INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by a novel beta coronavirus, namely, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 originated in Wuhan, Hubei province of China in 2019 and was declared a pandemic by the World Health Organization on March 12, 2020[1]. It is an acute atypical respiratory disease targeting the lungs, and symptoms range from mild to severe. Common symptoms of COVID-19 include cough, fever, nasal congestion, shortness of breath, and difficulty breathing, and severe COVID-19 can lead to pneumonia, kidney failure, acute severe respiratory syndrome, septic shock, multiple organ failure, and death[2,3]. Gastrointestinal symptoms include abdominal pain, vomiting, and diarrhoea[4]. The risk factors associated with COVID-19 are older age and comorbidities (diabetes, hypertension, obesity, and coronary artery disease) leading to multi-organ failure and death[5]. In addition, SARS-CoV-2 activates antiviral immune response and releases proinflammatory cytokines causing an uncontrolled inflammatory response leading to immune abnormalities, which may lead to septic shock, multiple organ failure, and infection by microbes[6].
Metabolic dysfunction associated fatty liver disease (MAFLD) (formerly named non-alcoholic fatty liver disease) poses an economic and health burden on society, affecting a quarter of the world population[7]. There is a dramatic increase in the prevalence of obesity and metabolic syndrome in Western and Asian countries due to changes in diet and lifestyle that have significantly increased the occurrence of MAFLD. Based on histopathological features, it can be classified into simple steatosis and non-alcoholic steatohepatitis that can progress to liver cancer and cirrhosis. Obesity, increasing age, and diabetes are the major risk factors associated with cirrhosis in MAFLD patients[8]. MAFLD is a hepatic manifestation of various metabolic dysfunctions, including obesity, dyslipidemia, type 2 diabetes mellitus, insulin resistance, oxidative stress, adipokines, and apoptosis[9].
MAFLD AND COVID-19
MAFLD is associated with fat accumulation and inflammation in hepatocytes, thus compromising liver function and making people more susceptible to SARS-CoV-2 infection. COVID-19 induces liver apoptosis and is observed with hepatic steatosis and damaging hepatocytes due to increased levels of inflammatory mediators, such as IL-1, IL-6, and IL-10. MAFLD patients are at a 4 to 6-fold higher risk of severe COVID-19 infection[10] as it exacerbates the virus-related cytokine storm[11] and carries a combination of comorbidities which is also a potential risk factor for COVID-19. Obese and advanced fibrosis MAFLD patients are more prone to severe COVID-19. The severity of COVID-19 in MAFLD patients is not associated with advanced liver diseases, and the mortality in the MAFLD group is associated with enhanced and pronounced inflammatory response in the host[12]. MAFLD patients with COVID-19 tend to have more severe symptoms and require more intensive hospital care and long-term monitoring than non-MAFLD patients[13]. Morbid obesity, older age, multi-morbidity scores, elevated FIB-4 scores, and hypoxia are independent predictors of mortality in hospitalized MAFLD patients[14]. MAFLD is associated with a higher risk of hospitalization in COVID-19 patients, and metabolic syndrome treatment with metformin, GLP-1RA, and bariatric surgery can mitigate the risk[15].
A preliminary analysis was done by Ji et al[16] on the implications of MAFLD in 202 COVID-19 patients. Persistent abnormal liver function was observed in 33% of patients from hospitalization to follow-up. MAFLD is an independent risk factor for the progression of COVID-19, causing liver damage and more prolonged viral shedding. The rate of liver abnormalities was 50% at the time of hospitalization and increased to 75% during the hospital stay. The same observation was supported by Huang et al[17] on 280 patients with COVID-19; 35.7% of patients reported abnormal liver function during admission. The alanine aminotransferase (ALT) level was higher in MAFLD patients compared to non-MAFLD ones on admission and during hospitalization. Older age (> 50 years) and concurrent MAFLD are risk factors for liver damage in patients suffering from COVID-19. A meta-analysis of 14 studies, including 1851 MAFLD patients, concluded that there is an increased risk of severe COVID-19 and ICU admission compared to the non-MAFLD group with no difference in mortality between MAFLD and non-MAFLD patients[18,19]. A multicenter cohort study on 65 MAFLD and 65 non-MAFLD patients demonstrated a positive correlation between MAFLD and metabolic risk factors and COVID-19 in non-diabetic patients. MAFLD was associated with a four-fold risk of severe COVID-19 in non-diabetic patients[20].
FACTORS INFLUENCING INTERACTION BETWEEN MAFLD/MAFLD AND COVID-19
A combination of risk factors itself accompanies MAFLD. Hence, several risk factors are allied with the interaction between MAFLD and COVID-19, which will be discussed in this section.
Influence of age
Age is a circuitously affecting factor for the rapport between MAFLD and COVID-19. People with comorbidities like diabetes, hypertension, chronic lung disorder, etc. are susceptible to COVID-19, particularly elderly patients. Thus, elderly patients are more susceptible to MAFLD and COVID-19. Hence, ageing can be considered as one of the perpetrators of the speedy attack of both diseases as mentioned above, and their alliance can also be affected by ageing very symptomatically.
A systematic multicenter analysis supported that younger patients with COVID-19 are more prone to gain MAFLD than aged patients. The study included 327 patients of different age groups. The patients younger than 60 years were grouped as younger, while the patients more than 60 years of age were grouped as older or aged patients[21]. According to the data, people with severe COVID-19 who had MAFLD made up 24% of the elderly patients and 55.9% of the younger patients. It is conspicuous that in young patients, but not in elderly ones, MAFLD was interrelated to the sternness of COVID-19. The influence of age on the alliance of MAFLD and COVID-19 still needs a proven mechanism for support. In comparison to younger patients, older patients have more comorbid conditions that involve many organs and greater death rates, which may outweigh the effect of MAFLD on COVID-19[22,23].
Impact of hypertension
In the general population, the prevalence of MAFLD is comparatively much higher in patients suffering from COVID-19. Studies prove that MAFLD plays a significant role in vulnerability to COVID-19. Sequentially, COVID-19 exacerbates MAFLD development. The relationship between MAFLD and COVID-19 is like a web, and both are interconnected with each other very intricately. The metabolic hitches like hypertension and lipid cholesterol levels get promoted by COVID-19. The development of COVID-19 is also accelerated in MAFLD patients due to metabolic comorbidities such hypertension and high lipid or cholesterol levels. In MAFLD patients with COVID-19, monitoring and treating these metabolic conditions can reduce the risk of a deprived projection.
One of the metabolic comorbidities, hypertension, is a crucial menace that affects the incidence and advancement of COVID-19. In a cohort study of patients with COVID-19 and chronic metabolic diseases, COVID-19 had the highest incidence in patients with hypertension, and 49.7% of patients with COVID-19 suffer from hypertension[24]. Hypertension is allied with the anomalous instigation of the renin-angiotensin system and the lessened countenance of angiotensin converting enzyme-2 (ACE-2)[25]. Therefore, ACE-2 levels get reduced in hypertension patients. Hence as per the hypothesis, these decreased ACE-2 Levels are responsible for the easy vulnerability towards COVID-19. Inversely, it has been anticipated that COVID-19 decreases ACE-2 bustle and its receptors, resulting in a higher incidence of hypertension in COVID-19 patients. Hypertension may enhance the menace of MAFLD in COVID-19 patients, with the reason being subordinate aspects, such as the instigation of systemic inflammatory reactions in the case of hypertension rather than ACE-2 receptors directly[26]. Around 126 out of 251 COVID-19 patients had hypertension in a retrospective study. The study exhibited that COVID-19 patients with hypertension had higher levels of interleukin -6 (IL-6) and a higher sensitivity to C-reactive protein and procalcitonin than the non-hypertensive patients[27].
These statistics suggested that COVID-19 patients with hypertension have a more unadorned systemic inflammatory retort than non-hypertension patients. The retort may affect liver metabolism in unembellished cases and cause subordinate liver damage or organ dysfunction.
Impact of dyslipidemia
Dyslipidemia is a metabolic comorbidity comprising anomalous upgradation in triglycerides (TG) and anomalous reduction in high-density lipoprotein. Dyslipidemia was found to be the subsequent recurrent complication after hypertension in a study among COVID-19 patients. An investigation has revealed that after the commencement of the disease, significant lowering of different cholesterols in the body (total cholesterol (TC)[28,29], TG, high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) occurs. The changes in these levels were responsible for brutality and the impermanence of the disease. Like the SARS virus, the SARS-CoV-2 also assaults the host cells through synthesizing and packaging virus particles using lipids, leading to a decree in the blood lipid components, which is counted as one of the consistent characteristics of dyslipidemia. Increased incidences of COVID-19 were observed due to an anomalous rise in triglycerides and total cholesterols in MAFLD patients. Therefore, patients with metabolic syndrome and dyslipidemia repeatedly have liver lipid metabolic surplus that may have a harmonious effect when combined with COVID-19, which may also be one of the reasons for liver injury. However, more specific studies are essential to authenticate this concept.
Impact of obesity
Obesity is a sovereign menace aspect of COVID-19. A cohort study, including around 350 patients infected by SARS-CoV-2, suggested that 52% of the patients were intricated with obesity[30]. A similar study revealed that around 19% of obese patients with COVID -19 suffered from MAFLD[31]. The exact mechanism for this has not been explained to date though numerous probable reasons may enlighten this rapport. The foremost reason is that obese patients frequently suffer from MAFLD due to visceral fat accretion. The inflammatory response of MAFLD itself can result in chronic liver injury. On this ground, COVID-19 and the drugs used to treat COVID-19 may additionally exacerbate liver grievance. The second reason is a liver injury resulting from weakened aeration in obese patients, leading to sleep apnea syndrome and hypoxemia, and further an anoxic atmosphere for liver metabolism. The third reason is the affected immune system due to obesity[32]. Numerous inflammatory responses are produced due to adipocytes and immune cells serving comparable functions[31].
In obese patients, the disparity in the regulation of adipocytes and the immune system may endorse the incidence of a provocative tempest due to COVID-19. Thus, the situation may lead to multisystem organ injury as the ultimate consequence.
Impact of diabetes
One relevant study revealed that around 74% of diabetes patients with COVID-19 suffer liver injury. The results claim that diabetes mellitus is one of the prime factors responsible for COVID-19 and MAFLD[33]. The mechanism for the same is still not very clear. However, the influence of high glucose levels over SARS-CoV-2 replication may be considered one of the strong bases influencing the degree of viral incursion. This phenomenon results in an aggravative systemic inflammatory response, resulting in multiple organ impairments, including the liver. The other reason is diabetes as an autoimmune and enduring inflammatory disorder (disparities of CD4+ or CD8+ T lymphocytes), further exaggerated by COVID-19. The liver is one of the most critical immune organs participating in the phagocytosis of exogenous microorganisms and releasing cytokines, and the hepatic immunomodulatory load may increase in diabetic patients with COVID-19. The last reason is anti-diabetic drugs that cause an irregular upsurge in transaminase and intensified immune-mediated hepatic injury[34]. Diabetes is a sovereign risk feature for COVID-19.
Impact of gender
Primary research findings recommend inequality in occurrences of COVID-19 gender-wise. According to these findings, men were usually at more considerable peril than women. For example, a clinical study in China reported that around 58% of patients were men, and as per WHO records, fatality of COVID-19 was more (approximately 65%) in men than women. This gender inequality was justified through the following clarifications:
Due to the heavy load of non-communicable diseases such as cardiac disorders, cancers, diabetes, etc. in males, mortality rates are higher in male COVID-19 patients than in females.
Men are more careless towards good health habits and are more fascinated by lifestyle-generated habits like addiction, smoking, irregular sleep cycle, and more fascination for media, which makes men more prone to SARS-CoV-2 infection.
Much research supports the stronger immune system of women than men, which is also one of the causes of easy COVID-19 and MAFLD attacks.
Instead of a higher percentage of men at higher risk for COVID-19 and MAFLD, few groups of women, like pregnant females, are at greater risk for both diseases.
Nevertheless, advanced studies suggested no gender bar for COVID-19 and MAFLD, as women also have many personal and social responsibilities compared to men, leading them to mental trauma with weak immunity leading to impaired hepatic function and easy attack of COVID-19[35,36].
DRUG-INDUCED LIVER INJURY IN COVID-19 TREATMENT
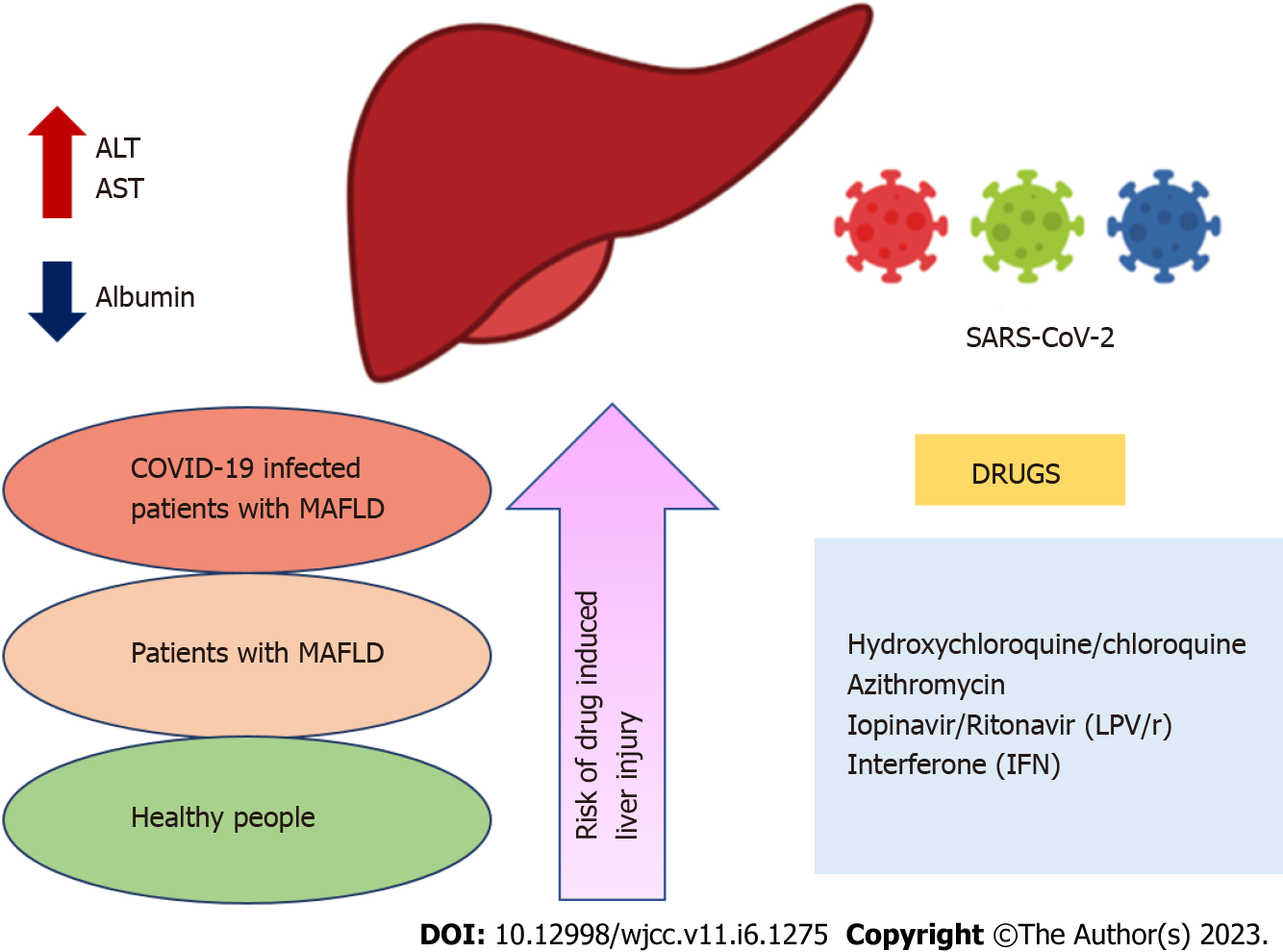
Drug-induced liver injury (DILI) represents liver lesions or liver dysfunction due to medications. The most probable cause of liver injury in COVID-19 patients may be due to the use of multiple drugs for COVID-19 treatment, like antibiotics, antivirals, analgesics, antipyretics, many traditional Chinese medicines, ayurvedic medicines, etc. The order of risk of DILI is depicted in Figure 1. To support this fact, one recent study, including liver biopsy of a patient with COVID-19, was performed, which displayed raised liver enzymes partially due to the drugs used in the treatment of COVID-19 leading to liver dysfunction as a consequence of sepsis and shock.
Figure 1 Order of risk of drug-induced liver injury.
ALT: Alanine transaminase; AST: aspartate transaminase; COVID-19: Coronavirus disease 2019; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; MAFLD: Metabolic associated fatty liver disorder.
Although DILI occurrences are rare, due to difficult diagnosis and dangerous consequences of liver failure with mortality or sometimes liver transplantation, it is very alarming and challenging for the medical fraternity. Polytherapy in COVID-19 treatment makes DILI more intricate, as drug toxicity may differ by sex, age, and race. Numerous medicines can impair liver function and harm the liver; some of them may asymptomatically increase hepatic enzymes; in other instances, acute hepatitis may manifest. Furthermore, liver damage range is consistent with the dose of the drug used, while in some instances, the drug dosage is not related to liver injury in all cases. The most common drugs responsible for liver injury or hepatotoxicity are antibiotic, anti-inflammatory, antimalarial, and antiviral agents[37].
MECHANISM OF LIVER INJURY IN PATIENTS WITH COVID-19
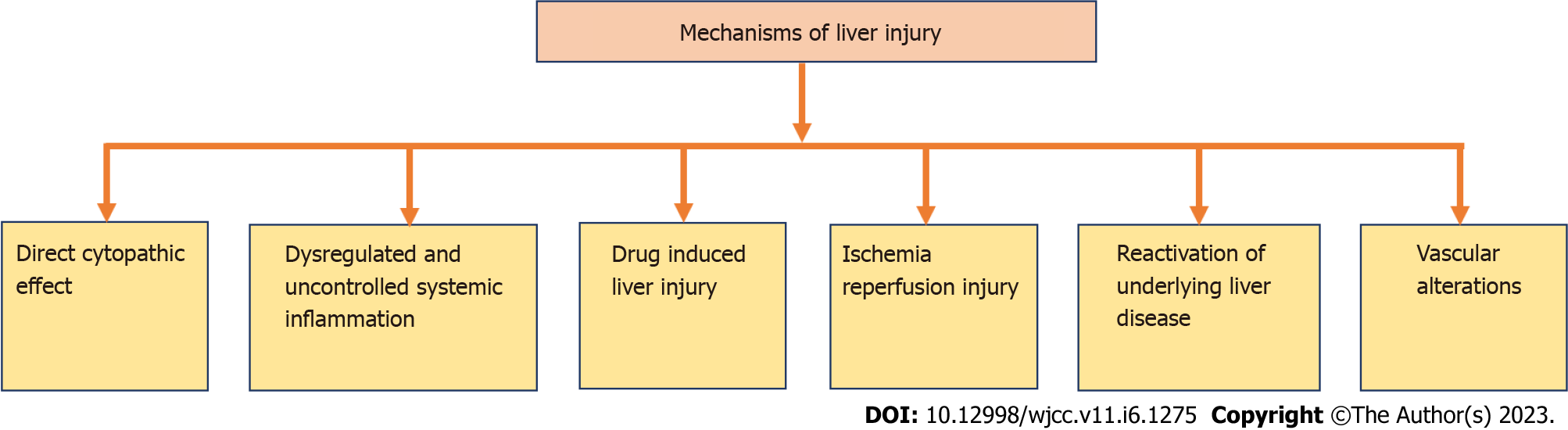
More severe cases of COVID-19 can be found in MAFLD patients due to impaired innate immunity. Various mechanisms for liver injury in patients with COVID -19 are listed in Figure 2[38].
Figure 2
Mechanisms of liver injury.
Direct cytopathic effect
In direct cytopathic effect, the host cells are being besieged by the entry of corona virus through ACE-2. The virus then infects the upper respiratory tract and lung cells. In severe COVID-19 patients, serum gamma-glutamyl transferase (GGT) (a latent investigative indicator for cholangiocyte injury) has been found at amplified levels up to 72%. This ACE-2 receptor binding to cholangiocytes leads to liver dysfunction[39].
Dysregulated and uncontrolled systemic inflammation
Dysregulated and uncontrolled systemic inflammation is an extremely credible reason for hepatic injury in patients with COVID-19. This systemic inflammation is not a major disease. However, the root cause behind this uncontrolled systemic inflammation is the unregulated activation of natural and cellular immunity, which results in multiple organ damage, including hepatic dysfunction owing to abandoned cytokine "hurricane". Again, impaired innate immunity is the main culprit for this uncontrolled systemic inflammation. The latest research findings declare that obesity is undeniably associated with MAFLD and endorses the swing of M2 macrophages (that are inflammation suppressing) to proinflammatory M1 macrophages. This exclusive divergence of macrophages is instigated by fatty acids that advance to ectopic lipid accretion and local and systemic chronic inferior tenderness. This metabolic and immunological dysregulation might aggravate infection caused by SARS-COV-2 that may lead to more unembellished COVID-19 disease[38].
Drug-induced liver injury
The most probable cause of liver injury in COVID-19 patients may be due to the use of multiple drugs for COVID-19 treatment, like antibiotics, antivirals, analgesics, antipyretics, many traditional Chinese medicines, and ayurvedic medicines. To support this, one recent finding, including liver biopsy of a patient with COVID-19, displayed raised liver enzymes partially due to the drugs used in the treatment of COVID-19 Leading to liver dysfunction as a consequence of sepsis and shock. Although DILI occurrences are rare, due to difficult diagnosis and dangerous consequences of liver failure with mortality or liver transplantation, it is very alarming and challenging for the medical fraternity. Polytherapy in COVID-19 treatment makes DILI more intricate, as the liver toxicity of drugs may differ per sex, age, and race[39]. Many drugs that we used may alter the hepatic function leading to liver damage. Asymptomatic elevation of hepatic enzymes and acute hepatitis are some examples for such condition. Hepatic injury may vary according to the dose of the drug; for example, paracetamol in excess dose produces acute hepatotoxicity. It is assumed that pre-existing metabolic liver disorders can increase medication hepatotoxicity because they impair the operation of digestive enzymes, cause oxidative stress, impair mitochondria, and affect lipid balance. Conversely, some xenobiotics might lead to the waning of MAFLD, i.e., prompting a changeover of fatty liver to non-alcoholic steatohepatitis (NASH) and thus persuading necroinflammation and subsequently fibrosis and speedy expansion of liver cirrhosis[39].
Ischemia-reperfusion injury
Complementary to the dysregulated and uncontrolled immune system, one more phenomenon is ischemia-reperfusion injury through respiratory disappointment or sepsis. When systemic inflammatory response syndrome develops in severe COVID-19 instances, the uncontrolled release of proinflammatory cytokines causes peripheral arteries to dilate, which lowers blood pressure and may result in comprehensive tissue hypoxia[40]. If acute respiratory distress syndrome (ARDS) befalls concurrently, it leads to deprived blood oxygenation that intensifies liver ischemia and previously underprivileged blood supply. Furthermore, reactive oxygen species (ROS) increase in the presence of stress and hypoxia, accelerating the oxidation of proteins, DNA, and lipids. Peroxidation products and ROS themselves trigger hepatic stellate cells to generate the extracellular matrix and innate liver immune cells that further result in liver injury through the generation of proinflammatory cytokines[40].
Reactivation of underlying liver diseases
One of the probable causes of liver injury in COVID-19 patients might be the renaissance of primary liver disorder. According to clinical studies, 20%-35% of patients with COVID-19 had altered liver enzymes upon admission, and 15%-30% of patients with COVID-19 had primary liver disease. COVID-19 patients with preliminary liver disease displayed greater elevated liver enzymes compared to the COVID-19 patients without any pre-existing liver disease. Hepatitis B recurrence may be brought on by immunosuppressive medications used to treat severe COVID-19, such as corticosteroids, IL-1 receptor antagonists, IL-6 receptor antagonists, and JAK inhibitors. However, the risk of recurrence is modest to little. Recent strategies endorse screening for HBsAg and anti-HBc prior to the use of immunosuppressive therapy. Prophylactic anti-HBV therapy is required for all patients who are in danger of developing hepatitis B again, ranging from low to high[41].
Vascular alterations
Vascular modifications are a probable alternative source of liver injury. This is reinforced by the study in which analysis of liver samples of individuals who died with COVID-19 due to respiratory failure was performed and negligible inflammatory infiltrate was detected. It was seen that the portal and sinusoidal veins had partial or complete luminal thrombosis, the portal tract had fibrosis, and the portal vein branches had dilated. However, these were undoubtedly attributable to diminished flow inside the liver and clotting cascade tempted by the virus. While the sample size was small, another autopsy investigation in COVID-19 patients found that 88% of patients had fatty alterations in their livers despite having no hepatic occlusion[38].
LIVER ENZYME VARIATION IN COVID-19
SRAS-CoV-2 infection may trigger the pathogenesis involved in the multi-organ impairment, including liver injury. There are different reasons for liver injury in patients suffering from COVID-19, such as injury of cells by direct virus attack and entry of SARS-CoV-2 into hepatocytes via ACE2 expressed in liver and bile duct cells. The damage of hepatocytes, cell apoptosis, and lobular inflammation seen in specimens of liver biopsy and acidophilic bodies are the results due to SARS-CoV-2.
Omrani-Nava et al[42] evaluated the changes in hepatic enzymes in patients suffering from COVID-19. The researchers evaluated the laboratory investigation of direct bilirubin, total bilirubin (TBIL), ALT, aspartate aminotransferase (AST), and alkaline phosphatase (ALP). The levels of direct bilirubin, TBIL, ALT, ALP, and AST were higher in COVID-19 patients than in controls. When the levels of AST were elevated, the mortality rate also surged. The investigators found an abnormality in liver enzymes in COVID-19 patients. Yu et al[43] performed a cohort study in China with 1099 COVID-19 patients. The researchers found that 21.4% and 22.3% of patients have elevated AST and ALT levels and 10.7% have abnormal bilirubin levels. The TBIL levels were more than 17.2 mmol/L, ALP was greater than 135 U/L, and GGT was more than 50 U/L. The researchers concluded that liver enzyme levels were increased in patients suffering from COVID-19. Cai et al[44] also performed a clinical examination of COVID-19 patients, with special reference to an abnormality in liver enzymes. The laboratory outcomes and clinical investigation of 417 patients were obtained from a referral hospital in Shenzhen, China. The researchers observed that during 2 wk of hospitalization, 50 (24.4%), 33(15.2%), 27(11.7%), and 52 (24.5%) patients had elevated levels of ALT, TBIL, AST, and GGT three times from the normal limit. The seven large-scale hospital investigations have revealed that 15%-50% of patients suffering from COVID-19 have superior levels of ALT and AST. These enzymes increased significantly in patients with severe COVID-19[45]. Notwithstanding the potential association reported across the globe between COVID-19 and varying degrees of altered liver enzymes, more detailed study is required to establish the linkage between SARS-CoV-2 infection and liver damage.
RELATION BETWEEN MAFLD AND SARS-COV-2 INFECTION
Metabolic syndrome is a cluster of conditions such as obesity, diabetes, hyperlipidemia, hypertension, and insulin resistance that are responsible for or worsen the conditions of COVID-19 patients[46]. Diabetes mellitus was the second most common disease prevalent in COVID-19 patients and enhanced mortality in infected patients. Hu et al[47] performed a meta-analysis, having 40000 patients from Wuhan, China. The researchers found that 8% of total patients were diabetic. The second most prevalent comorbidity in the patients after DM was hypertension.
A study examined 214 patients suffering from COVID-19, belonging to three hospitals in Wenzhou, China. Out of the 214 COVID-19 patients, there were 66 with MAFLD (45 with obesity and 21 without obesity). The investigators observed that obese MAFLD patients had higher levels of ALT and AST as compared to non-obese MAFLD patients. The researchers found that obese MAFLD patients had a six times more risk of COVID-19 as compared to non-obese MAFLD patients[48]. A meta-analysis was performed by Pan and his colleagues about the association between the severity of COVID-19 and MAFLD. The researchers performed this meta-analysis based on PubMed, Medline, EMBASE, and MedRxiv. After screening, the researchers included a total of six studies, and employed 1293 participants. The meta-analysis studies revealed a high percentage of COVID-19 patients suffering from MAFLD. The researchers observed that MAFLD enhanced the risk of disease progression in patients suffering from COVID-19. The researchers concluded that patients with MAFLD who have been exposed to SARS-COV-2 require better intensive treatment and monitoring[49]. Another study in Chinese hospitals reported that individuals with MAFLD had increased serum IL-6 compared to patients without. The researchers concluded that individuals with MAFLD and enhanced serum IL-6 risk develop severe illness from COVID-19[50]. Zhou et al[22] investigated 327 adult patients more than 18 years old suffering from COVID-19 from four different centres (Wenzhou Central Hospital, Hospital of Wenzhou Medical University, Ningbo No. 2 Hospital, and Ruian People's Hospital) in China in January 2020. The 74 patients (23%) were above the age of 60 suffering from MAFLD, and the rest 93 patients also had MAFLD. Moreover, in elderly patients, 18 suffered from diabetes and 32 were diagnosed with hypertension. Of the younger patients (less than 60 years of age), 45 suffered from hypertension and 29 were diagnosed with diabetes. Hypertension and diabetes prevalence was higher in elderly patients than in younger patients. In contrast, the researchers found a strong correlation between MAFLD and COVID-19 in younger patients (χ2 test P = 0.001) compared to elderly patients (χ2 test P = 0.66). The investigators observed that the rate of severe COVID-19 was two times higher in younger MAFLD patients (age less than 60 years) than in non-MAFLD patients. The researchers recapitulated that younger MAFLD patients were at higher risk of COVID-19 than elderly MAFLD patients. In an investigation of 310 COVID-19 patients, out of 310, 94 suffered from MAFLD. The researchers used fibrosis-4 (FIB-4) index as a prime criterion for fibrosis evaluation. The researchers observed that the FIB-4 index was less than 1.3 in 44 patients, while this value was between 1.3 to 2.6 in 36 patients. The MAFLD patients with a high FIB-4 value were more likely to be obese, older, and diabetic, and had higher C reactive protein, elevated liver enzyme level, and lower lymphocyte count and platelet count as compared to MAFLD with a low score of FIB-4. It is evident from the studies that MAFLD patients having high FIB-4 scores were more prevalent to have COVID-19[51]. Ji et al[16] studied 202 MAFLD patients suffering from COVID-19. Most patients had a liver injury with a mild hepatocellular pattern, and only 3% had a mixed or ductular pattern. The different liver enzymes were elevated in patients, such as TBIL at 9%, ALP at 2.5%, AST at 17%. and ALT at 50%. The researchers observed that MAFLD patients had a higher risk of COVID-19 progression and longer shedding time of the virus than non-MAFLD patients.
COVID-19 was found to alter glucose homeostasis, induce cytokine storm, and increase oxidative stress[52]. The elevated levels of IL-6 have been seen in MAFLD patients, mainly in obese ones, contributing to an increased risk of COVID-19[53]. MAFLD can characterize an enhanced predisposition to cytokine storm syndrome, increased C-reactive protein and IL-6 levels, and activation of NLR family pyrin domain containing 3 (NLRP3). An individual with pre-existing MAFLD makes him/her more vulnerable to infection caused by SARS-CoV-2 and its associated complications. Different studies pointed out that severe COVID-19 is more common in people with MAFLD, which results in critical illness and even the development of non-alcoholic steatohepatitis[54]. A critical analysis of the PubMed database reported that patients less than 60 years and suffering from MAFLD (obesity and severe fibrosis) are more prevalent to have severe COVID-19. The investigators found that the severity of COVID-19 was enhanced by 4 to 6 fold in MAFLD patients compared to non-MAFLD patients[10]. Thus, elevated levels of cytokines and altered liver function can be conceded as important with the severe illness of COVID-19 in MAFLD patients.
LIVER INJURY MECHANISM IN COVID-19 AND MAFLD
The potential mechanism responsible for COVID-19 facilitating MAFLD progression includes direct toxicity of the virus, systemic inflammatory response syndrome, DILI, hypoxic injury, intestinal microbiota imbalance, and hepatic lipid metabolism dysregulation[55]. The expression of hepatic ACE2 and transmembrane protease serine 2 (TMPRSS2) is enhanced in COVID-19 patients who already have MAFLD. These two factors may be responsible for escalated susceptibility of MAFLD patients towards COVID-19[56]. Different innate immune cells such as natural killer cells, natural killer T cells, and macrophages are present abundantly in the liver[57]. MAFLD and obesity are commonly associated with enhanced production of proinflammatory cytokines by Kupffer cells and adipose cells (TNF-α). There are two types of responses from stimulated macrophages, M1 and M2. The M1 macrophages are responsible for initiating the inflammatory processes, while M2 macrophages have reparative and anti-inflammatory functions with high expression of chemokines. It is predicted that dysregulated hepatic innate immunity is responsible for the pathogenesis of MAFLD[58]. Possibly hepatic macrophages are more likely to shift from M1 macrophages (promoting inflammation) to M2 macrophages (suppressing inflammation), leading to COVID-19 progression. MAFLD with noteworthy fibrosis may intensify the virus-induced cytokine storm, probably by the hepatic release of proinflammatory cytokines, significantly contributing to severe COVID-19.
It was reported that ACE2 receptors are present in the liver in hepatocytes and cholangiocytes. The coronavirus targets these ACE2 receptors to enter and it is assumed that it leads to damage to hepatocytes and cholangiocytes[59]. In contrast, another research group recapitulated that MAFLD is not associated with enhanced expression of genes encoding for protein receptors necessary for coronavirus infection such as TMPRSS2, ACE2, and phosphatidylinositol 3-phosphate 5-kinase (PIKfyve). The researchers concluded that enhanced ACE2 expression in MAFLD-COVID19 patients is not relevant justification for escalated liver injury[60]. The SARS-CoV-2 infection reduced the hepatic mitochondrial activity and facilitated the mitochondrial swelling of hepatocytes confirmed by ultrastructural examination and transcriptomic analysis. These results strongly recommend that SARS-CoV-2 is directly responsible for cytopathic effects and positively contributes to MAFLD progression[61]. SARS-CoV-2 infection encourages activation of cGAS-STING in endothelial cells by releasing mitochondrial DNA, resulting in type I IFN production and cell death. Mitochondrial swelling in hepatocytes happens due to SARS-CoV-2 infection, signifying that cGAS-STING signalling activation may aggravate MAFLD in patients suffering from COVID-19[62]. The upsurge of inflammatory cytokine levels was reported in patients suffering from MAFLD and COVID-19[10,63]. Thus, COVID-19 in MAFLD patients resulted in serious illness from cytokine storm. Altered expression of host ACE2 receptor, direct viral attack on hepatocytes, interruption of cholangiocyte function, dysregulated immune responses, hyperinflammation, hepatic ischemic and hypoxic injury, abnormal coagulation and thrombosis, DILI, and altered glucose and lipid hemostasis are some multifactorial mechanisms that can explain the worse outcome of COVID-19 and MAFLD[21].
MANAGEMENT OF COVID-19 PATIENTS ALREADY SUFFERING FROM MAFLD
The first line of management is early and accurate liver biochemical monitoring of COVID-19 patients. The tests related to MAFLD should be carried out as early as possible to monitor the proper functioning of the liver. MAFLD patients may be vulnerable to DILI, so repeated medication should be avoided and focus should be given to dosage and duration of medication. Metabolic control should be enhanced in diabetic patients, and is a primary preventive step for SARS-CoV-2 infection. The influenza vaccination declines the risk of pneumonia by 45%-50% among people suffering from diabetes mellitus, which can be employed for patients suffering from COVID-19[64]. ARDS developed in COVID-19 patients commonly due to dysregulated immune response facilitating cytokine release syndrome. Metformin, the first-line treatment for T2DM, enhanced the immune response and prevented the ARDS compared to other anti-hyperglycemic drugs. Moreover, glucagon-like peptide-1 receptor agonists such as SGLT2 and GLP-1RA also potentially manage hyperglycemia in COVID-19 patients[21,65]. The management of COVID-19 includes improvement in targeted interventions for metabolic pathologies. The vaccination response against SARS-CoV-2 should be carefully examined in obesity and DM patients[66]. COVID-19 in MAFLD patients requires special attention and early hospital admission is recommended. Further, in such patients, treatment of arterial hypertension should be continued. Rigorous lifestyle modification, including follow-up of nutritional guidance, measures for weight loss, and management of hyperglycemia, is required to prevent the development of a severe illness in case of SARS-CoV-2 infection[67]. Due to SARS-CoV-2 infection, hypoxia may occur in hepatocytes of MAFLD patients, followed by severe lung damage leading to enhanced expression of hypoxia-induced factors and ACE2 receptors. The MAFLD patients suffering from fibrosis are another challenge to manage in COVID-19. Thus, the MAFLD patients require special care during SARS-CoV-2 infection and adequate lifestyle intervention to avert the consequence of COVID-19.
CONCLUSION
MAFLD patients are at higher risk of COVID-19, and adequate lifestyle intervention is essential to minimize the damage caused by SARS-CoV-2 infection. MAFLD patients suffering from COVID-19 are at greater risk of hepatic damage. COVID-19 was found to alter glucose homeostasis, enhance the generation of inflammatory cytokines storm, and increase oxidative stress, thus worsening the situation in MAFLD patients. Abnormalities in cardiac, kidney, and liver function markers as well as muscle injury and coagulation parameters must also be monitored in patients with COVID-19. In addition, COVID-19 induces liver injury with an elevated level of ALT and AST. Polytherapy in COVID-19 patients is common; thus, such patients are also vulnerable to DILI as some of them can cause the elevation of liver enzymes asymptomatically. Thus, SARS-CoV-2 infected MAFLD patients are considered severe and require urgent attention.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gutierrez-Castrellon P, Mexico; Leowattana W, Thailand S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH