Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.952
Peer-review started: November 16, 2022
First decision: November 25, 2022
Revised: December 11, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 6, 2023
Processing time: 81 Days and 20.2 Hours
The incidence of pulmonary embolism (PE) in children is low, but its mortality is high. Hypereosinophilic syndrome (HES) is a group of diseases caused by an abnormal increase in eosinophilic granulocytes resulting in multiple-organ dysfunction. The urgent event of thromboembolism in the pulmonary region provoked by eosinophils in idiopathic HES (IHES) is relatively unusual. This article reports a case of IHES with multiple PEs and left leg venous thrombosis as the first manifestation. One month later, the patient developed Henoch-Schonlein purpura (HSP), which is very rare.
We report the case of a 12-year-old boy who was admitted to the hospital with dyspnea, left leg pain, and aggravation. He had bilateral PE and left leg venous embolism with mild eosinophilia. Low-molecular-weight heparin and urokinase were given. At the same time, the interventional department was contacted about filter implantation, followed by urokinase thrombolysis. The left leg thrombus was aspirated under ultrasound guidance. He was discharged from the hospital on rivaroxaban. One month later, he developed a rash on both legs and ankle pain consistent with HSP, with severe eosinophilia and motor and sensory distu
We report a rare and life-threatening case of IHES with multiple embolisms associated with HSP. A mild elevation of eosinophils early in the disease leads to difficulties in diagnosis and delayed treatment.
Core Tip: Pulmonary embolism (PE) in children usually occurs in the presence of an underlying condition, systemic disease, or other risk factors. Idiopathic PE accounts for less than 4% of these. The child has no risk factors for thrombosis other than obesity at the first hospitalization. A month later, he developed a purpuric rash on both legs, and pain in his ankles consistent with Henoch-Schönlein purpura (HSP) was accompanied by severe eosinophilia and motor and sensory impairments. Persistent eosinophilia in peripheral blood can lead to tissue infiltration and even organ damage. If end-organ damage occurs, hypereosinophilic syndrome (HES) can be diagnosed immediately. According to monist principles, patients are diagnosed with idiopathic HES with multiple embolisms complicated by HSP. After glucocorticoids, eosinophils quickly return to normal, neurological symptoms gradually improve, and the rash disappears. Eosinophils are only mildly elevated in PE, making clinical diagnosis more difficult.
- Citation: Xu YY, Huang XB, Wang YG, Zheng LY, Li M, Dai Y, Zhao S. Development of Henoch-Schoenlein purpura in a child with idiopathic hypereosinophilia syndrome with multiple thrombotic onset: A case report. World J Clin Cases 2023; 11(4): 952-961
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/952.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.952
Hypereosinophilic syndrome (HES) is a group of clinical syndromes characterized by a persistent and significant increase in eosinophilic granulocytes in the peripheral blood and surrounding tissues, resulting in organ dysfunction. This condition, which has an unknown cause, is called idiopathic HES (IHES). This disease mostly occurs in people between 20 years and 50 years of age, with an incidence rate of 0.04/100000 and a mortality rate of 9.3%[1]. It is rare in children, in whom its incidence is unknown. Pulmonary embolism (PE) caused by IHES is even rarer. Patients with HES also have autoimmune diseases such as ulcerative colitis, autoimmune hepatitis, autoimmune thyroiditis, multiple sclerosis, systemic lupus erythematosus, antiphospholipid syndrome, myasthenia gravis, and rheumatoid arthritis[2-4]. Henoch-Schoenlein purpura (HSP) is a common vasculitis in school-aged children that can affect the skin, joints, kidneys, and other organs. Children diagnosed with IHES with multiple embolisms complicated by HSP are very rare. This case extends the list of HES patients with autoimmune diseases. To improve the understanding of this disease among pediatric clinicians, a case of IHES with dyspnea and leg pain as the initial presentation and subsequent concomitant HSP was reported in our hospital.
A 12-year-old boy was admitted to our hospital for the first time on January 11, 2021 because of dyspnea for 2 wk, left leg pain for 1 wk, and aggravation for 3 d. He also had a fever and occasional cough. On February 24 and April 2, 2021, the patient was admitted to the hospital with a symmetrical, dark red rash on both legs that did not face when pressed, accompanied by ankle swelling and pain.
The child had a history of respiratory infections prior to labored breathing.
The child was previously healthy.
First hospital admission: The patient was 168 cm tall, weighed 76 kg, and had a body mass index (BMI) of 26.9 kg/m2. His vital signs were as follows: Body temperature, 37.4 °C; blood pressure 128/81 mmHg; heart rate, 104 beats per min; respiratory rate 32/min; and SpO2 92% (room air). The patient’s skin was normal, but he displayed slight shortness and exertion of breath. The circumference of his left leg was 39.5 cm and right leg 37.5 cm, and his limb muscle tension was normal.
Second and third admissions: His legs showed a scattered, symmetrical, pressing dark red rash that did not fade, and both ankles were swollen and painful. On April 4, 2021, the child had decreased pain and temperature sensation in the right plantar, the toe could not bend to the ventral side, and the skin temperature of the right plantar was higher than that of the opposite side.
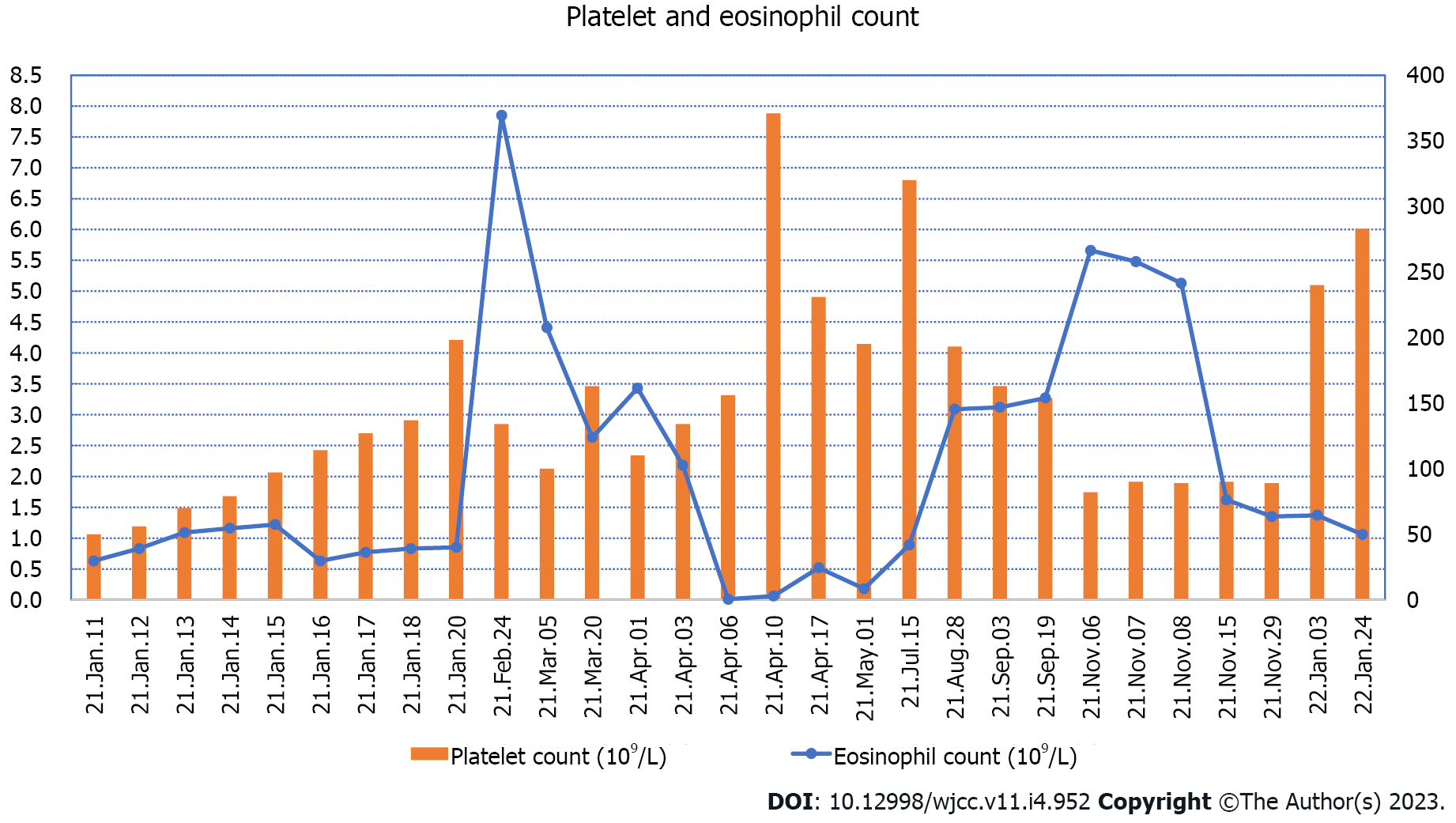
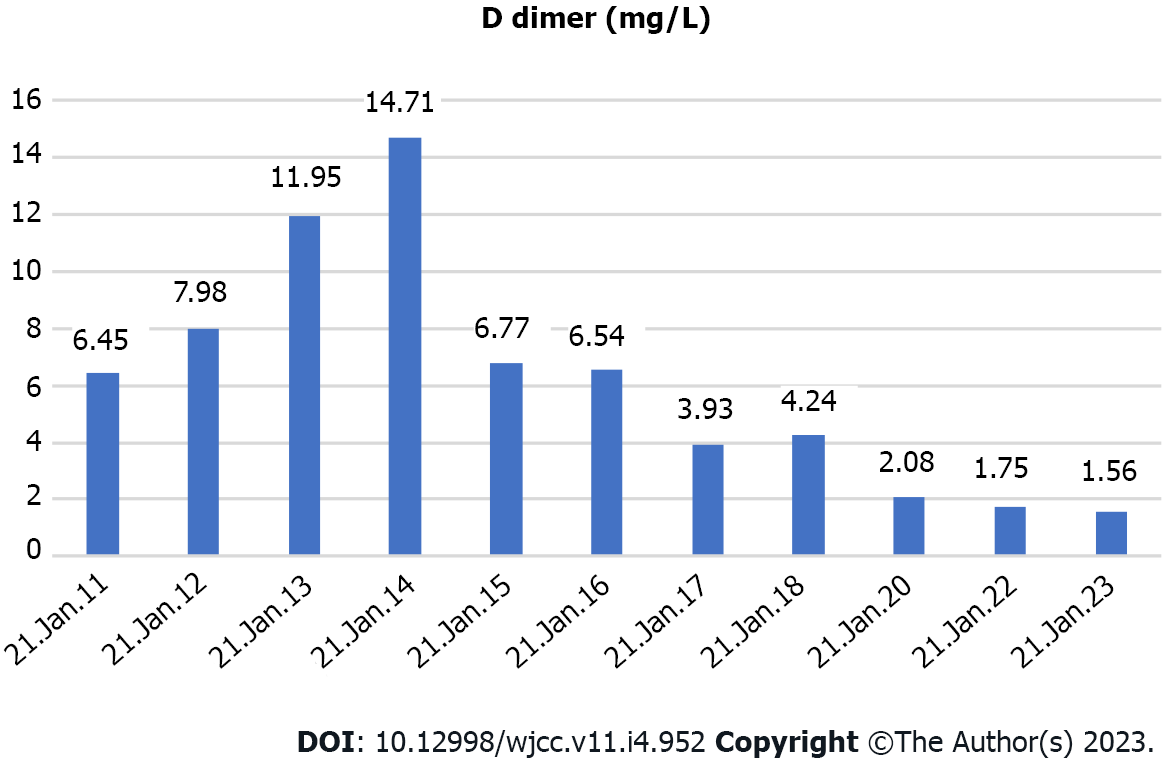
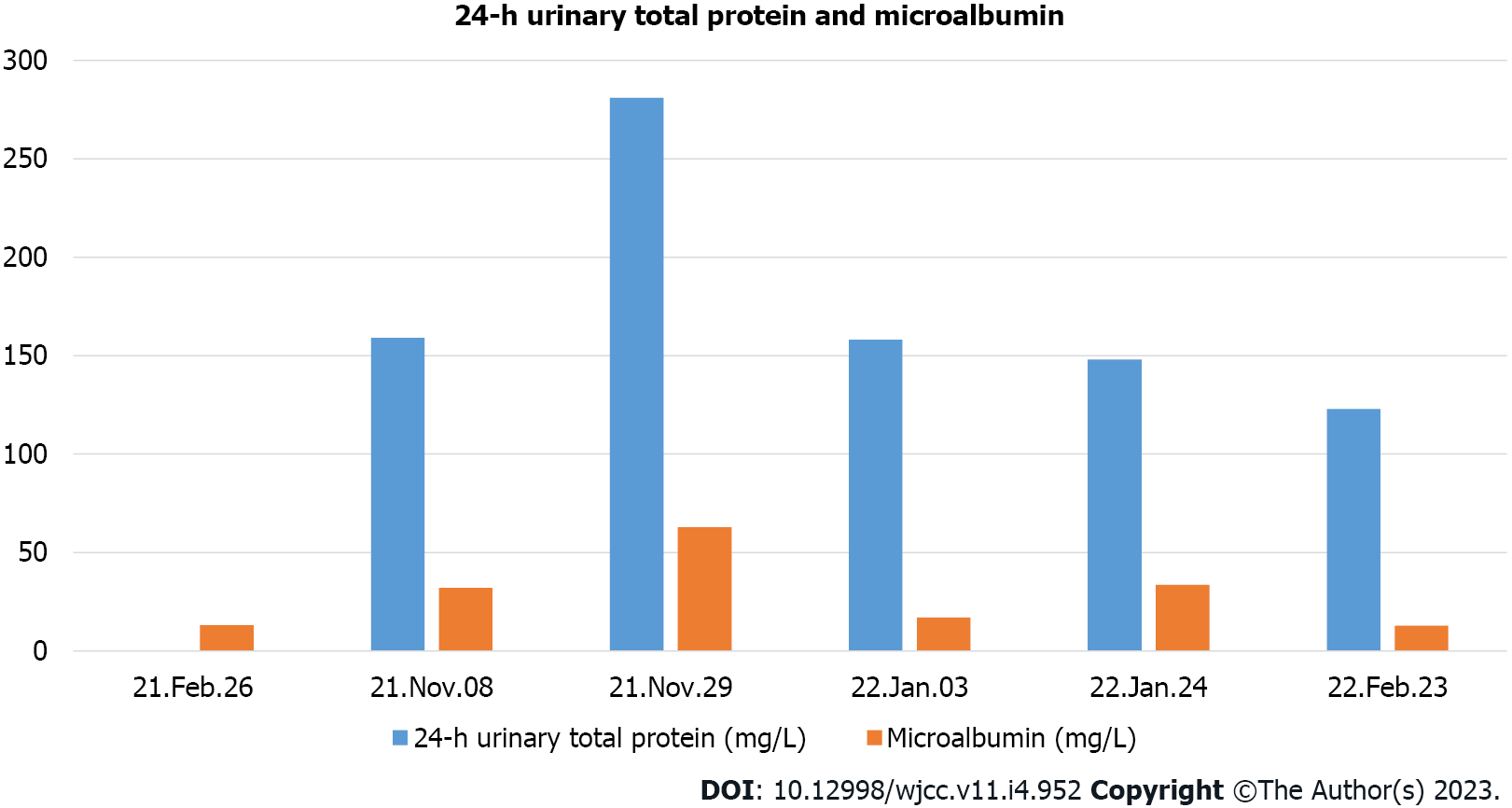
His platelet count was 50 × 109/L, and his eosinophil count (EC) was slightly high at 0.63 × 109/L (Figure 1). On the other hand, his D-dimer level was 11.12 μg/mL (Figure 2). Serum potassium was 3.32 mmol/L. On day 8, blood tests showed normal levels of potassium. C4 was slightly high at 0.46 g/L (normal: 0-0.4). Protein C was 50.2% (normal: 75-130). Neuronal enolase was slightly high at 21.96 μg/L (normal: 0-16.3). N-terminal pro-brain natriuretic peptide (NT-proBNP) and troponin I (TnI) were high at 3116 pg/mL and 0.145 ng/mL, respectively, on January 11, 2021. No abnormalities were found in TnI on January 16, 2021 or in NT-proBNP on January 19, 2021. On February 24, 2021, the patient’s IgE level was significantly high at 531 IU/mL (normal: 0-52). No abnormalities were found in hemoglobin, glucose, lipid, liver function, renal function parameters, C-reactive protein, activated partial thromboplastin time, or protein C. Anti-neutrophil cytoplasmic antibody, antinuclear antibodies, anticardiolipin antibody, HIV tests, hepatitis B, hepatitis C, mycoplasma pneumonia antibody, borrelia, and treponema pallidum were negative. Chlamydia pneumoniae IgG was positive. Human chorionic gonadotropin, alpha-fetoprotein, carcinoembryonic antigen, ferritin, and C3 were within the reference ranges. Allergen IgE detection, food intolerance, stool for Giardia lamblia, cryptosporidium, pinworm, and genetics were negative. On February 26, 2021, the microalbumin level in urine was 13.01 mg/L, but total urinary protein was not found due to the problem of specimen retention. The 24-h urinary total protein and microalbumin levels reviewed at later stages are shown in Figure 3.
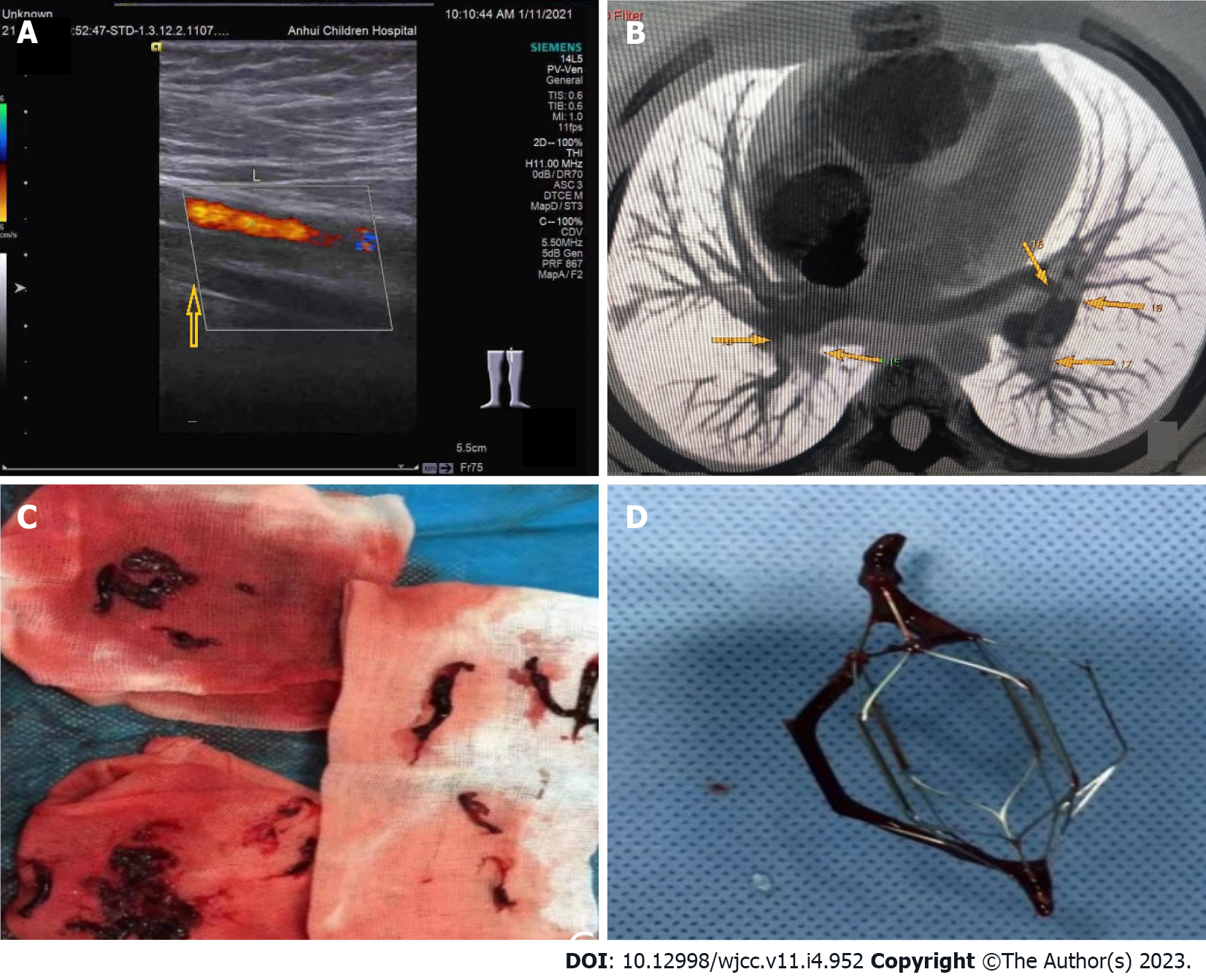
On January 11, 2021, color Doppler echocardiography revealed right atria and ventricle enlargement, mild tricuspid regurgitation with insufficiency, and pulmonary hypertension. Color Doppler ultrasonography of the blood vessels of the legs showed thrombosis of the superficial femoral vein, popliteal vein, and posterior tibial vein of the left leg (Figure 4A). The lung computed tomography (CT) showed scattered wedge-shaped consolidation of both lungs with an outward-facing base and apex pointing to the hilum, a relatively widened pulmonary artery, and pulmonary infarction cannot be excluded. CT angiography of the pulmonary artery showed extensive embolization of both pulmonary arteries with enlargement of the pulmonary artery and the right heart (Figure 4B). On February 4, 2021, color Doppler ultrasound of the left lower extremity vein showed patency of blood flow. On April 6, 2021, color Doppler echocardiography showed a plump right atrium, mild tricuspid regurgitation with regurgitation insufficiency, and mild pulmonary hypertension. Bone marrow puncture and cranial and spinal MRI were not performed.
HSP was diagnosed given the child's typical symmetry of the two legs, nonfading compression, dark-red rash, and ankle swelling and pain. Symptomatic treatment was recommended, and glucocorticoids were added when necessary.
The patient was diagnosed with IHES with multiple embolisms complicated by HSP.
First hospital admission: Immediately afterward, he was given low-molecular-weight heparin and urokinase. At the same time, the interventional department was contacted for inferior vena cava filter implantation followed by urokinase thrombolysis. Angiography showed a marked reduction in filling defects. Rivaroxaban tablets were taken orally after the operation. On January 15, 2021, the left leg thrombus was aspirated under ultrasound guidance, and numerous long strips of thrombus were extracted (Figure 4C). Postoperative angiography showed that the filling defect had disappeared. On January 21, 2021, the filter was removed, and a thrombosis was attached to the filter (Figure 4D). He was discharged from the hospital on rivaroxaban.
Second hospital admission: He was given anti-anaphylactic treatment while rivaroxaban was continued orally.
Third hospital admission: Methylprednisolone sodium succinate (2.0 mg/kg/d) was administered. Prednisone (0.5 mg/kg/d) and rivaroxaban were administered orally at discharge. Prednisone and rivaroxaban were gradually discontinued over 3 mo.
After methylprednisolone sodium succinate treatment was administered, EC decreased significantly, and the patient’s skin rash, arthralgia, pain, temperature sensation, and toe activity gradually recovered. After following the child for one year, on November 8, 2021, the child showed signs of respiratory tract infection, skin rash in the legs again, and ankle pain. On November 10, 2021, purpura nephritis was diagnosed, and prednisone was given (0.2 mg/kg/d). The child has no symptoms at present, and his EC has decreased. His total urinary protein and micro urinary albumin levels gradually returned to normal. Presently, prednisone is being gradually reduced.
A 30% mortality rate in patients with PE was reported in some studies that included autopsy-based PE diagnosis[5]. PE in children is associated with central venous catheterization, malignancy, congenital heart disease, systemic lupus erythematosus, trauma, surgery, long-term total parenteral nutrition, dehydration, infection, hospitalization with a state of acquired or congenital hypercoagulability, oral contraceptive usage, and inactivity. There were no risk factors in our patient. One month later, the child had significantly elevated eosinophils (EOSs) and a symmetrical, dark-red rash on both legs that did not fade when pressed, accompanied by ankle swelling and pain. The rash was diagnosed as HSP after consultation with the nephrology department. HES can cause thrombi. A retrospective review of the English literature (Table 1) found 16 articles[6-21], a total of 21 patients were diagnosed with HES and complicated with thrombotic events, of which deep venous thrombosis accounted for 38%, and PE 33%. The mortality rate was 19%. HES is characterized by a consistently elevated EC of 1.5 × 109/L or more that lasts for at least 6 mo or across 2 examinations (≥ 1 mo between tests) and can be diagnosed immediately in the event of life-threatening end-organ injuries, such as PE and cerebrovascular thromboembolism, to avoid delayed treatment[22]. HES is more common in men than in women, and fever is the most common symptom, which can cause functional impairment of the skin, heart, nerves, lungs, digestive tract, and other organs. Thrombotic events are rare. The patient reported here presented with PE and left leg venous thrombosis as the first manifestation, and the peripheral blood eosinophil level was only slightly elevated at this time. Rato reported a case of IHES with a maximum peripheral eosinophil count of 0.83 × 109/L, but the pleural pathological examination was consistent with eosinophil pleurisy. Bronchoalveolar lavage revealed EOSs with a large pericardial effusion and left ventricular perforation. A pericardial biopsy indicated the presence of many EOSs[23]. According to the principles of monism, HES was considered likely in this case. HES has a complex etiology and can be divided into primary (mainly hematological tumors), secondary (allergic diseases such as asthma, parasitic or fungal infections, drugs, and rheumatic diseases), and idiopathic[24]. Our patient was negative for HES-related etiology and screening indicators, including infection, allergens, rheumatic diseases, immune deficiency, and tumors. The child was healthy and showed normal growth and development. He denied a history of rhinitis, asthma, intractable eczema, or repeated bacterial infections. He did not lose weight recently, nor did he have an enlargement of the liver, spleen, or lymph nodes, so primary HES was not supported. The onset age was young, there was no history of thrombotic disease in his lineal or collateral relatives, and no positive results were found in the genetic test. The diagnosis was IHES with multiple embolisms associated with HSP.
| No. | Ref. | Number of cases | Symptom | Thrombotic events | Treat | Prognosis |
| 1 | [6] | 2 | Papules/multiple painful, papules and; plaques over both legs | DVT of the lower extremities, PE, left renal vein thrombosis/dermal microthrombi | Prednisolone and anticoagulants/unknown | Died/unknown |
| 2 | [7] | 1 | Pain and swelling of both legs accompanied with fever | DVT of the lower extremities, PE, portal thrombosis, mesenteric venous thrombosis | Prednisone, coumadin | Improved |
| 3 | [8] | 2 | Progressive chest pain, cough, hemoptysis, painless right leg swelling and 10 d history of intermittent diarrhea/pain and swelling of right leg, hemoptysis | DVT of the right lower extremity, PE | Anticoagulant, corticosteroids | Improved |
| 4 | [9] | 1 | Shortness of breath, chest pain, digital ischaemia | DVT of the lower extremities, PE, portal vein; thrombus, vena cava thrombus | Inferior vena cava filter insertion, thrombolysis, prednisolone, anticoagulation | Improved |
| 5 | [10] | 1 | Distal lower extremity paresthesias and fatigue | Cerebral arteriolar thromboembolism | Methylprednisolone | Died |
| 6 | [11] | 1 | Headaches, vomiting, a rise in blood pressure | Sagittal sinus vein thrombosis | Anticoagulation | Died |
| 7 | [12] | 1 | Fever, respiratory distress | Right atrial and ventricular thrombosis | Anticoagulation | Improved |
| 8 | [13] | 2 | Pain in the left inguinal region/palsy and pain in the right middle finger | Thrombus formation from the trunk of the portalvein, superior mesenteric vein, splenic vein, and right hepatic vein to deep veins in the lower extremities/A thrombus of the muscular artery in the subcutaneous region | Splenectomy, prednisolone, anticoagulation/prednisolone, cyclosporin A, prostaglandin E1, the finger was amputated | Improved |
| 9 | [14] | 1 | Left upper abdominal pain, dry cough, a painful, swollen left leg | DVT of the left lower extremity | Prednisolone, anticoagulation | Improved |
| 10 | [15] | 1 | Headache, vomiting | Superior sagittal sinus thrombosis | Anticoagulation | Improved |
| 11 | [16] | 1 | Fever | Portal vein thrombosis | Corticosteroids, anticoagulation | Improved |
| 12 | [17] | 1 | Disorientation, decreased muscle strength in the upper limbs, nonspecific chest pain | Arch of the aorta thrombus | Corticosteroids, anticoagulation | Aggravation |
| 13 | [18] | 1 | Cough, malaise, loss of appetite, and fever | DVT of the lower extremities, PE, and both right and left biventricular mural thrombi | Glucocorticoids | Improved |
| 14 | [19] | 1 | Swelling in the right parotid gland | Transverse and sigmoid sinuses thrombosis | Methylprednisolone | Died |
| 15 | [20] | 1 | Fever, hemoptysis, and chest pain | DVT of the lower extremities, PE | Dexamethasone, anticoagulation | Improved |
| 16 | [21] | 3 | Unknown | Superficial venous thrombophlebitis of the lower extremities | Prednisone, anticoagulation | Improved |
In most cases, PE originates from deep vein thrombosis of the legs. The child first had dyspnea, followed by left leg pain. PE may come from in situ thrombosis. In HES, symptoms similar to PE are usually caused by a large increase in EOSs in the pulmonary vasculature. Thus, this “thrombus” that is subsequently detected clinically is not a true thrombus but rather an accumulation of EOSs[25]. This pulmonary accumulation leaves fewer EOSs in peripheral blood, which may explain the EOS reduction in the PE with leg thrombosis in the present case. Furthermore, EOS release eosinophilic cationic protein (ECP), EOS peroxidase (EPO), major basic protein (MBP), and EOS-derived neurotoxin (EDN) to damage vascular endothelial cells through degranulation. MBP, ECP, and EPO can also improve the activity of tissue factors. Coagulation factors VI and X activate the endogenous coagulation pathway, inhibit the production of activated protein C, and cause hypercoagulability. In addition, EOSs can directly activate tissue factor, platelet-activating factor, and leukotriene; activate the exogenous coagulation pathway; activate and aggregate platelets; and promote thrombosis, while direct EC infiltration leads to vascular endothelial cell injury[26]. These mechanisms suggest that IHES may involve blood vessels and cause thrombi. The child's EC fluctuated between 2.63 × 109/L-7.85 × 109/L in the late stage, and neurological damage occurred. EOS particles contain EDN and MBP, which may be associated with neurological damage[27].
Glucocorticoids (0.5-1.0 mg/kg/d) are currently recommended as the mainstream treatment for IHES. It has been reported all patients’ EC decreased, and 98.3% achieved complete remission after treatment with glucocorticoids. Standard anticoagulation treatments also effectively prevented the recurrence of venous thromboembolism, and no severe bleeding events were observed during the short-term follow-up. There is no published information about the long-term outcomes of glucocorticoids and anticoagulant therapy in patients with idiopathic eosinophilia (including HES) and venous thromboembolism[28]. Glucocorticoids inhibit EOS maturation and the production of cytokines and chemokines, induce EOS apoptosis, and reduce tissue and organ damage. Interferon-α, imatinib, hydroxyurea, or mepolizumab is recommended for glucocorticoid deficiency, intolerance, or long-term maintenance therapy[24]. In the case of thrombosis, anticoagulation, catheter interventional thrombolytic therapy, and surgical treatment can be selected, according to the location of the thrombosis, the time of embolization, and the specific situation of the patient[29]. The first symptom in the present child was multiple systemic embolisms. After the elimination of anticoagulant contraindications, anticoagulant and thrombolytic therapy were administered, and catheter interventional thrombolytic therapy was performed simultaneously. When IHES was combined with HSP, there was no recurrence of the rash after glucocorticoid administration. With anticoagulant therapy, the neurological damage gradually recovered, and the EOSs quickly returned to normal.
HSP, also known as IgA vasculitis, is the most common small vasculitis in childhood. It may affect the skin, joints, gastrointestinal tract, and kidneys. HSP can be complicated by venous thrombosis[30,31], but there was no evidence of HSP before the occurrence of thrombosis in this child. It is not clear whether IHES and HSP have a common pathogenesis. Interleukin-4 (IL-4) and IL-5 are cytokines secreted by Th2 cells. IL-5 specifically promotes terminal differentiation and proliferation of EOSs and is an important chemokine in EOSs. When inflammation occurs, EOSs aggregate and become activated, and a large amount of IL-5 is secreted, which leads to increased EOS proliferation and infiltration and leukotriene synthesis, resulting in a self-amplification effect and aggravation of the inflammatory response[32,33]. IL-5 and IL-4 can promote the expression of vascular endothelial cell adhesion molecules, thereby enhancing EOS, basophil, and endothelial cell binding and causing inflammatory cells to infiltrate local tissues. IL-5 not only activates EOSs but can also recruit transforming growth factor β and various cytokines to promote IgA production in B lymphocytes, all of which play an important role in the pathogenesis of HSP[34]. IL-5 and ECP-activated EOSs may be factors in the pathogenesis of purpura nephritis[35]. The serum ECP of children with HSP during the acute episode was significantly higher than that of healthy children and children in remission with hormone therapy. The serum ECP of those with kidney involvement was significantly higher than that of those without kidney involvement. Late respiratory tract infection in children leads to recurrent rash, joint pain, increased urinary protein, and EC. This leads to the progression of purpura nephritis, in which IL-5, ECP, and mast cells also play a certain role[36]. The current case was isolated, and whether there are other pathways between eosinophilia and HSP needs to be further investigated.
In this case, a 12-year-old boy diagnosed with IHES presented with dyspnea and lower limb pain as the first presentation, with only mild eosinophilia, and later developed HSP. Respiratory infections can cause recurrence of HSP and an increase in eosinophils. For atypical rare diseases with multidisciplinary treatment and poor prognosis, clinical expertise should be paid more attention.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dragonieri S, Italy; Ji X, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 2. | Markusse HM, Schravenhoff R, Beerman H. Hypereosinophilic syndrome presenting with diarrhoea and anaemia in a patient with systemic lupus erythematosus. Neth J Med. 1998;52:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kane SP. Ulcerative colitis with chronic liver disease, eosinophilia and auto-immune thyroid disease. Postgrad Med J. 1977;53:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Chaudhuri K, Dubey S, Zaphiropoulos G. Idiopathic hypereosinophilic syndrome in a patient with long-standing rheumatoid arthritis: a case report. Rheumatology (Oxford). 2002;41:349-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Arch Intern Med. 1999;159:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 489] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Liao YH, Su YW, Tsay W, Chiu HC. Association of cutaneous necrotizing eosinophilic vasculitis and deep vein thrombosis in hypereosinophilic syndrome. Arch Dermatol. 2005;141:1051-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Sui T, Li Q, Geng L, Xu X, Li Y. A case of hypereosinophilic syndrome presenting with multiorgan thromboses associated with intestinal obstruction. Turk J Haematol. 2013;30:311-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Li D, Xu L, Lin D, Jiang S, Feng S, Zhu L. Acute pulmonary embolism and deep vein thrombosis secondary to idiopathic hypereosinophilic syndrome. Respir Med Case Rep. 2018;25:213-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Todd S, Hemmaway C, Nagy Z. Catastrophic thrombosis in idiopathic hypereosinophilic syndrome. Br J Haematol. 2014;165:425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Grigoryan M, Geisler SD, St Louis EK, Baumbach GL, Davis PH. Cerebral arteriolar thromboembolism in idiopathic hypereosinophilic syndrome. Arch Neurol. 2009;66:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Schulman H, Hertzog L, Zirkin H, Hertzanu Y. Cerebral sinovenous thrombosis in the idiopathic hypereosinophilic syndrome in childhood. Pediatr Radiol. 1999;29:595-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Tai CP, Chung T, Avasarala K. Endomyocardial fibrosis and mural thrombus in a 4-year-old girl due to idiopathic hypereosinophilia syndrome described with serial cardiac magnetic resonance imaging. Cardiol Young. 2016;26:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Fujita K, Ishimaru H, Hatta K, Kobashi Y. Hypereosinophilic syndrome as a cause of fatal thrombosis: two case reports with histological study. J Thromb Thrombolysis. 2015;40:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Gao SJ, Wei W, Chen JT, Tan YH, Yu CB, Litzow MR, Liu QJ. Hypereosinophilic syndrome presenting with multiple organ infiltration and deep venous thrombosis: A case report and literature review. Medicine (Baltimore). 2016;95:e4658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Sakuta R, Tomita Y, Ohashi M, Nagai T, Murakami N. Idiopathic hypereosinophilic syndrome complicated by central sinovenous thrombosis. Brain Dev. 2007;29:182-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Yamada Y, Hoshino K, Shimojima N, Shinoda M, Obara H, Kawachi S, Fuchimoto Y, Tanabe M, Kitagawa Y, Morikawa Y. Idiopathic hypereosinophilic syndrome in a case with ABO-incompatible liver transplantation for biliary atresia complicated by portal vein thrombosis. Pediatr Transplant. 2010;14:e49-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Silva MS, Ramalho C, Ferreira F, Maia I, Joosten A. Idiopathic Hypereosinophilic Syndrome Presenting With Embolic Stroke. Cureus. 2021;13:e19307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Eisa N, Shaheen K, Alraiyes AH, Alraies MC. Loeffler's endocarditis with biventricular mural thrombi. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Numagami Y, Tomita T, Murakami K, Masaki I, Kubo K, Michiharu N. Sinus thrombosis in idiopathic hypereosinophilic syndrome causing fatal cerebral haemorrhage. J Clin Neurosci. 2008;15:585-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Chen TS, Xing LH, Wang SL, Liu QH, Zhao SL, Yuan CC. Pulmonary embolism, deep vein thrombosis and recurrent bone cysts in a patient with hypereosinophilic syndrome. Blood Coagul Fibrinolysis. 2016;27:831-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Terrier B, Piette AM, Kerob D, Cordoliani F, Tancrède E, Hamidou L, Lebbé C, Blétry O, Kahn JE. Superficial venous thrombophlebitis as the initial manifestation of hypereosinophilic syndrome: study of the first 3 cases. Arch Dermatol. 2006;142:1606-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Akuthota P, Weller PF. Spectrum of Eosinophilic End-Organ Manifestations. Immunol Allergy Clin North Am. 2015;35:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Rueff Rato I, Rigor J, Ferreira P, Laranjinha J, Santos-Silva G, Martins-Mendes D. Investigating Febrile Polyserositis: An Unusual Case of Idiopathic Hypereosinophilic Syndrome. Eur J Case Rep Intern Med. 2021;8:002426. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Butt NM, Lambert J, Ali S, Beer PA, Cross NC, Duncombe A, Ewing J, Harrison CN, Knapper S, McLornan D, Mead AJ, Radia D, Bain BJ; British Committee for Standards in Haematology. Guideline for the investigation and management of eosinophilia. Br J Haematol. 2017;176:553-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Karnak D, Kayacan O, Beder S, Delibalta M. Hypereosinophilic syndrome with pulmonary and cardiac involvement in a patient with asthma. CMAJ. 2003;168:172-175. [PubMed] |
| 26. | Noh HR, Magpantay GG. Hypereosinophilic syndrome. Allergy Asthma Proc. 2017;38:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Moertel CL, Kazacos KR, Butterfield JH, Kita H, Watterson J, Gleich GJ. Eosinophil-associated inflammation and elaboration of eosinophil-derived proteins in 2 children with raccoon roundworm (Baylisascaris procyonis) encephalitis. Pediatrics. 2001;108:E93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Meng X, Feng J, Zhou X, Zhu H. Hypereosinophilia with Concurrent Venous Thromboembolism: Clinical Features, Potential Risk Factors, and Short-term Outcomes in a Chinese Cohort. Sci Rep. 2020;10:8359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S-e496S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2569] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 30. | Diana A, Gaze H, Laubscher B, De Meuron G, Tschantz P. A case of pediatric Henoch-Schönlein purpura and thrombosis of spermatic veins. J Pediatr Surg. 2000;35:1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Dhaliwal KK, Lile NA, Tan CL, Lim CH. Life-threatening complications of Henoch-Schönlein purpura: diffuse alveolar haemorrhage, venous thrombosis and bowel ischaemia. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Horii Y, Muraguchi A, Iwano M, Matsuda T, Hirayama T, Yamada H, Fujii Y, Dohi K, Ishikawa H, Ohmoto Y. Involvement of IL-6 in mesangial proliferative glomerulonephritis. J Immunol. 1989;143:3949-3955. [PubMed] |
| 33. | Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N, Tominaga A, Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988;167:43-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 382] [Cited by in RCA: 398] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989;170:1415-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 298] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Kawasaki Y, Hosoya M, Suzuki H. Possible pathologenic role of interleukin-5 and eosino cationic protein in Henoch-Schönlein purpura nephritis. Pediatr Int. 2005;47:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Namgoong MK, Lim BK, Kim JS. Eosinophil cationic protein in Henoch-Schönlein purpura and in IgA nephropathy. Pediatr Nephrol. 1997;11:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |