87 g/L, white blood cell counts 0.071 x 109/L, glucose 4.31 mmol/L, chloride 117.9 mmol/L, negative test for herpes simplex virus DNA and fungus, and deliver Cerebrospinal fluid and plasma autoimmune encephalitis antibody profile. During the period of waiting for the results of autoimmune encephalitis, after receiving communication with the patient, he was given methylprednisolone 1000 mg qd intravenous drip shock combined with gamma globulin 25 g qd treatment. On the 8th day of admission, the patient's level of consciousness was reduced to lethargy, spontaneous eye-opening, and body temperature gradually decreased too normal. On the same day, the patient's delivery of autoimmune encephalitis antibody showed positive plasma CASPR-2 antibody (1:320), The cerebrospinal fluid was negative for CASPR-2 antibody (Figure 1A and B). At this point, the patient was considered to have CASPR-2 antibody encephalitis, and then plasma exchange was performed (the plasma volume was calculated according to 40 m/kg). A total of 4 plasma exchanges were performed on the next 7 d. After the above treatment, the patient's condition gradually improved and the ventilator treatment was discontinued.
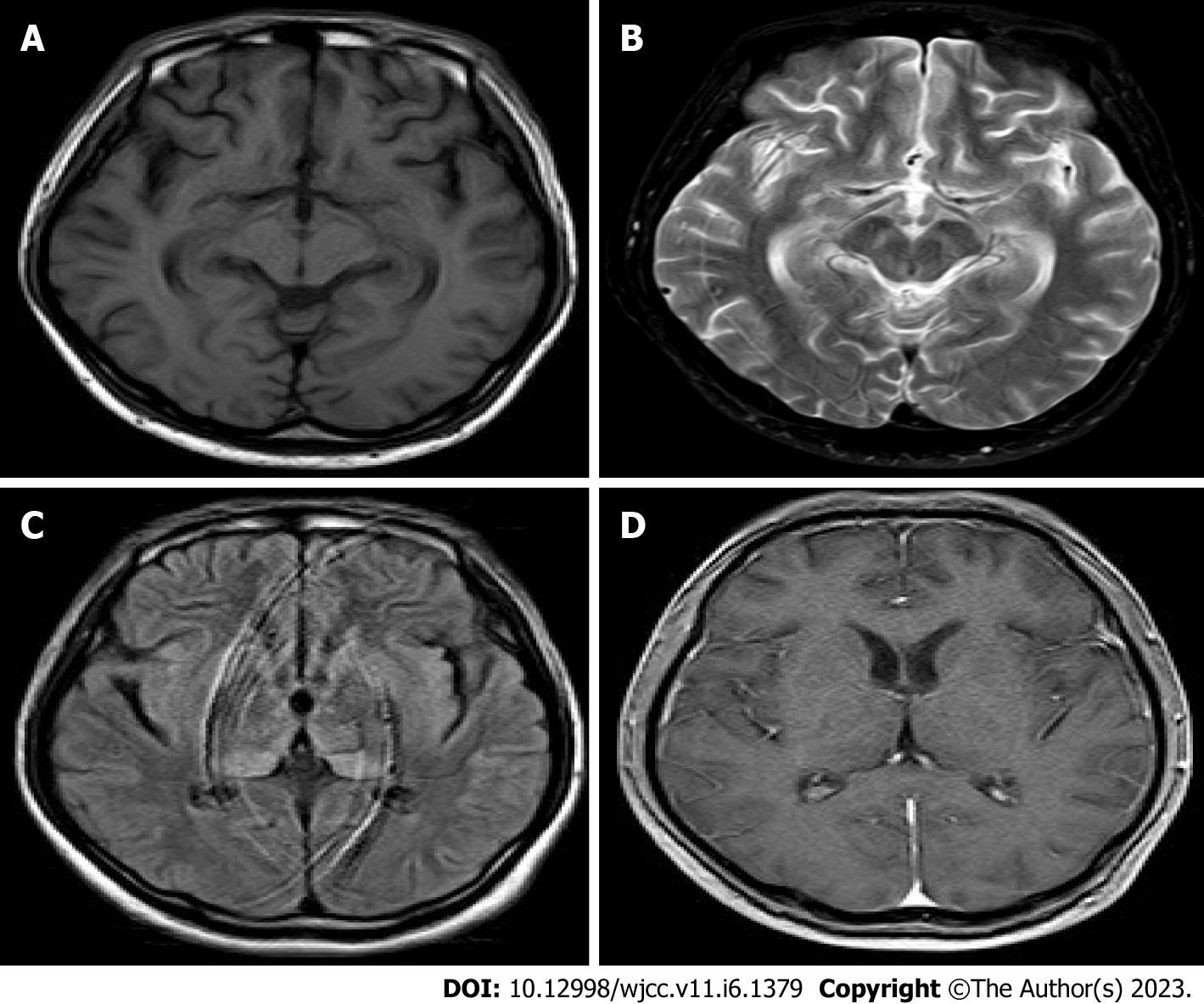
After the patient's condition was stable, a head magnetic resonance image (MRI) examination revealed mild brain atrophy and abnormal symmetry signals in the medial temporal lobe, bilateral thalamus, and basal ganglia (Figure 2A-D).
Epidemic Japanese B encephalitis combined with CASPR-2 antibody-positive autoimmune encephalitis.
Combined with the patient's head MRI image results and the onset time is summer, it is highly suspected to be Epidemic Japanese B encephalitis, Cerebrospinal fluid is sent to the disease control center, and after 5 d, the report is Japanese encephalitis. At this point, the diagnosis of Epidemic Japanese B encephalitis combined with CASPR-2 antibody-positive autoimmune encephalitis was clear. After the above comprehensive treatment, the patient's condition improved 42 d after admission and was discharged. The patient's consciousness and speech were clear when discharged, but the senior Knowing functional decline, limb strength is 5 grades, bilateral pathological signs are negative, and meningeal irritation signs are negative.
After discharge from the hospital, he was followed up in the outpatient clinic every month, and there were no fluctuations in his condition. After 3 mo, the serum and cerebrospinal fluid autoimmune encephalitis antibody CASPR-2 antibody turned negative (Figure 1C and D).
In recent years, autoimmune encephalitis has gradually attracted people's attention[1] . CASPR-2 is the main target antigen for the autoantibody of the voltage-gated potassium channel complex of neurons in and around the central nervous system The nervous system is expressed, so its clinical manifestations are central and peripheral nerve symptoms[2,3]. Peripheral nerve symptoms are due to neurological and muscle rigidity, muscle convulsions caused by overexcitation of peripheral nerves, such as Morvan syndrome and Isaacs syndrome[4,5], The central symptoms are often due to cognitive decline and seizures caused by the limbic system. The imaging findings of CASPR-2 antibody-positive autoimmune encephalitis are mainly manifested as abnormalities of the limbic system, accounting for about one-third of patients with positive findings on the head MRI. Compared with other types of encephalitis, the incidence of CASPR-2 antibody-positive autoimmune encephalitis is not high, about 3%, but the treatment effect is relatively significant[6,7]. The diagnostic criteria for CASPR-2 antibody-positive autoimmune encephalitis are as follows[8]: (1) Have anti-CASPR2 antibody positive; and (2) Rule out other possible causes such as: Morvan syndrome, primary central nervous system vasculitis, Rasmussen encephalitis, etc. In treatment, anti-CASPR2 antibody-positive autoimmunity is treated with high-dose corticosteroids (oral or intravenous), immunoglobulin, and/or plasma exchange as first-line treatment, while cyclophosphamide and rituximab are refractory cases's choice[9].
Epidemic Japanese B encephalitis is an acute zoonotic disease caused by Japanese encephalitis virus (JE virus). Epidemiology shows that there are currently 67900 new cases worldwide each year, of which about 50% occur in China, and the mortality rate is 25%-30%[10,11]. JE virus infection expands in peripheral tissues and enters the central nervous system, causing extensive central nervous system inflammation and destruction of the blood-brain barrier, thereby triggering central nervous system symptoms. Its typical clinical manifestations include recurrent seizures, paralysis and Knowledge barrier[12]. The imaging findings are mainly manifested as abnormal signals involving the thalamus and limbic system. The magnetic resonance T2-Flair and diffusion weighted imaging images are more likely to detect the lesion. In terms of treatment, vaccine immunoprevention is the main measure against Japanese encephalitis. Intravenous administration of methylprednisolone combined with human immunoglobulin can also improve the clinical symptoms and long-term recovery of some Japanese encephalitis patients. The diagnosis of Japanese B encephalitis in this case was based on the following: The patient's onset of illness was in the summer, the favourable season for Japanese B encephalitis, with the typical clinical presentation of high fever/impaired consciousness/convulsions and, most importantly, a polymerase chain reaction test of the patient's cerebrospinal fluid by the Centre for Disease Control that found positive for Japanese B encephalitis virus.
There have been reports of Herpes simplex encephalitis combined with autoimmune encephalitis, and Epidemic Japanese B encephalitis combined with autoimmune encephalitis has only been reported in a few cases, but Epidemic Japanese B encephalitis combined with CASPR-2 antibody-positive autoimmunity No related reports of encephalitis. From the diagnostic criteria, the patient not only met the diagnostic criteria for Epidemic Japanese B encephalitis, but also met the diagnostic criteria for CASPR-2 antibody-positive autoimmune encephalitis, However, the relationship between the two in this patient needs further study. By consulting the literature, we preliminarily speculated that this patient may be secondary to CASPR-2 antibody-positive autoimmune encephalitis after Japanese encephalitis infection: The first is a molecular simulation mechanism, that is, the JE virus protein that infects the central nervous system has the same or similar antigen structure cluster as CASPR-2[13]. The second is that after infection of the central nervous system JE virus leads to destruction of brain tissue, a large number of CASPR-2 antigen structure clusters are exposed, thereby inducing the body to produce autoanti
In summary, this article reports a case of Epidemic Japanese B encephalitis combined with CASPR-2 antibody-positive autoimmune encephalitis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kenzaka T, Japan S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Hoffman WE, Seals C, Miletich DJ, Albrecht RF. Plasma and myocardial catecholamine levels in young and aged rats during halothane anesthesia. Neurobiol Aging. 1985;6:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Bastiaansen AEM, van Sonderen A, Titulaer MJ. Autoimmune encephalitis with anti-leucine-rich glioma-inactivated 1 or anti-contactin-associated protein-like 2 antibodies (formerly called voltage-gated potassium channel-complex antibodies). Curr Opin Neurol. 2017;30:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133:2734-2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1088] [Cited by in RCA: 961] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 4. | Song J, Jing S, Quan C, Lu J, Qiao X, Qiao K, Xi J, Zhao C. Isaacs syndrome with CASPR2 antibody: A series of three cases. J Clin Neurosci. 2017;41:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Vale TC, Pedroso JL, Dutra LA, Azevedo L, Filho LH, Prado LB, Hoftberger R, Prado GF, Barsottini OG. Morvan syndrome as a paraneoplastic disorder of thymoma with anti-CASPR2 antibodies. Lancet. 2017;389:1367-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Brown MP, Hissaria P, Hsieh AH, Kneebone C, Vallat W. Autoimmune limbic encephalitis with anti-contactin-associated protein-like 2 antibody secondary to pembrolizumab therapy. J Neuroimmunol. 2017;305:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2680] [Article Influence: 297.8] [Reference Citation Analysis (0)] |
| 9. | Nosadini M, Mohammad SS, Ramanathan S, Brilot F, Dale RC. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. 2015;15:1391-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Wang Y, Li K, Mehmood K, Gui R, Li J. Epidemiology of Japanese Encephalitis in China (2004-2015). Travel Med Infect Dis. 2019;28:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Cheng Y, Tran Minh N, Tran Minh Q, Khandelwal S, Clapham HE. Estimates of Japanese Encephalitis mortality and morbidity: A systematic review and modeling analysis. PLoS Negl Trop Dis. 2022;16:e0010361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 13. | Titulaer MJ, Leypoldt F, Dalmau J. Antibodies to N-methyl-D-aspartate and other synaptic receptors in choreoathetosis and relapsing symptoms post-herpes virus encephalitis. Mov Disord. 2014;29:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Linnoila JJ, Binnicker MJ, Majed M, Klein CJ, McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |