Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1372
Peer-review started: October 29, 2022
First decision: January 12, 2023
Revised: January 17, 2023
Accepted: February 2, 2023
Article in press: February 2, 2023
Published online: February 26, 2023
Processing time: 117 Days and 18.6 Hours
Gemcitabine is an antimetabolite used in the treatment of pancreatic cancer. One of the side effects of gemcitabine is vascular toxicity. Here, we report the case of a patient treated with gemcitabine who had peripheral vascular disease concomi
A 75-year-old man was diagnosed with locally recurrent pancreatic cancer. Partial response was achieved after 9 mo of gemcitabine. At the same time, the patient reported peripheral vascular disease without necrosis. Chemotherapy was suspended, and after one month the Positron Emission Tomography (PET) scan showed locoregional tumor recurrence. Gemcitabine was resumed and partial response was obtained, but peripheral vascular disease occurred.
Our results suggest that the appearance of peripheral vascular disease may be related to a prolonged response to gemcitabine.
Core Tip: Gemcitabine is known for vascular side effect. In this case, we report a vascular acrosyndrome that occurred during first-line with Gemcitabine for pancreatic adenocarcinoma. In this case, the patient experienced prolonged tumor response. Immunological phenomena could be responsible for this double effect.
- Citation: Fabien MB, Elodie P, Anna S, Addeo P, Meher B. Gemcitabine-induced peripheral vascular disease and prolonged response in a patient with metastatic pancreatic adenocarcinoma: A case report. World J Clin Cases 2023; 11(6): 1372-1378
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1372.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1372
Gemcitabine is a nucleoside metabolic inhibitor. This antimetabolite drug has displayed significant antitumor activity in pancreatic adenocarcinoma[1]. Gemcitabine causes often myelosuppression, influenza-like syndrome and vascular toxicity[2]. Among toxic vascular effects of gemcitabine, we find venous and arterial events, digital ischemia and necrosis, vascular inflammation, and thrombotic microangiopathy. We report a case of locoregional recurrent pancreatic adenocarcinoma in a patient treated with gemcitabine who experienced severe peripheral vascular disease and prolonged antitumor response.
A 75-year-old man presented with a diagnosis of borderline adenocarcinoma of the pancreatic body in April 2019.
In July 2021, during Gemcitabine, the patient reported the appearance of Raynaud’s phenomenon-like symptoms.
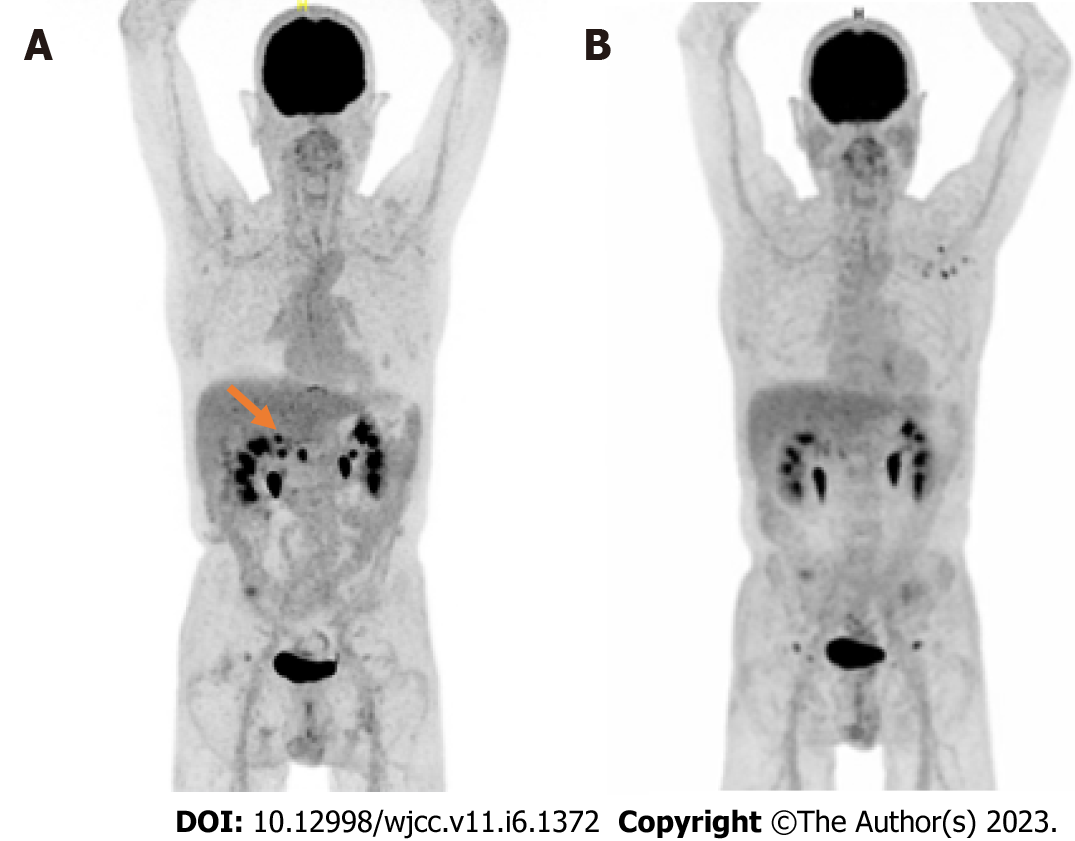
For borderline adenocarcinoma of the pancreatic body, he underwent neoadjuvant chemotherapy by FOLFIRINOX (12 cycles) with stable disease. He underwent pancreaticoduodenectomy in January 2020 (ypT2N2R1). A PET scan showed locoregional recurrence during a follow-up in August 2020 (Figure 1). In accordance with ESMO guidelines, chemotherapy with gemcitabine was initiated. Partial objective response was observed after 9 mo and gemcitabine was continued as maintenance therapy (Figure 1).
A 75-year-old man had a history of smoking (15 pack-year), and stopped in 1976. He was treated with verapamil for hypertension and with tinzaparine for a deep vein thrombosis of the lower left extremity since 2019.
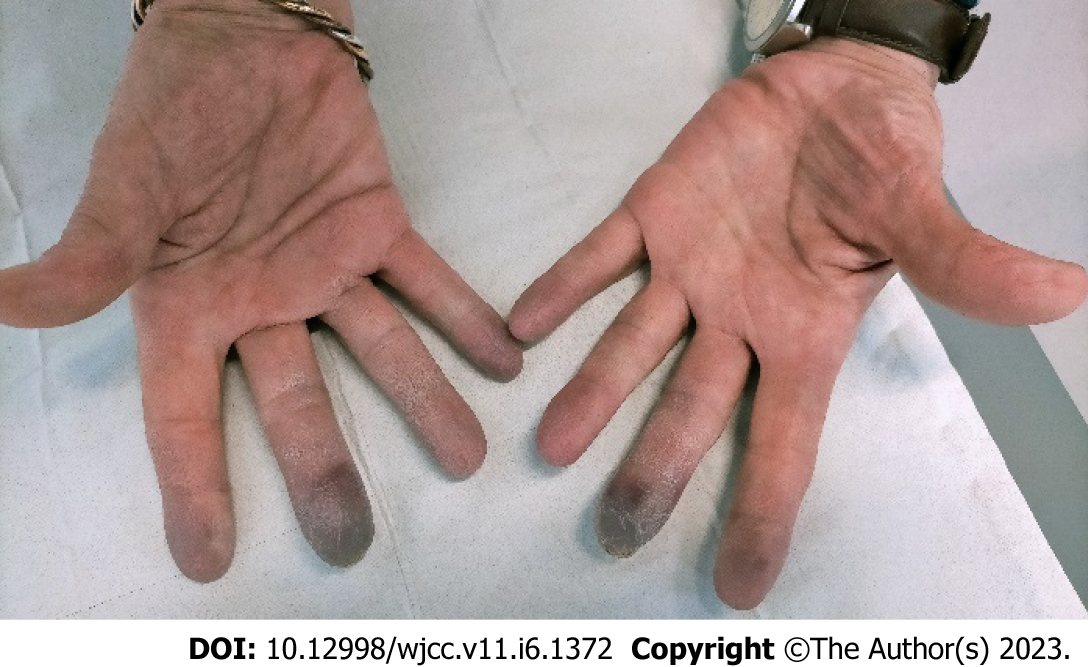
The symptoms consisting of loss of sensitivity and cold-induced cyanosis of the left middle finger matching with a typical syncopal phase of the Raynaud’s phenomenon. Other arguments in favor of this phenomenon were sparing of the thumb and absence of digital pulp ulceration. Allen's test showed pathological results at the radial and ulnar levels. There were no megacapillaries or flame hemorrhage, cupuliform ulceration, or rectangular telangiectasia. There was no toe involvement.
Laboratory analyses showed normal hemogram, electrolytes, creatinine, liver function, and hemostasis. C3- and C4-complement, cryoglobulin, ANCA and CPK did not show any abnormality. Anti-extractable nuclear antigen antibodies and antinuclear antibody (ANA) were negative. The specific absence of anti-Scl70 or anti-centromere antibodies was noted. Other antiphospholipid antibodies were not detected either.
An arterial and venous Doppler ultrasound found no abnormality.
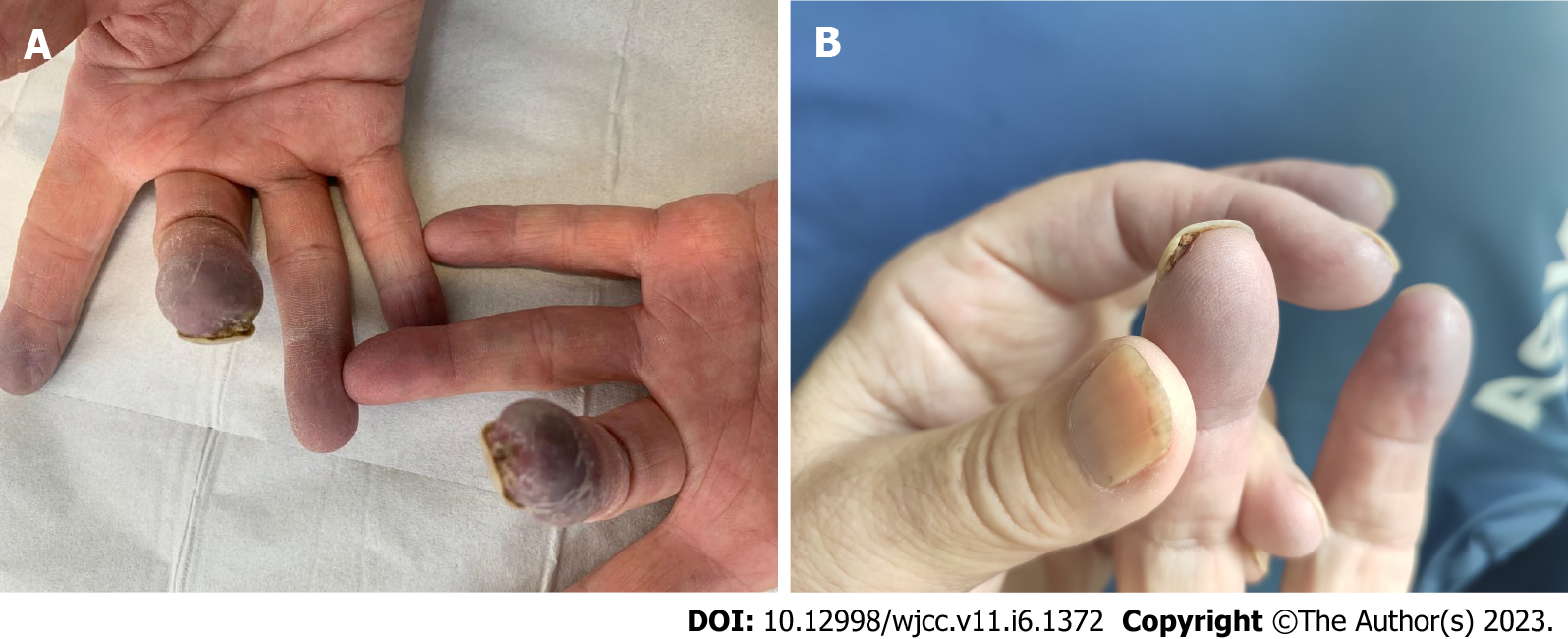
At the patient’s request, chemotherapy was suspended for 4 wk after the onset of symptoms. Paraneoplastic syndrome was initially suspected. PET scan in August 2021 showed locoregional tumor recurrence coincident with an elevation of CA 19-9 blood level at 893 ng/mL. Weekly gemcitabine chemotherapy was consequently resumed, and partial response was obtained after 3 mo of chemotherapy. CA 19-9 blood levels gradually decreased to 380 ng/mL. Gemcitabine was eventually interrupted in December 2021 after 13 cycles because of resurgence of the vascular acrosyndrome (permanent cyanosis and pain) then affecting the distal phalanx of both left and right 2nd and 3rd fingers (Figure 2) and causing great repercussions on daily activities. Symptoms showed little to no improvement after 2 mo with the appearance of ulceration of the 3rd digits (Figure 3). A Doppler echocardiography showed no macrovascular abnormalities but capillary microscopy revealed impaired microcirculation.
The patient was then referred to the cardiovascular department where a treatment with iloprost (prostacyclin analog) was introduced for a duration of 28 days.
We noticed clinical improvement after 1 mo of treatment, and the disappearance of the ulceration (Figure 3). Gemcitabine was not resumed and disease progression was observed on the March 2022 CT scan. The patient died in June 2022.
In the present study, we report a case of metastatic pancreatic adenocarcinoma in a patient presenting with peripheral vascular disease that occurred during first-line chemotherapy. The vascular symptoms improved after discontinuation of gemcitabine. In this case, the patient experienced prolonged tumor response. The median survival time was 5.6 mo in historical studies using gemcitabine. Here, the patient showed no sign of progressive disease 17 mo after treatment initiation. Cases of Raynaud’s phenomenon and digital necrosis after receiving gemcitabine for bladder cancer and lung cancer have been reported[3-6]. Three cases of Raynaud’s phenomenon and/or digital ischemia have also been described in patients with pancreatic cancer[6-8]. Peripheral vascular disease is a rare and painful condition that impairs the patient quality of life. The most frequent etiologies are connective diseases, vasculopathies, hematological diseases, paraneoplastic syndromes, drugs, infectious diseases, and embolic diseases. They can all be complicated by secondary vasospasm[9]. In this case, we discuss the multifactorial mechanisms underlying peripheral vascular disease, aggravated by the administration of antimetabolites, and the relationship to the associated better outcomes.
Antimetabolites can have cumulative toxicity leading to endothelial dysfunction and hypercoagulability. Several chemotherapies can induce endothelial lesions or cause thromboembolic events[10-15].
Many vascular side effects have been reported in the literature as related to gemcitabine treatment. We note venous and arterial events, vasculitis with necrosis, thrombotic microangiopathy, severe capillary leak syndrome, and digital necrosis[5,6,16,17]. Here, chemotherapy was stopped, resulting in the improvement of symptoms despite cancer progression. The occurrence of peripheral vascular disease in patients with cancer can also be considered a paraneoplastic disorder, natably in the case of adenocarcinoma, squamous cell carcinoma or hematological diseases[18]. Several mechanisms have been proposed to explain peripheral vascular disease associated with cancer. It is suggested hypothesis a peripheral vasospasm or larger production of vasoconstrictor substances by tumor cells following neoplastic involvement of the cervical sympathetic trunk[19]. A thromboembolic mechanism with either migration of tumor fragments or hyperviscosity, hypercoagulability and spontaneous platelet aggregation has also been suggested[20]. In many case-report of patients with paraneoplastic peripheral vascular disease, vasospastic complications improve after initiation of suitable anticancer treatment[21]. For our patient, this etiology was unlikely to be the cause of the patient's digital manifestations, as he had an radiologic response at the time of symptoms worsening.
The hypothesis immunological’s mechanism has also been suggested. In fact, cancer diseases can promote autoimmunity by generating autoantibodies against different autoantigens, leading to the activation of the complement upon contact with the arterial wall[22].
The association between toxicity and treatment efficacy has long been a concern in cancer patients. Better outcomes associated with immune-related adverse events is well described in cancer patients treated with immunotherapy. For example, vitiligo is significantly correlated with a better outcome to ICI in melanoma[23].
The restoration of antitumor immunity during treatment with immunotherapy leads to multiples manifestations, including vasculitis of the medium and large vessels but rarely of the small vessels[24]. Several recent studies have described the development of acral vascular necrosis with immunotherapy, without history of autoimmune disease[25,26]. The mechanism of action of immunotherapy could lead to a disturbance of immune tolerance with stimulation of T population of lymphocytes or to the formation of autoantibodies against many antigens such as endothelial cells and be at the origin of the disorder’s vascularization. Additionally, an autoimmune etiology of digital ischemic symptoms during treatment of immunotherapy is supported, as a steroids treatment might improve acral necrosis[27,28].
One study postulated that antimetabolites induced both vascular and immunological adverse effects and prolonged response as shown with ICI[29]. Gemcitabine has the capacity to activate the immune system and create an inflammatory tumor microenvironment[30,31]. In particular, it depletes regulatory T lymphocytes and selectively kills immunosuppresive cells, thereby alleviating immunosuppression and enhancing cytotoxic T-cell-dependent anti-cancer immune responses[32].
Peripheral vascular disease is a rare complication of antimetabolite chemotherapeutic drugs. This is the second study to report the case of peripheral vascular disease and prolonged response with gemci
The authors gratefully thank Lisa Schohn for her contribution to the proofreading of the English version.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghazanfar A, United Kingdom; Ungureanu BS S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4351] [Cited by in RCA: 4179] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 2. | Aapro MS, Martin C, Hatty S. Gemcitabine--a safety review. Anticancer Drugs. 1998;9:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | D'Alessandro V, Errico M, Varriale A, Greco A, De Cata A, Carnevale V, Grilli M, De Luca P, Brucoli I, Susi M, Camagna A. [Case report: Acro-necrosis of the upper limbs caused by gemcitabine therapy]. Clin Ter. 2003;154:207-210. [PubMed] |
| 4. | Yamada Y, Suzuki K, Nobata H, Kawai H, Wakamatsu R, Miura N, Banno S, Imai H. Gemcitabine-induced hemolytic uremic syndrome mimicking scleroderma renal crisis presenting with Raynaud's phenomenon, positive antinuclear antibodies and hypertensive emergency. Intern Med. 2014;53:445-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Blaise S, Appeltants H, Carpentier PH, Debru JL. [Digital ischaemia and gemcitabine. Two new cases]. J Mal Vasc. 2005;30:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kuhar CG, Mesti T, Zakotnik B. Digital ischemic events related to gemcitabine: Report of two cases and a systematic review. Radiol Oncol. 2010;44:257-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Zaima C, Kanai M, Ishikawa S, Kawaguchi Y, Masui T, Mori Y, Nishimura T, Matsumoto S, Yanagihara K, Chiba T, Mimori T. A case of progressive digital ischemia after early withdrawal of gemcitabine and S-1 in a patient with systemic sclerosis. Jpn J Clin Oncol. 2011;41:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Vénat-Bouvet L, Ly K, Szelag JC, Martin J, Labourey JL, Genet D, Tubiana-Mathieu N. Thrombotic microangiopathy and digital necrosis: two unrecognized toxicities of gemcitabine. Anticancer Drugs. 2003;14:829-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | McMahan ZH, Wigley FM. Raynaud's phenomenon and digital ischemia: a practical approach to risk stratification, diagnosis and management. Int J Clin Rheumtol. 2010;5:355-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Doll DC, List AF, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 1986;105:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 155] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Robben NC, Pippas AW, Moore JO. The syndrome of 5-fluorouracil cardiotoxicity. An elusive cardiopathy. Cancer. 1993;71:493-509. [PubMed] [DOI] [Full Text] |
| 12. | Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res. 1993;53:3028-3033. [PubMed] |
| 13. | Tonato M, Mosconi AM, Martin C. Safety profile of gemcitabine. Anticancer Drugs. 1995;6 Suppl 6:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Tempero MA, Brand R. Fatal pulmonary toxicity resulting from treatment with gemcitabine. Cancer. 1998;82:1800-1801. [PubMed] [DOI] [Full Text] |
| 15. | Dobbie M, Hofer S, Oberholzer M, Herrmann R. Veno-occlusive disease of the liver induced by gemcitabine. Ann Oncol. 1998;9:681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Viguier JB, Solanilla A, Boulon C, Constans J, Conri C. [Digital ischemia in two patients treated with gemcitabine]. J Mal Vasc. 2010;35:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Holstein A, Bätge R, Egberts EH. Gemcitabine induced digital ischaemia and necrosis. Eur J Cancer Care (Engl). 2010;19:408-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Racanelli V, Prete M, Minoia C, Favoino E, Perosa F. Rheumatic disorders as paraneoplastic syndromes. Autoimmun Rev. 2008;7:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Poszepczynska-Guigné E, Viguier M, Chosidow O, Orcel B, Emmerich J, Dubertret L. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Le Besnerais M, Miranda S, Cailleux N, Girszyn N, Marie I, Lévesque H, Benhamou Y. Digital ischemia associated with cancer: results from a cohort study. Medicine (Baltimore). 2014;93:e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Naschitz JE, Rosner I, Rozenbaum M, Zuckerman E, Yeshurun D. Rheumatic syndromes: clues to occult neoplasia. Semin Arthritis Rheum. 1999;29:43-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Abu-Shakra M, Buskila D, Ehrenfeld M, Conrad K, Shoenfeld Y. Cancer and autoimmunity: autoimmune and rheumatic features in patients with malignancies. Ann Rheum Dis. 2001;60:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Ouwerkerk W, van den Berg M, van der Niet S, Limpens J, Luiten RM. Biomarkers, measured during therapy, for response of melanoma patients to immune checkpoint inhibitors: a systematic review. Melanoma Res. 2019;29:453-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 1451] [Article Influence: 241.8] [Reference Citation Analysis (0)] |
| 25. | Gambichler T, Strutzmann S, Tannapfel A, Susok L. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer. 2017;17:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Khaddour K, Singh V, Shayuk M. Acral vascular necrosis associated with immune-check point inhibitors: case report with literature review. BMC Cancer. 2019;19:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Le Burel S, Champiat S, Routier E, Aspeslagh S, Albiges L, Szwebel TA, Michot JM, Chretien P, Mariette X, Voisin AL, Lambotte O. Onset of connective tissue disease following anti-PD1/PD-L1 cancer immunotherapy. Ann Rheum Dis. 2018;77:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Comont T, Sibaud V, Mourey L, Cougoul P, Beyne-Rauzy O. Immune checkpoint inhibitor-related acral vasculitis. J Immunother Cancer. 2018;6:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Geier M, Babey H, Monceau-Baroux L, Quéré G, Descourt R, Cornec D, Robinet G. Vascular Acrosyndromes Associated With Prolonged Tumor Response in Advanced Lung Cancer Patients During Treatment With Antimetabolites: A Report of Two Cases. Front Oncol. 2021;11:644282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Sen T, Della Corte CM, Milutinovic S, Cardnell RJ, Diao L, Ramkumar K, Gay CM, Stewart CA, Fan Y, Shen L, Hansen RJ, Strouse B, Hedrick MP, Hassig CA, Heymach JV, Wang J, Byers LA. Combination Treatment of the Oral CHK1 Inhibitor, SRA737, and Low-Dose Gemcitabine Enhances the Effect of Programmed Death Ligand 1 Blockade by Modulating the Immune Microenvironment in SCLC. J Thorac Oncol. 2019;14:2152-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 31. | Parente P, Parcesepe P, Covelli C, Olivieri N, Remo A, Pancione M, Latiano TP, Graziano P, Maiello E, Giordano G. Crosstalk between the Tumor Microenvironment and Immune System in Pancreatic Ductal Adenocarcinoma: Potential Targets for New Therapeutic Approaches. Gastroenterol Res Pract. 2018;2018:7530619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 32. | Zheng H, Zeltsman M, Zauderer MG, Eguchi T, Vaghjiani RG, Adusumilli PS. Chemotherapy-induced immunomodulation in non-small-cell lung cancer: a rationale for combination chemoimmunotherapy. Immunotherapy. 2017;9:913-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |