Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1356
Peer-review started: October 2, 2022
First decision: January 5, 2023
Revised: January 18, 2023
Accepted: February 3, 2023
Article in press: February 3, 2023
Published online: February 26, 2023
Processing time: 145 Days and 0.4 Hours
Post-traumatic cauda equina nerve calcification is extremely rare in clinical practice, and its etiology, pathogenesis, treatment and prognosis are unclear. There are few studies and reports on Post-traumatic cauda equina nerve calci
A 52-year-old patient presented to our hospital with a history of lumbar spinal stenosis and a lumbar vertebral fracture caused by trauma. The patient's right lower limb had weakness in hip flexion, knee extension and plantarflexion with muscle strength grade 3, right ankle dorsiflexion and thumb dorsiflexion with muscle strength grade 0. The patient's skin sensation below the right knee plane disappeared. The patient's Computed tomography (CT) data showed signs of cauda equina nerve calcification and the terminal filaments in the plane of the third to fifth lumbar vertebrae. After treatment the patient's symptoms were slightly relieved.
We provide an extremely rare case of Post-traumatic cauda equina nerve calcification and offer a conservative treatment plan. However, the etiology, mechanism and treatment of Post-traumatic cauda equina nerve calcification are still unclear. This requires scholars to conduct more research and exploration in this area.
Core Tip: We report an extremely rare case of Post-traumatic cauda equina nerve calcification and speculate on its etiology and mechanism, providing material for scholars to study the etiology, mechanism and treatment of post-traumatic cauda equina nerve calcification.
- Citation: Liu YD, Deng Q, Li JJ, Yang HY, Han XF, Zhang KD, Peng RD, Xiang QQ. Post-traumatic cauda equina nerve calcification: A case report. World J Clin Cases 2023; 11(6): 1356-1364
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1356.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1356
Post-traumatic cauda equina nerve calcification refers to the deposition of calcium in ten pairs of lumbosacral nerve roots below the conus medullaris, resulting in various clinical symptoms[1]. The cauda equina nerve is the bridge between the spinal cord and the peripheral nerve, connecting the pseudounipolar neurons of the dorsal root ganglion and the spinal cord neurons. Because of its special anatomical structure (only a layer of nerve inner membrane, lack of corresponding protective tissue), it is vulnerable to mechanical injury. The nutrition of cauda equina nerve comes from cerebrospinal fluid and blood, and there is a relatively anemia area in the anastomosis between the spinal artery and the root artery supplying the cauda equina nerve, so the cauda equina nerve is more prone to decompensation due to mechanical compression[2]. Post-traumatic cauda equina nerve calcification is extremely rare clinically, and its etiology, pathogenesis, treatment, and prognosis are unclear. No studies or reports on Post-traumatic cauda equina nerve calcification have been inquired, and a case of Post-traumatic cauda equina nerve calcification is reported here for reference.
On May 13, 2022, a 53 years female community worker with 35 years of progressive lumbar kyphosis was admitted to our hospital with symptoms worsening over 7 mo, claudication and right knee retroflexion.
The patient complained of lumbar spine fracture and bilateral lower limb paralysis due to injury from fall more than 35 years ago, and underwent emergency surgery in other hospitals. Two years after the surgery, the motor and sensory functions of the left lower limb gradually recovered, while the right lower limb had weakness in hip flexion, knee extension and plantarflexion, with muscle strength of about grade 3. He was admitted to the hospital 7 mo ago after a fall during a walk in the park, resulting in right knee retroflexion and right ankle sprain. In the last week, the patient's symptoms were aggravated without any obvious cause, and the right hip was accompanied by radiating pain and numbness in the right lower limb, especially in the lateral and posterior side of the right lower limb. During the pain attack, the patient took metamizole sodium tablets (0.5g po st). After taking metamizole sodium tablets, the patient's pain was slightly relieved. The patient complained of numbness and trapped pain in the lumbosacral region with radiating pain and numbness in the right lower extremity, especially the lateral and posterior sides of the right lower limb. The patient has difficulty squatting down, and the pain increases when bending down. The patient had no symptoms such as chills, high fever, spontaneous sweating, night sweating, dizziness, nausea and vomiting. The patient's sleep and urination were normal. Since the patient's stool is dry, laxatives are used to help him defecate for 3 days.
The patient had a history of hypertension for more than 3 years, with blood pressure up to 150/100 mmHg. Patients regularly take nifedipine sustained-release tablets to control their blood pressure, which has been controlled at present. The patient underwent internal fixation of the lumbar spine at an another hospital 35 years ago and had no adverse blood transfusion reactions during the operation. Had a cesarean section in another hospital more than 30 years ago.
The patient had no relevant personal or family history.
The patient's Lumbar kyphosis and right knee retroflexion. The patient had limited anterior flexion and posterior extension of the lumbar region with percussion pain and pressure pain at the spinous process and paraspinal process of the third lumbar vertebra - fifth lumbar vertebra (L3-5). The patient's right lower limb was positive for lasegue sign and supine abdominal sticking test. Patient's muscle strength: iliopsoas (Second lumbar vertebra /fourth lumbar vertebra) (L2/4) muscle strength, quadriceps (L2/4) muscle strength, gluteus maximus Fifth lumbar vertebra/second sacral vertebra (L5/S2) muscle strength: right: grade 3, left: grade 4+. biceps femoris (L5/S2) muscle strength, tibialis anterior L4/5 muscle strength, thumb extension (L4/S1) muscle strength: right: grade 0, left: grade 4+. gastrocnemius, hallux valgus (L5/S2) muscle strength: right: grade 3, left: grade 4+. The patient's right Achilles tendon reflex disappeared, the skin sensation below the knee joint plane of the right lower limb disappeared, and the skin sensation below the knee joint plane of the left knee decreased. The patient's pathological reflex was not elicited.
Erythrocyte Sedimentation Rate: 24 mm/h. Complete biochemistry: Apolipoprotein A1: 0.77 g/L, High density lipoprotein-C: 0.84 mmol/L, Albumin ratio (A/G): 1.18, Triglycerides (TG): 2. 78 mmol/L, Homocysteine (HCY): 21 μmol/L, Glutamyl transpeptidase (γ-GT): 218 U/L, Creatinine (CREA): 34 μmol/L, Rheumatoid factor (RF):42.26, C-reactive protein (CRP): 11.12 mg/L. Bone densitometry suggested decreased bone mass.
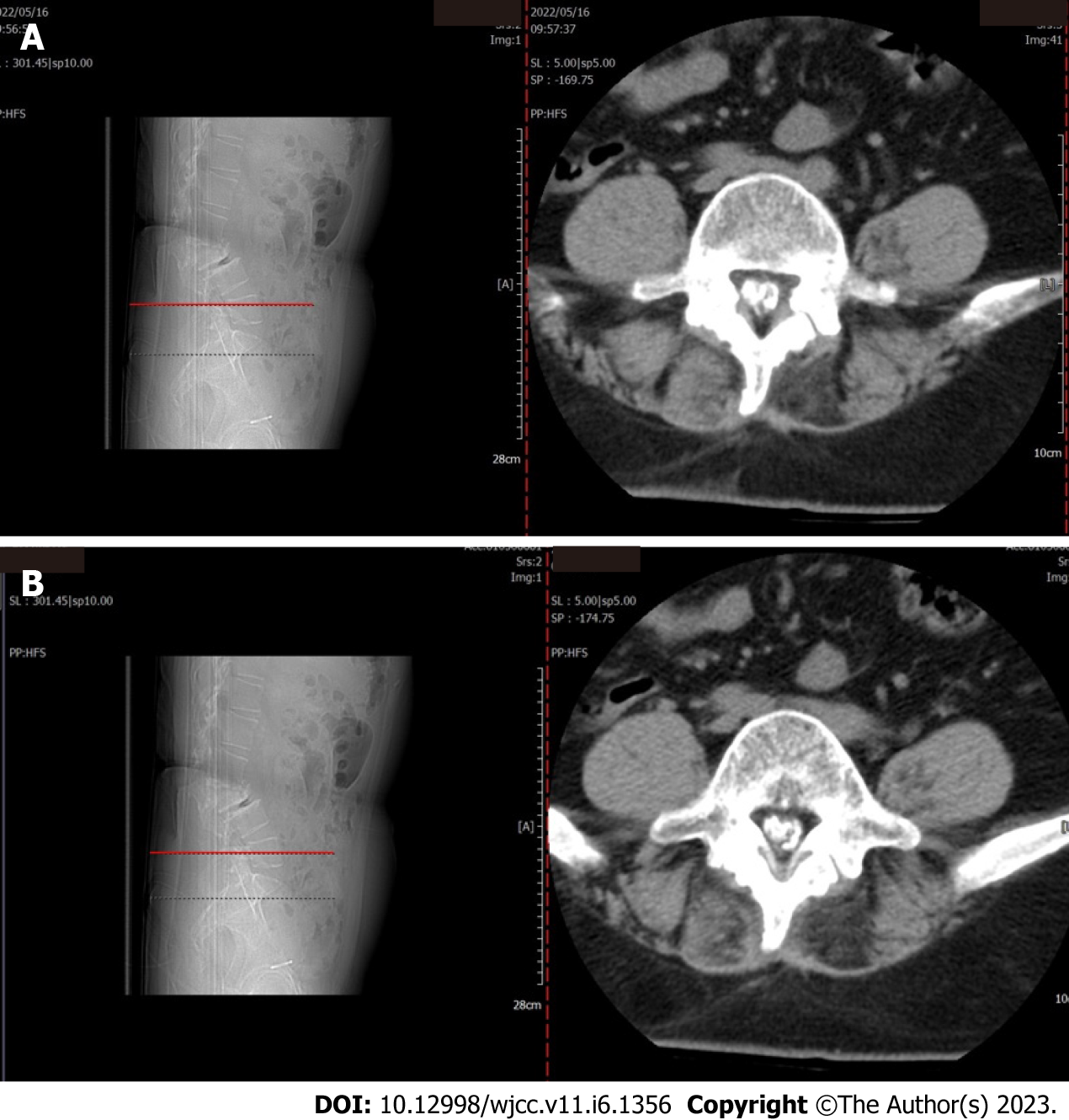
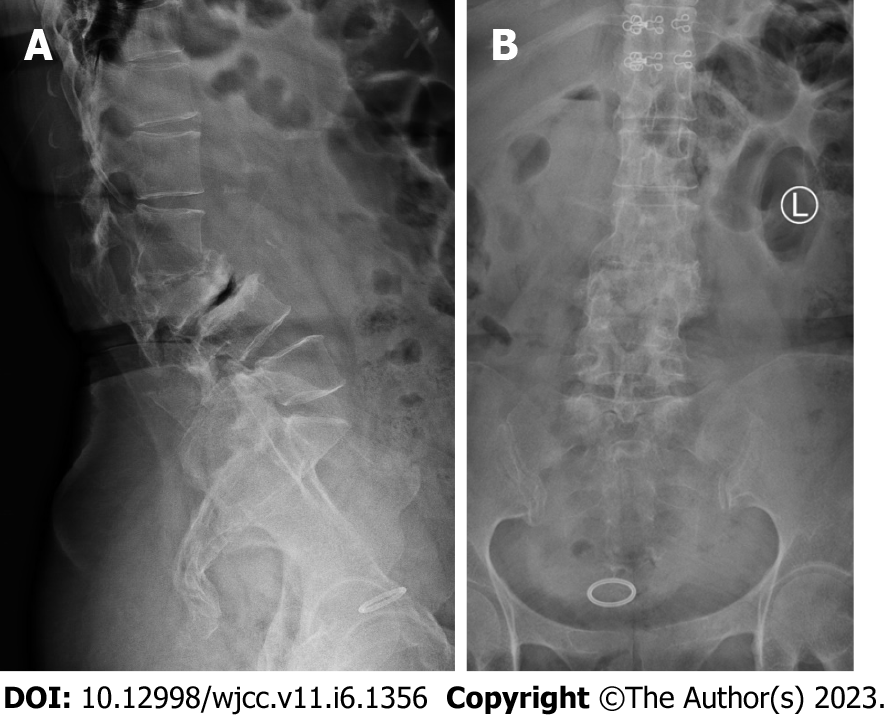
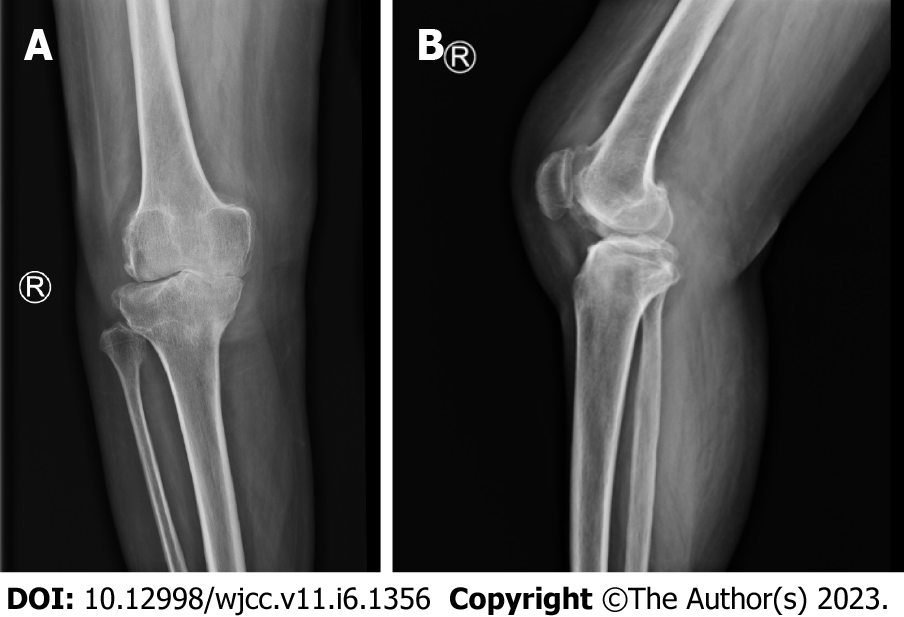
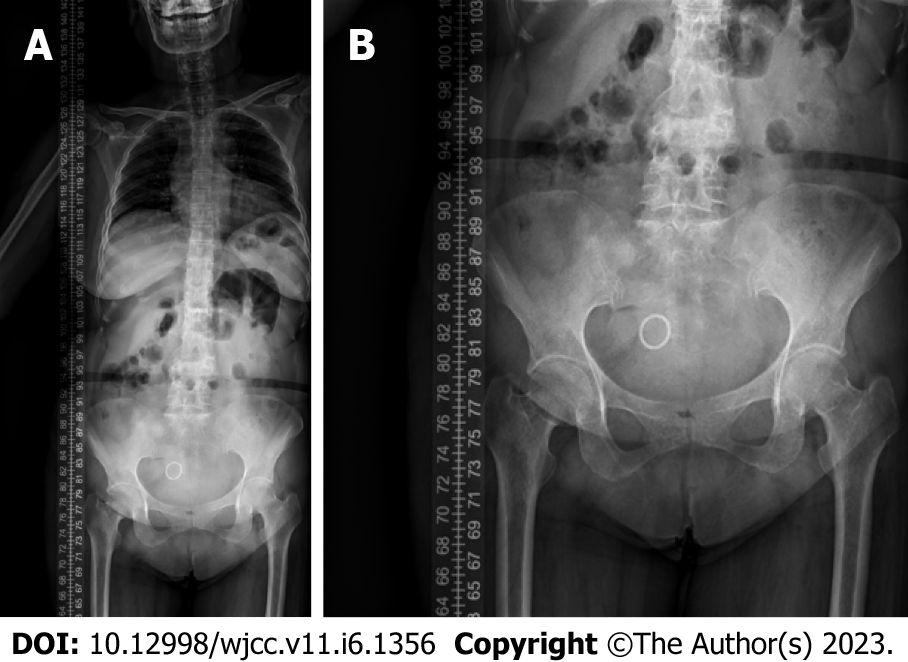
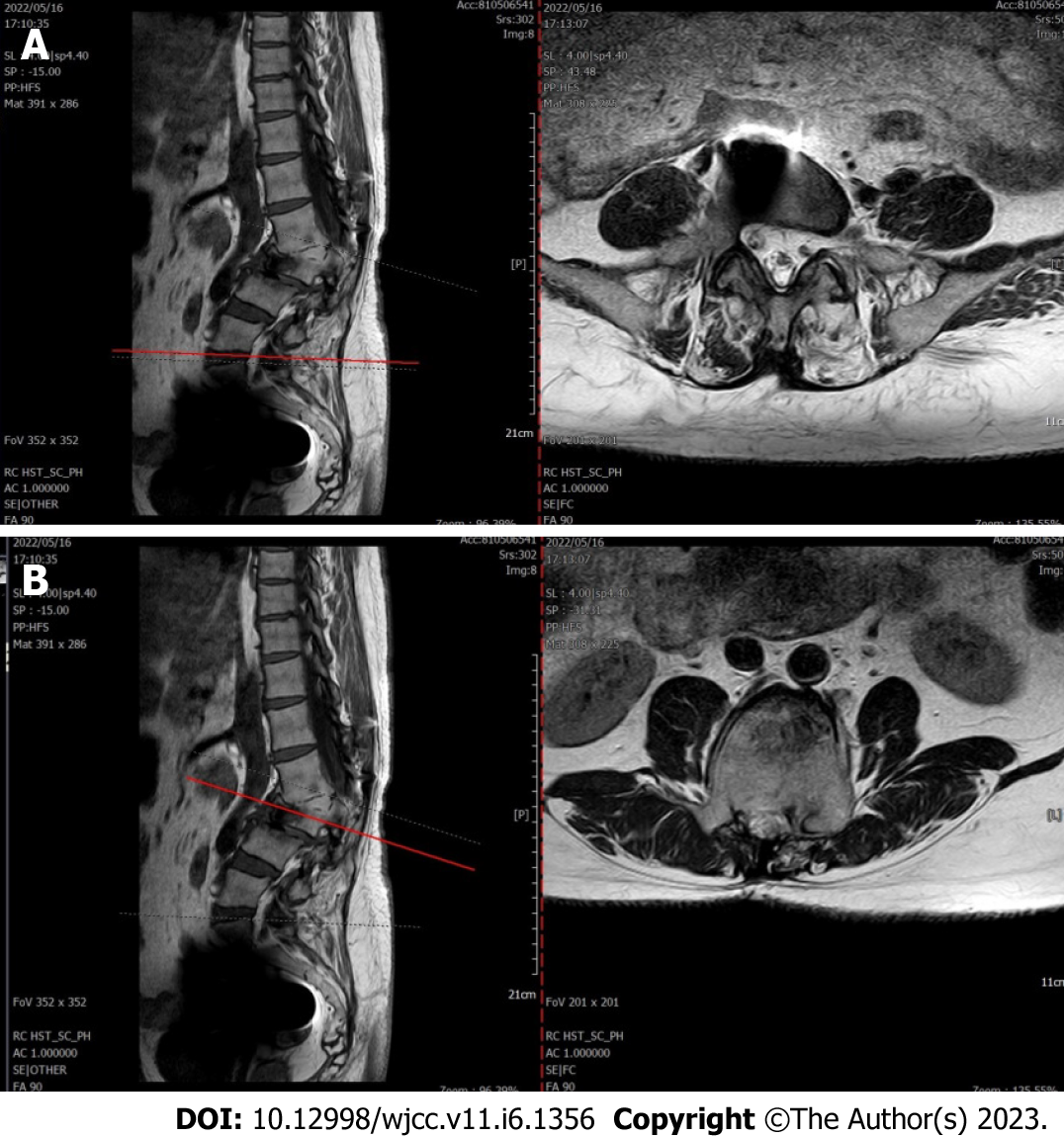
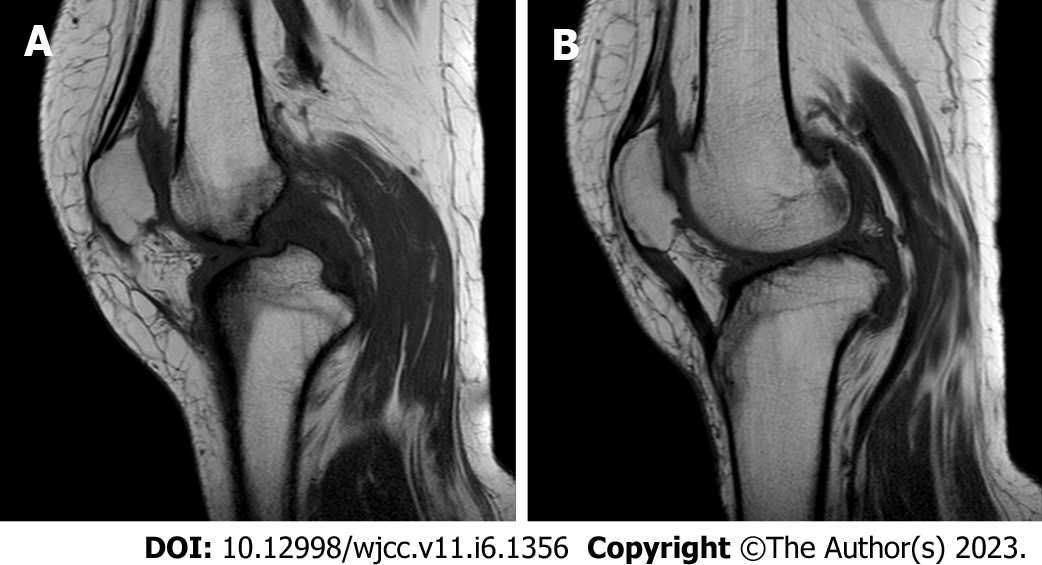
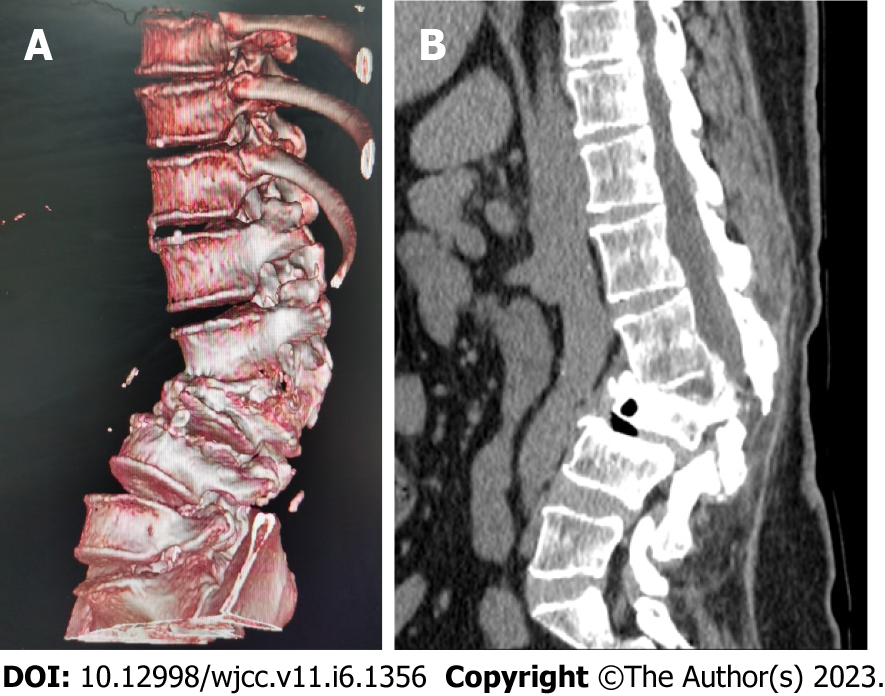
Lumbar spine Computed tomography (CT) showed: L5 plane end filament and cauda equina nerve calcification signs (Figure 1). The X-ray film of lumbar vertebrae shows that the sequence of lumbar vertebrae is abnormal, L3 vertebral body is diseased and shifted backward, L2-3 intervertebral space disappears, L2-3, L3-4, L4-5, L5-S1 intervertebral space narrows, and all vertebrae and vertebral facet joints have hyperosteogeny (Figure 2). The X-ray film of the right knee joint showed that the right knee joint space was narrow inside and wide outside, the articular surface was sclerotic, and the lower edge of the articular surface was spotted with low density. Hyperosteogeny appeared on the upper edge of patella, tibial plateau, tibial intercondylar crest and femoral condyle. The density of the suprapatellar bursa is increased and the surrounding soft tissue is swollen (Figure 3). The X-ray film of the full length of the spine shows that L3-4 vertebral body is diseased and shifted backward, the lumbar spine protrudes backward at the L2-3 plane, the intervertebral space at the L2-3 segment is narrowed, and the L3 vertebral body is wedge-shaped and flattened (Figure 4). The lumbar spine Magnetic Resonance Imaging (MRI) showed: Lumbar vertebrae protrude backward, L3 vertebrae show wedge-shaped changes, spinal canal stenosis on the same level of L3, intervertebral space narrowing on the same level of L2-3, endplate inflammation in the L3-4 intervertebral space, Schmorl node formation near T10-11 vertebral body, degeneration and bulge of L3-4 and L4-5 intervertebral discs, and many abnormal strip signals in the filum terminale (Figure 5). The right knee MRI showed: The cartilage of the medial femoral condyle and tibial plateau is worn, the cartilage is denatured, the posterior cruciate ligament of the right knee is torn, the medial collateral ligament is injured, the anterior and posterior corners of the medial and lateral meniscus of the right knee are worn, the right knee joint has effusion, and the medial head bursa of the gastrocnemius has effusion (Figure 6). Lumbar vertebral body (plain scan + 3D reconstruction) CT showed: Lumbar vertebral body (plain scan + 3D reconstruction) CT showed: L2-3 plane lumbar lordosis, L2-3 vertebral space narrowing, L3 vertebral body wedge-shaped flattening, L3, 4 vertebral body adjacent edge patchy high density. There are some bone defects in the lamina and spinous process of L3 and L4 vertebrae, and the vertebral canal at the same plane is deformed (Figure 7).
Motor nerve conduction velocity measurement (both lower extremities), sensory nerve conduction velocity measurement (both lower extremities), neuroelectrogram, needle electrode electromyography results showed: Neurogenic damage in both lower extremities.
Based on this patient's imaging data and his symptoms, we diagnosed this patient with Post-traumatic cauda equina nerve calcification.
We use the combination of traditional Chinese and western medicine to treat the patient's clinical symptoms symptomatically. Use mannitol injection, dexamethasone sodium phosphate injection, pregabalin capsules, etocoxib, mecobalamin tablets, sodium glutamate injection, and compound betamethasone injection according to scientific drug use rules to dehydrate and detumescence, nourish nerves, diminish inflammation and pain. In addition, we also use radio frequency electrotherapy, traditional Chinese medicine directional penetration, intracutaneous acupuncture, radioactive shock wave and other physical therapy methods to activate blood circulation and remove blood stasis, warm meridians and relieve pain.
We advise patients to maintain a light diet and recommend active functional exercise. Continue to take etoricoxib tablets, methylcobalamin tablets, and pregabalin capsules. The patient was also advised to carry out a variety of blood-activating and stasis-removing types of physical therapy. After completing regular follow-up (1st, 2nd, 3rd and months after discharge), the patient's limb muscle strength changed to grade II and limb sensation appeared. The patient had good compliance.
Nerve calcification usually occurs in the cranial nerve and the dental nerve. It is generally accepted that nerve calcification is divided into two main types: Physiological and pathological calcification. Physiological calcification is due to the natural formation of calcified spots on the nerve with age. Pathological calcification is caused by degenerative diseases, parasitic infections, ischemic necrosis of the surrounding tissue of the nerve, oxidative stress, and apoptosis[3]. However, pathological calcification of cauda equina nerve is extremely rare.We speculate that cauda equina nerve calcification in this patient may be secondary to acute trauma and surgical scar, thus stimulating local lesions. In addition, lumbar spinal stenosis, lumbar disc herniation, spinal tumors and spinal infection are also possible causes of cauda equina nerve calcification[4]. L2-5, S1-5 and the 10 pairs of nerve roots emanating from their caudal segments together form the cauda equine nerve. The cauda equina nerve, in the usual sense, is the nerve root below L2 that innervates the pelvis as well as the perineum. It is a very important nerve in the human body[5]. Once the cauda equina nerve undergoes pathological changes, it will lead to dysfunction of sensory nerve, motor nerve, autonomic nerve, etc., thus causing other body dysfunction. as opposed to the cauda equina nerve calcification, Cauda equina injury is relatively common in clinical practice[6]. The clinical symptoms of cauda equina compression were first reported by Verbiest in 1949 and were named Cauda Equine Syndrome (CES). The clinical symptoms of cauda equina nerve injury mainly include Low back pain, numbness of one or both lower limbs, weakness of lower limbs, weakness or disappearance of tendon reflex, sensory abnormalities in the sellar region, sphincter dysfunction, urinary incontinence, and sexual dysfunction. The cauda equina nerve is easy to be damaged (for example, the free nucleus pulposus and epidural hematoma will cause the cauda equina nerve to be compressed), because the cauda equina nerve has no protective sheath of connective tissue and is particularly sensitive to compression. The recovery after peripheral nerve injury is very slow, which may be due to the lack of blood supply. The nutrition of cauda equina nerve also comes from cerebrospinal fluid and blood, and the recovery of cauda equina nerve after injury is slower than that of peripheral nerve. Therefore, we can boldly speculate that the mechanism of cauda equina nerve calcification may be that the surrounding tissues of cauda equina nerve suffer from mechanical compression or tissue necrosis, which affects the blood supply of cauda equina nerve. Due to long-term poor blood supply, nerve dehydration and poor calcium and phosphorus metabolism lead to calcium salt deposition, which eventually leads to cauda equina nerve calcification[7,8].
There are many reports on Idiopathic Basal Ganglia Calcification (IBGC) in the existing literature, but the specific cause is still unclear, and scholars believe that it is related to the following factors: (1) Genetic studies show that IBCG patients have autosomal recessive or dominant inheritance; (2) Vitrification during atherosclerosis can lead to calcium deposition, so some scholars believe that local calcium accumulation is related to changes in vascular osmotic pressure; (3) The abnormal level metabolism of iron and calcium phosphate, especially the decrease of serum ferritin level and iron binding capacity, will also lead to IBGC; (4) Exogenous toxic substances continuously stimulate and activate glutamate receptor, thus producing neurotoxin effect, leading to calcium ion deposition; (5) It has been reported in the literature that during brain biopsy of patients with IBGC, there are immune inflammatory cells infiltrating around calcification points and ESR is accelerated, while C-reactive protein, rheumatoid factor (RF) and antinuclear antibody (ANA) are significantly increased. So the occurrence of IBGC is also related to the immune system. It is worth noting here that the ESR, C-reactive protein, RF and ANA of the cases reported by us are higher than the normal values. This is consistent with the study of IBCG; and (6) The IBCG has also been suggested to be associated with carbonic anhydrase II deficiency. Regrettably, the epidemiological characteristics and specific treatment methods of basal ganglia calcification have not been studied[9,10,11].
The benign tumor of the cauda equina nerve may also lead to the calcification of the cauda equina nerve. We call this benign tumor the neurilemmoma of the cauda equina nerve. spinal cord neurilemmoma often occur in the spinal cord nerve roots, but only a few occur in the cauda equina nerve. Because the neurilemmoma of the cauda equina nerve has good activity and broad intradural space, it usually has no obvious pain symptoms. The report of neurilemmoma calcification is extremely rare. In 2012, Dr. Seung Jae Hyun and others from South Korea reported that a 21 year old patient with dystrophic neurilemmoma of cauda equina had calcification, and the neurilemmoma was completely removed by surgery. However, this report does not explain the relationship between trauma and calcification of cauda equina neurilemmoma[12]. Paraganglioma is a neuroendocrine tumor located outside the adrenal gland. Paragangliomas originating from filum terminale or cauda equina nerve are rare (accounting for 1% of all paragangliomas and 3%-4% of all tumors in the lower lumbar spine). Calcification of cauda equina nerve paraganglioma is very rare. It is noteworthy that Professor M Vural once reported cauda equina paraganglioma with obvious calcification characteristics, but did not clarify its cause and pathogenesis[13]. Professor J Rot é s Querol once reported a case of cauda equina syndrome caused by ankylosing spondylitis, which caused calcification of lumbosacral meninges. However, the CT images of the cases reported by Professor J Rot é s Querol showed perispinal calcification rather than central calcification[14]. Intradural lumbar disc herniation is another important cause of intraspinal calcification and even cauda equina nerve calcification. Intradural lumbar disc herniation means that the free nucleus pulposus punctures the fibrous ring, posterior longitudinal ligament and dura mater, and directly compresses the spinal cord or nerve after entering the subdural cavity, resulting in acute spinal cord or cauda equina nerve injury. Intradural disc herniation is a rare type of disc herniation, with a incidence rate of 0.26%-0.3%. The most common location of the disease was the lumbar spine, accounting for 92%[15]. Surgical exploration is the only standard for the diagnosis of intradural lumbar disc herniation. Although it is difficult to make a definite diagnosis of intradural lumbar disc herniation through imaging and symptoms, some special signal signs shown by imaging data can well indicate the existence of free intradural nucleus pulposus. For example, the Y sign refers to the prominent nucleus pulposus separating the dura and arachnoid membranes into two lines in a "Y" shape. Over time, the free nucleus pulposus entering the dura will directly compress the cauda equina nerve and calcification will occur to some extent[16]. We speculate that the calcification of the free nucleus pulposus will probably cause the calcification of the cauda equina nerve compressed by it.
Since cauda equina nerve calcification in this patient is secondary to trauma stress, it is necessary to discuss arachnoiditis during injury. There are also some literatures that study the problems related to cauda equina nerve calcification from this perspective. In 1999, I. G. Bilgen reported a case of cauda equina syndrome caused by ankylosing spondylitis, a patient with adhesive arachnoiditis. CT data of the patient showed signs of dural calcification.His MRI data showed that the cauda equina had adhesions with the dorsal arachnoid membrane. They believe that arachnoiditis is responsible for cauda equina syndrome and curvilinear dural calcification[17]. In 2021, Brunner A et al[18] introduced a case of intradural calcification caused by chronic adhesive arachnoiditis and performed a dural incision exploration, and found nerve calcification structures in the dural sac. Brunner A et al[18] believed that the main cause of calcification was the inflammation of arachnoid membrane. Arachnoid inflammation leads to excessive proliferation of arachnoid cells, which leads to excessive production of collagen tissue and rapid formation of intradural scars. Subsequently, osteoblasts are generated, leading to progressive intradural ossification. However, the pathogenesis of ossifying arachnoiditis has not been completely clarified. El Asri AC et al[19] reported a rare case of post-traumatic Arachnoiditis ossifi cans of the cauda equina nerve. El Asri AC et al[19] clearly proposed that trauma, surgery, infection and subarachnoid hemorrhage are the main causes of post traumatic arachnoiditis ossifi cans of the cauda equina nerve.
Since cases of Post-traumatic cauda equina nerve calcification are extremely rare in clinical practice, our research on this disease has not been carried out and the literature that we have been able to study is very scarce. Therefore, it is necessary that we carry out studies on the etiology, mechanisms, treatment and prognosis of this disease.
We provide a rare case of Post-traumatic cauda equina nerve calcification. This case reports the symp
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohey NM, Egypt; Zharikov YO, Russia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Joshi A, Chitale N, Phansopkar P. The Impact of Physical Therapy Rehabilitation on Pain and Function in a Patient With Cauda Equina Syndrome. Cureus. 2022;14:e28131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Fukatsu S, Ogihara S, Imada H, Ikemune S, Tamaru JI, Saita K. Chronic spontaneous epidural hematoma in the lumbar spine with cauda equina syndrome and severe vertebral scalloping mimicking a spinal tumor: a case report. BMC Musculoskelet Disord. 2022;23:508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 3. | Isen DR, Gaddamanugu S, Kline LB. Optic Nerve Sheath Calcification. Ophthalmology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Nasim O, Eskander B, Rustam Z, Pantelias C, Moverley R. Expediting the Management of Suspected Cauda Equina Syndrome (CES) in the Emergency Department Through Clinical Pathway Design at a District General Hospital: A Quality Improvement Project. Cureus. 2022;14:e32722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Ando T, Watanabe H, Riku Y, Yoshida M, Goto Y, Ando R, Fujino M, Ito M, Koike H, Katsuno M, Iwasaki Y. Neurogenic intermittent claudication caused by vasculitis in the cauda equina: an autopsy case report. Eur Spine J. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Khashan M, Ofir D, Grundshtein A, Kuzmenko B, Salame K, Niry D, Hochberg U, Lidar Z, Regev GJ. Minimally invasive discectomy vs open laminectomy and discectomy for the treatment of cauda equina syndrome: A preliminary study and case series. Front Surg. 2022;9:1031919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Rascón-Ramírez FJ. Spinal cord stimulation and cauda equina syndrome: Could it be a valid option? Neurocirugia (Astur: Engl Ed). 2022;33:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Moussa MK, Alkefrawi P, Elkhalil JK. Large central disc herniation causing cauda equina syndrome in an adolescent. A case report. Int J Surg Case Rep. 2021;79:119-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Li M, Fu Q, Xiang L, Zheng Y, Ping W, Cao Y. SLC20A2-Associated Idiopathic basal ganglia calcification (Fahr disease): a case family report. BMC Neurol. 2022;22:438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Durante A, Audino N, Cristiano M, Tanga M, Martino MT, Noschese I, D'Auria D, Pinto F. Basal ganglia calcification: a Fahr's disease case report. Radiol Case Rep. 2021;16:3055-3059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Peters MEM, de Brouwer EJM, Bartstra JW, Mali WPTM, Koek HL, Rozemuller AJM, Baas AF, de Jong PA. Mechanisms of calcification in Fahr disease and exposure of potential therapeutic targets. Neurol Clin Pract. 2020;10:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Hyun SJ, Rhim SC. Giant cauda equina schwannoma with dystrophic calcifications : case report and review of the literature. J Korean Neurosurg Soc. 2012;51:105-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Vural M, Arslantas A, Isiksoy S, Adapinar B, Atasoy M, Soylemezoglu F. Gangliocytic paraganglioma of the cauda equina with significant calcification: first description in pediatric age. Zentralbl Neurochir. 2008;69:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Rotés-Querol J, Tolosa E, Roselló R, Granados J. Progressive cauda equina syndrome and extensive calification/ossification of the lumbosacral meninges. Ann Rheum Dis. 1985;44:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Francio VT, Wie CS, Murphy MT, Neal MT, Lyons MK, Gibbs WN, Strand NH. Multispecialty perspective on intradural disc herniation: diagnosis and management - A case report. Anesth Pain Med (Seoul). 2022;17:221-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Thohar Arifin M, Ikbar K N, Brilliantika SP, Bakhtiar Y, Bunyamin J, Muttaqin Z. Challenges in intradural disc herniation diagnosis and surgery: A case report. Ann Med Surg (Lond). 2020;58:156-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Bilgen IG, Yunten N, Ustun EE, Oksel F, Gumusdis G. Adhesive arachnoiditis causing cauda equina syndrome in ankylosing spondylitis: CT and MRI demonstration of dural calcification and a dorsal dural diverticulum. Neuroradiology. 1999;41:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Brunner A, Leoni M, Eustacchio S, Kurschel-Lackner S. Spinal Arachnoiditis Ossificans: A Case-Based Update. Surg J (N Y). 2021;7:e174-e178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 19. | El Asri AC, El Mostarchid B, Akhaddar A, Baallal H, Dao I, Naama O, Gazzaz M, Boucetta M. Arachnoiditis ossificans of the cauda equina. Br J Neurosurg. 2012;26:547-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |