Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.922
Peer-review started: September 29, 2022
First decision: December 13, 2022
Revised: December 31, 2022
Accepted: January 10, 2023
Article in press: January 10, 2023
Published online: February 6, 2023
Processing time: 129 Days and 15 Hours
Klippel-Trenaunay syndrome (KTS) is a congenital vascular malformation with a complicated etiology. It is sporadic and clinically rare in occurrence. The typical characteristics are capillary malformation (also known as port-wine stain), varicose veins and malformations, and bony and/or soft tissue hypertrophy with or without lymphatic malformation, which are known as the “classic clinical triad”. Herein, a rare case of KTS characterized by crossed-bilateral limb hyp
We described a 37-year-old female with KTS. She was admitted to our hospital owing to the gradual enlargement of the left lower extremity along with intermittent hematochezia and hematuria. The patient was diagnosed to have hemorrhoid bleeding by other hospitals and treated with conventional hemostatic drugs, but continued to have intermittent gastrointestinal bleeding and hematuria. Therefore, she visited our hospital to seek further treatment. During hospitalization, relevant imaging and laboratory examinations and colonoscopy were performed. In combination with the patient’s history and relevant examinations, we considered that the patient had a complex form of KTS. We recom
The clinical manifestations of KTS are extensive and diverse and chiefly include the typical triad. However, Vascular malformations of KTS can also involve several parts and systems such as digestive and urogenital systems. Therefore, the atypical manifestations and rare complications necessitate the clinician’s attention and are not to be ignored.
Core Tip: Klippel-Trenaunay syndrome (KTS) is a complicated, mixed, low-flow vascular malformation syndrome. Vessels that show abnormalities include skin capillaries, veins, and lymphatic vessels. Vascular malformation can lead to soft tissue and/or bony hypertrophy and, hence, KTS is also known as venous malformation and bone hypertrophy syndrome. However, Vascular malformations of KTS can also involve several parts and systems such as digestive and urogenital systems. KTS is a rare congenital disease and the clinical manifestations of it are extensive and diverse. This patient that we reported was initially misdiagnosed to have filariasis and hemorrhoid bleeding. Therefore, the atypical manifestations and rare complications necessitate the clinician’s attention and are not to be ignored.
- Citation: Li LL, Xie R, Li FQ, Huang C, Tuo BG, Wu HC. Easily misdiagnosed complex Klippel-Trenaunay syndrome: A case report. World J Clin Cases 2023; 11(4): 922-930
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/922.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.922
First described by the French physicians Klippel and Trénaunay in 1900, Klippel-Trenaunay syndrome (KTS) is also known as congenital venous malformation and bone hypertrophy syndrome. KTS is a rare disease with an incidence rate of 2-5/100000[1], involving multiple factors and its pathogenesis is not well understood. It exhibits a series of clinical symptoms and complications, including capillary malformation, varicose veins and malformations, and bony and/or soft tissue hypertrophy with or without lymphatic malformation, which often affects only one lower extremity. These symptoms are denoted as the “classic clinical triad”[2]. The clinical diagnosis of KTS is based on the presence of two of these features[3]. Moreover, some atypical KTS cases need to be diagnosed based on a detailed medical history combined with physical and imaging examinations. The disease is sporadic and rare and lacks any obvious aggregation tendencies of family, sex, and race[1,4]. In addition, the clinical manifestations are extensive and vary immensely. Therefore, there is no standard treatment for KTS. Because the syndrome often presents with swelling and varicose veins in the lower extremities, it is imperative to distinguish it from non-gene related diseases, such as filariasis and varicose veins that are not induced by vascular malformations, to avoid misdiagnosis. Vascular malformations associated with KTS can affect the extremities, particularly one lower extremity and, rarely, bilateral or upper extremities. In addition to the extremities, the splanchnic system can be involved. The involvement of the gastrointestinal tract may appear in the form of intermittent or continuous bleeding in the digestive tract, whereas the involvement of the urogenital system may appear in the form of life-threatening hematuria with massive bleeding[5]. According to the literature, KTS marked by the involvement of both the gastrointestinal and urogenital systems is rare (about 1%) and only one case of KTS with crossed-bilateral extremity involvement has been reported[6]. Herein, we have reported a case of KTS characterized by crossed-bilateral extremity involvement combined with lower gastrointestinal hemorrhage and hematuria. Based on our literature search, we could not find any other similar case. This patient was initially misdiagnosed in other hospitals to have filariasis and hemorrhoid bleeding.
A 37-year-old female presented to the gastroenterology clinic because of the gradual enlargement of the left lower extremity along with intermittent hematochezia and hematuria for 5 mo.
The patient had repeated hematochezia with intermittent gross hematuria for 5 mo, and the symptoms gradually worsened.
The female patient was found to have several port wine stains on the skin of her left lower extremity and hip after birth. In addition, her left lower extremity was found to be larger than the right lower extremity since childhood, which gradually increased with age and was accompanied with pain and heaviness. At the age of 14 years, she was diagnosed with “filariasis” by other hospitals; however, no improvement was noted after the anti-filarial treatment. Repeated hematochezia began to appear at the age of 7 years. The bleeding frequency was low (3-5 times/mo), and the amount was small. As the problem was self-limiting and did not affect her normal life, the patient did not undergo enteroscopy or professional treatment. However, the hematochezia gradually worsened and intermittent gross hematuria appeared. She was admitted to another hospital and was diagnosed to have hemorrhoid bleeding. She was treated with conventional hemostatic drugs, but continued to have intermittent gastrointestinal bleeding and hematuria.
Her parents did not have a consanguineous marriage, and nobody else in the family had a similar medical history.
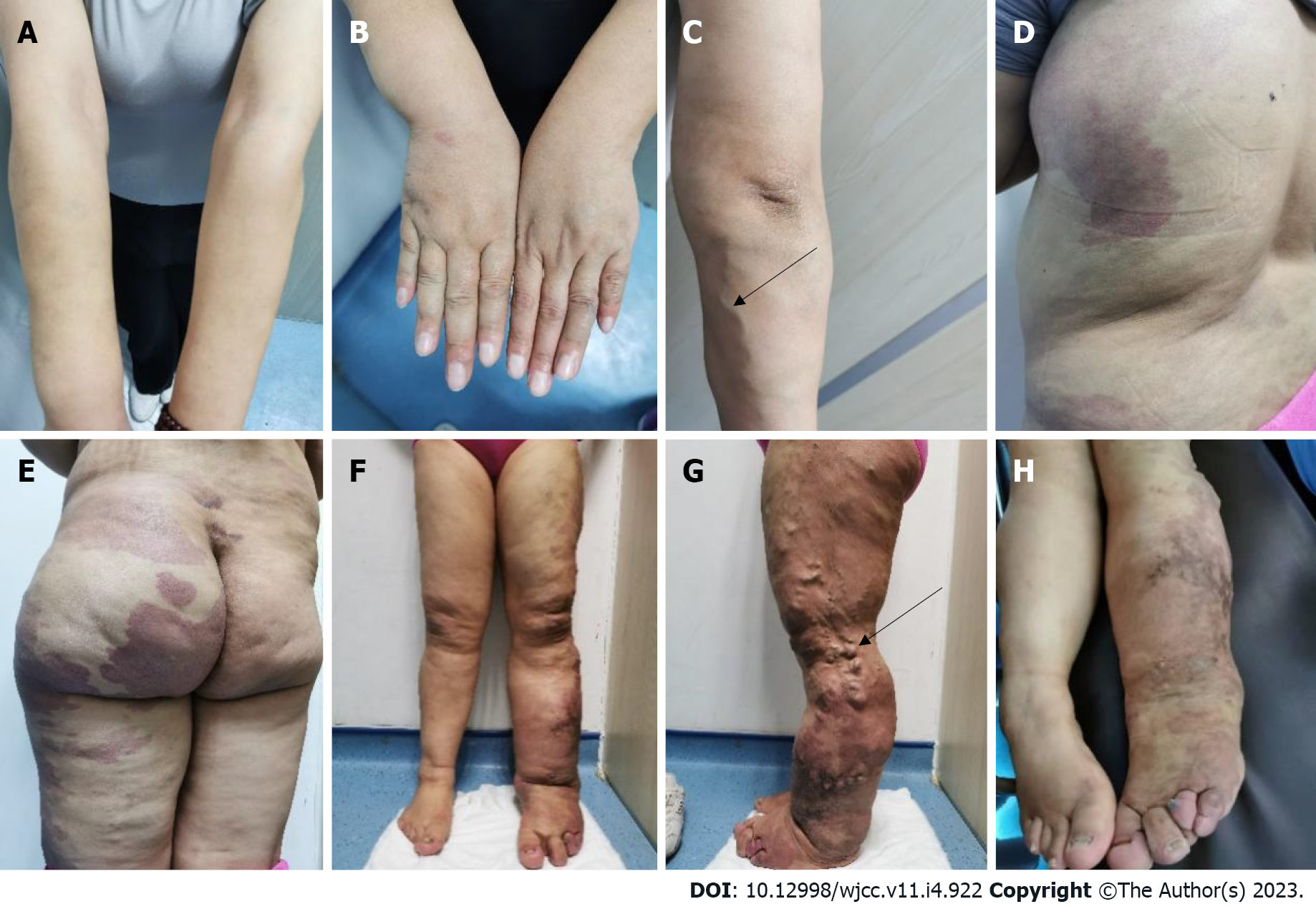
The patient walked with asymmetry in both lower extremities and mild claudication. Her skin and sclera were pale. There was a mild enlargement in the right arm, and her fingers were thickened. There was a small piece of port-wine stain on the back of her hand (Figure 1A and B), and the varicose veins were seen on the forearm (black arrows in Figure 1C). Furthermore, there were several map-like port wine stains on the left lower extremity, hip, and trunk (Figure 1D-H). The left hip and the left extremity were obviously enlarged, with giant deformed toes (Figure 1E-H) and atypical varicose veins on the outside of the leg (black arrows in Figure 1G).
Blood routine: Hemoglobin 88g/L. Liver function was normal.
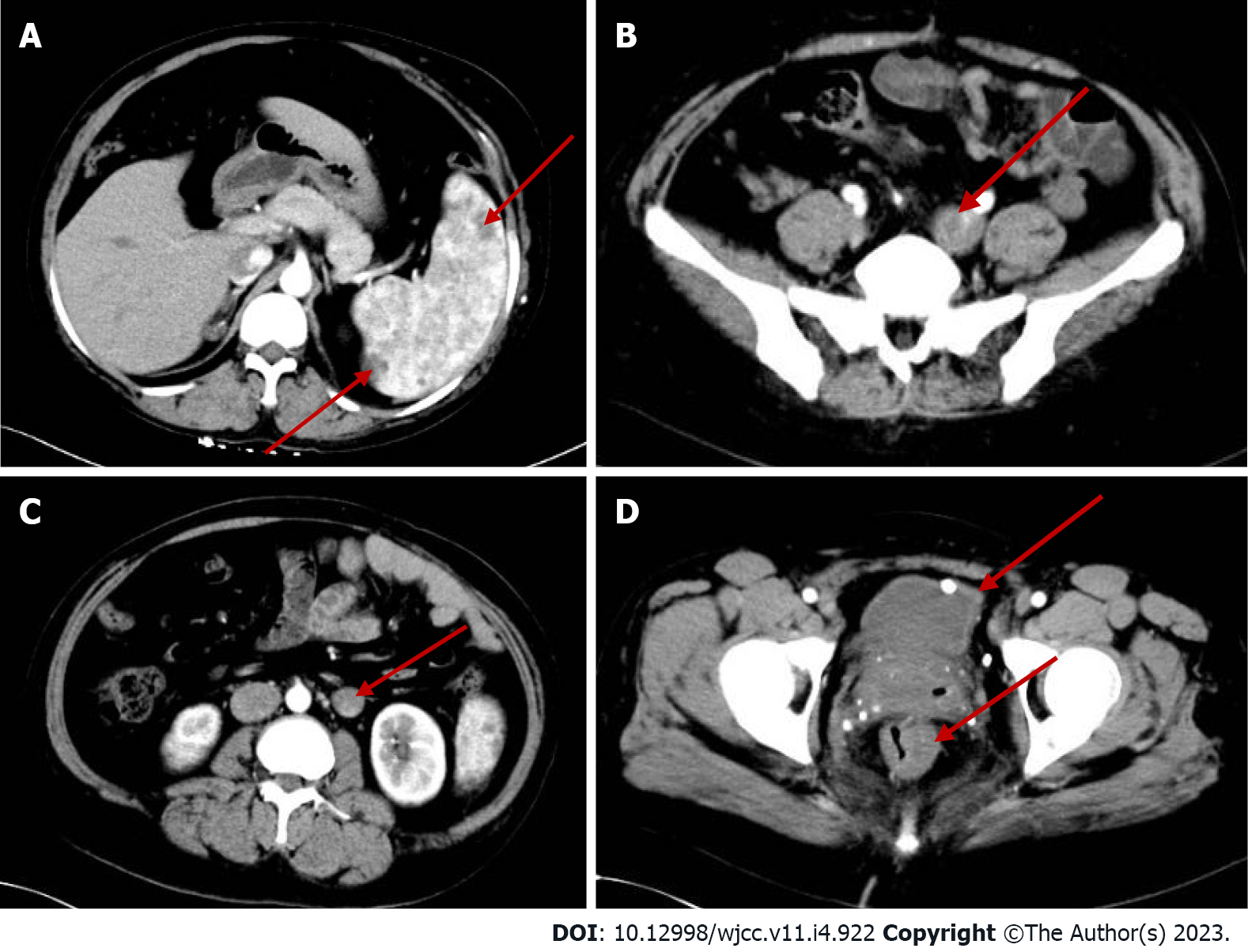
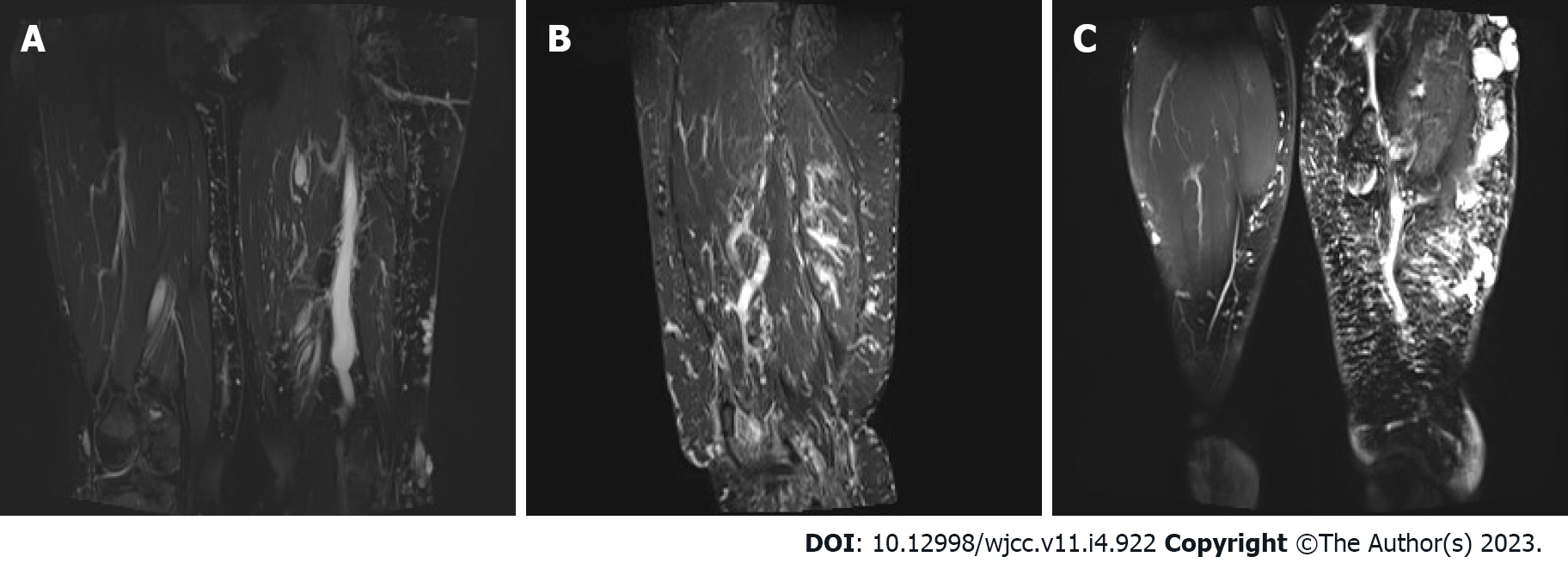
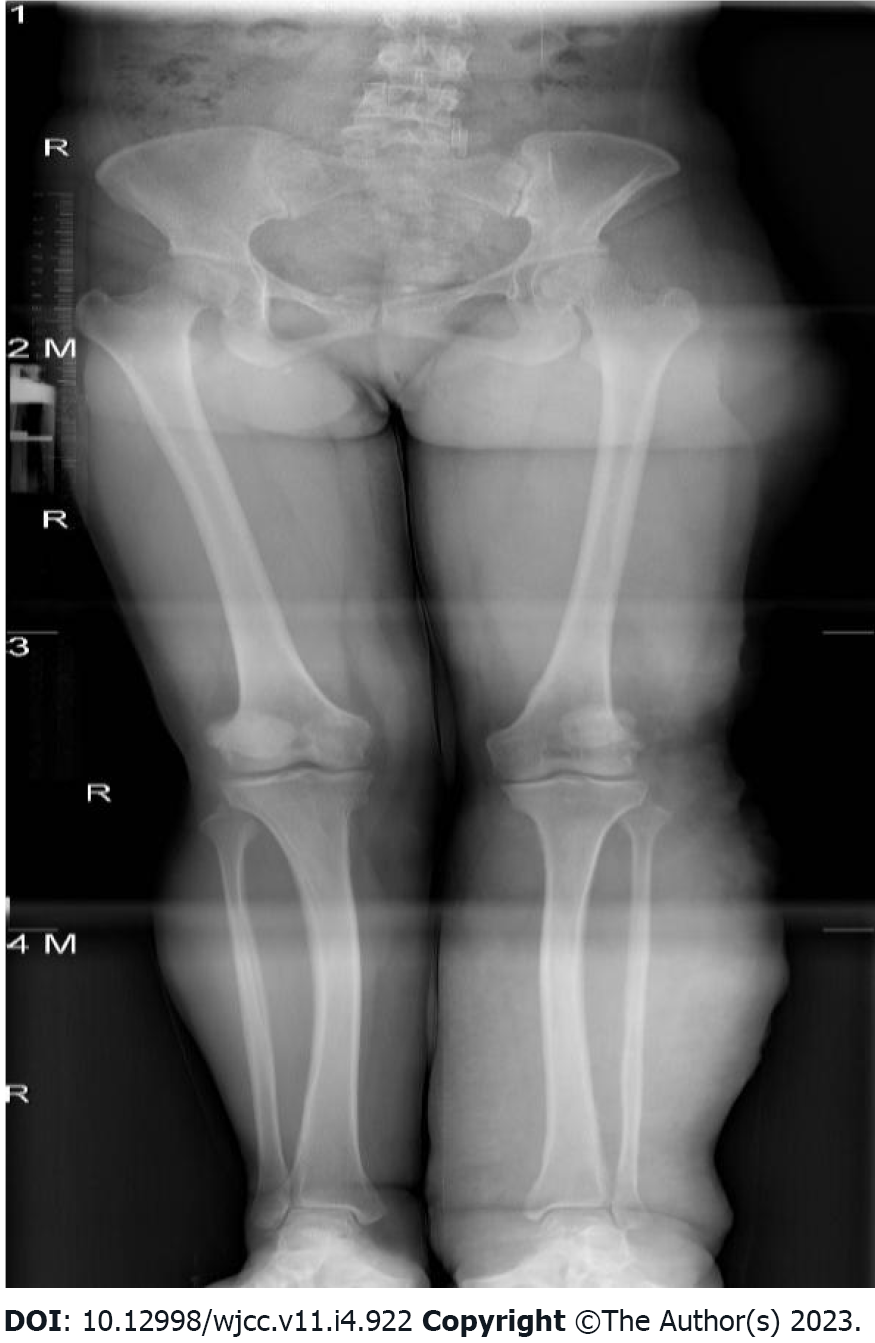
Abdominal computed tomography (CT)-plain scan + enhancement: The spleen was large, with multiple low-density shadows, which were considered to be vascular lesions (hemangioma). The rectal wall was unevenly thickened, and the enhancement was not uniform. The left ovarian vein was thickened, with a filling-defect area. The left iliac vein and pelvic floor vein were slightly thickened and tortuous. Multiple speckled calcium density shadows were observed in the pelvic cavity. The bladder wall was slightly thickened, and venous stones were detected (Figure 2). Left lower extremity magnetic resonance imaging (MRI)-plain scan + enhancement: There was an obvious enlargement in the left lower extremity, with multiple tortuous strip long T1/T2 and enhanced signals in the subcutaneous soft tissues, muscle space, and the muscle soft tissue area. Subcutaneous varicose veins were prominent on the left leg, with an obvious enhancement. Multiple reticular long T2WI signal shadows were observed in the surrounding muscles and soft tissues, with mild enhancement (Figure 3). X-ray of both lower extremities: The spine exhibited a slight compensatory deviation to the left. The soft tissue of the left lower extremity was thickened, with nonuniform density and a normal bone structure (Figure 4).
Based on the patient’s medical history and the various clinical presentation and related examinations and tests, she was diagnosed with a complex form of KTS.
As the patient was currently not showing any symptoms of hematuria and no active colonic bleeding was observed in the colonoscopy, no other drug or treatment was administered. However, in order to prevent the aggravation of the patient’s condition and avoid gastrointestinal rebleeding, we recommended a combined diagnosis and treatment from the vascular, interventional, anorectal, and other departments, although she declined any further treatment for financial reasons. Presently, the patient is on conventional hemostatic drugs and iron treatment.
The patient continues to experience intermittent and a small amount of hematochezia (similar to hemorrhoid bleeding), which is not life-threatening. The patient has accordingly been advised regular medical consultation. If the bleeding worsens, surgery can be considered.
KTS is a complicated, mixed, and low-flow vascular malformation syndrome. The vessels that displayed abnormalities include skin capillaries, veins, and lymphatic vessels. Vascular malformation can lead to soft tissues and/or bony hypertrophy; hence, KTS is also known as venous malformation and bone hypertrophy syndrome[7]. The etiology of KTS is complex and controversial. The somatic PIK3CA mutation is believed to cause abnormal hyperplasia of the blood vessels, bone, and soft tissues[6-8]. Furthermore, chromosome translocation may be involved[9]. A recent theory suggested that this disease is related to mesodermal dysplasia acting on angiogenesis[10].
The clinical manifestations of KTS are extensive and diverse and chiefly include the typical triad (port-wine stains, varicose veins with or without venous malformations, and bony and/or soft tissue hypertrophy). The complete triad occurs in 63% of the patients and is either found at birth or develops during infancy and becomes more and more evident with age[11,12]. The patient reported in this study also exhibited similar findings. Port-wine stain is considered to be caused by skin capillary malformation, while some scholars claim that it is related to lymphoid malformation[13]. The stain has a map-like, segmental distribution.
Sreekar et al[14] demonstrated that venous malformations in patients with KTS can involve multiple parts and systems. Nonetheless, most of the extremity involvement is unilateral (approximately 85%)[15], especially unilateral lower extremity. In rare cases, the upper extremities, trunk, head, and neck can be involved and is usually manifested as varicose veins. Two persistent embryonic veins are common in the abnormal veins of patients with KTS: The lateral marginal vein (or the Servelle vein) and the sciatic vein. The servelle vein is present on the outside of the thigh and displays obvious varicose and malformations. There is a controversy regarding whether patients with KTS exhibit deep venous abnormalities. In this case, Port-wine stain was observed in the trunk, left lower extremity, hip, and the back of the right hand (Figure 1). Mild varicose veins were observed on the right arm (Figure 1C). Obvious varicose Servelle veins were detected on the outside of the left lower extremity (Figure 1G). The right hip was more hypertrophic than the left one. This case demonstrated cross bilateral hypertrophy of the left lower and right upper extremities (Figure 1), which is quite rare and has only been reported once[6]. In addition, the patient’s left toe was giant and deformed (Figure 1H), possibly related to KTS[16]. Meanwhile, the MRI of the lower extremity demonstrated significant enlargement, varicose veins and malformations, and lymphatic abnormality in the left lower extremity (Figure 3), which is consistent with the clinical characteristics of KTS. In addition to the extremity involvement, the splanchnic system can be rarely involved in patients with KTS, including the gastrointestinal tract, urogenital tract, liver, and spleen. In this case, the patient displayed intermittent hematochezia and hematuria. The relevant examination, that is, abdominal CT splenomegaly, exhibited splenic hemangioma, thickened rectal wall, thickened bladder wall, venous stone, and thickened iliac vein and ovarian vein (Figure 2). Colonoscopy revealed extensive varicose vein malformations of the intestinal wall (Figure 5). All these findings proved that the patient had involvement of the intestine, urogenital tract, and spleen as well as a wide range of vascular lesions. Therefore, her condition was complex and serious, for which she needed active treatment. Intestinal involvements are mostly observed in the sigmoid colon and rectum, and only a few were recorded in the entire intestine. This problem is often manifested as asymptomatic-repeated hematochezia, which can, occasionally, be life-threatening and cause massive bleeding. In case of no bleeding, the intestinal involvement may be ignored. Bleeding often occurs in the first 10 years of life[5,17]. As for the involvement of the genitourinary system, it is often manifested in the form of bleeding, such as hematuria, increased menstruation, and massive uterine bleeding during childbirth. However, the splenic involvement may be more serious because the spontaneous rupture of splenic hemangioma is life-threatening and requires emergency surgery[18,19]. Although our patient showed abnormal blood vessels in the spleen, no such acute bleeding occurred.
The most common symptoms of KTS are swelling and pain in the extremity, accompanied by obvious heaviness caused by venous disfunction and abnormal lymphatic drainage[20,21]. Other atypical manifestations include infectious cellulitis, extremity ulcer, headache, intracerebral hemorrhage, hydrocephalus, seizures[14,22,23], and even poor vision when the eyes are involved[24]. In addition, other rare complications, such as deep venous thrombosis, venous thromboembolism, pulmonary embolism, thrombophlebitis, and gangrene, may occur in the patients[25]. The abovementioned atypical manifestations and rare complications necessitate the clinician’s attention and are not to be ignored.
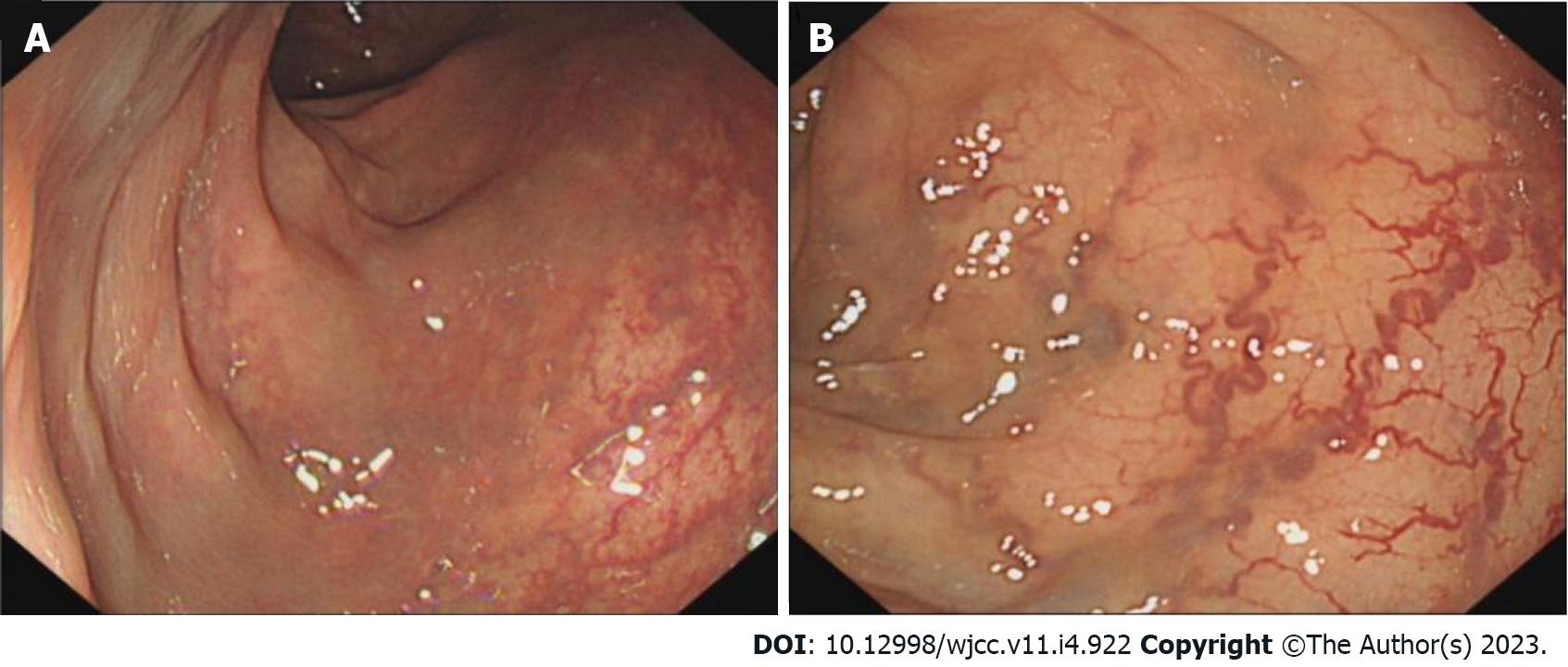
Being a rare disease with a complex etiology, KTS can only be diagnosed clinically as no universal diagnostic criteria have been reported and pathological diagnosis is barely possible. When the patients display some atypical symptoms, the diagnosis is made based on medical history and related examinations. Based on the clinical characteristics of KTS, noninvasive imaging is the first choice to facilitate the diagnosis. Lower extremity ultrasound is the preferred modality as it can clearly display the blood flow characteristics as well as venous malformations and exhibits high sensitivity and specificity[7]. Moreover, X-ray can judge the length of bones as well as bony hypertrophy and reveal the hypertrophy of the soft tissues. CT venography or magnetic resonance venography was performed when deep vein abnormalities were considered. Most scholars believe that MRI is necessary for the diagnosis of KTS owing to its high resolution for the soft tissues and its ability to demonstrate abnormalities in the lymphatic vessels. CT can also be employed for the diagnosis of KTS, albeit it is not the first choice and not employed in routine examination. The method should be avoided in children and patients with KTS who exhibited renal disfunction because of radiation hazard and possible renal damage. When considering KTS combined with pulmonary embolism, pulmonary CT angiography is preferred. If the gastrointestinal tract is involved or if the patient has hemochezia, endoscopy is essential because it can help directly visualize the blood vessels of the digestive tract and judge the bleeding site. If necessary, local hemostasis can also be performed and is of a high diagnostic value. In the present case, the patient underwent colonoscopy, which exhibited clustered abnormal expansion of the blood vessels in the intestinal wall below the descending colon (Figure 5). This finding suggested unique vascular manifestations and helped avoid misdiagnosis.
The mutations of somatic PIK3CA are considered to be one of the causes of vascular malformation and limb hypertrophy of KTS. Therefore, KTS is included in the PIK3CA-related overgrowth spectrum (PROS)[26]. PROS is defined as a series of rare congenital disease syndromes due to mutations in PIK3CA. PROS is one of the subcategories in the latest (2018) ISSVA classification, which, in addition, to including KTS, it also includes fibroadipose hyperplasia or overgrowth; congenital lipomatous overgrowth, vascular malformations, epidermal nevi, scoliosis/skeletal and spinal (CLOVES) syn
Until date, there is no consensus available on the treatment of KTS. The syndrome cannot be completely cured, and symptomatic treatment is mostly given. The disease is characterized by multiple involvements, which have enormous physical and mental impacts. Lameness due to limb deformity and hypertrophy poses inconvenience in daily living and mars the appearance, which may cause psychological disorders accompanied by long-term recurrent pain[26]. Therefore, multidisciplinary cooperative treatment aimed at alleviating the symptoms, delaying disease progression as well as treatment complications, and improving the quality of life of the patients is essential in managing KTS[3,25,28]. Varicose veins can be treated by methods such as compression with elastic socks, raising the affected extremity, and other physical therapies as well as changing the lifestyle and keeping the affected extremity clean. Port-wine stain can be treated with a laser. Infectious cellulitis and thrombophlebitis are usually treated with antibiotics and symptomatic analgesic treatment in case of pain. Anticoagulant therapy is used to treat acute thrombosis, and prophylactic medications are given before operation[29]. In some cases, surgery is needed; for instance, surgical treatment is required in case of life-threatening gastrointestinal and urogenital bleeding or splenic rupture and bleeding. To manage hypertrophy of the extremity, it is possible to reduce weight via surgery. Nevertheless, symptomatic treatment combined with psychotherapy is the best approach for KTS[30].
KTS is a congenital vascular malformation with a complicated etiology. The somatic PIK3CA mutation is believed to cause abnormal hypertrophy of the blood vessels, bone, and soft tissues. The clinical manifestations of KTS are extensive and diverse and chiefly include the typical triad. However, vascular malformations of KTS can also involve several parts and systems such as digestive and urogenital systems, which increases the complexity and severity of the disease. Therefore, the atypical manifestations and rare complications necessitate the clinician’s attention and are not to be ignored.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar S, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Permatananda PANK, I Gusti Agung Made Adnyana Putra. Klippel Trenaunay Syndrome: A Brief Overview. Bsm. 2021;5:387-394. [DOI] [Full Text] |
| 2. | Marvin EK, Schoch JJ, Nguyen H, Anderson KR, Driscoll DJ, Rose CH, Bendel EC, Tollefson MM. Venous thromboembolic and bleeding complications among pregnant women with Klippel-Trenaunay syndrome. J Am Acad Dermatol. 2019;81:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Lee A, Driscoll D, Gloviczki P, Clay R, Shaughnessy W, Stans A. Evaluation and management of pain in patients with Klippel-Trenaunay syndrome: a review. Pediatrics. 2005;115:744-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Wang ZK, Wang FY, Zhu RM, Liu J. Klippel-Trenaunay syndrome with gastrointestinal bleeding, splenic hemangiomas and left inferior vena cava. World J Gastroenterol. 2010;16:1548-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (3)] |
| 5. | Samo S, Sherid M, Husein H, Sulaiman S, Yungbluth M, Vainder JA. Klippel-Trenaunay Syndrome Causing Life-Threatening GI Bleeding: A Case Report and Review of the Literature. Case Rep Gastrointest Med. 2013;2013:813653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Al-Najjar RM, Fonseca R. An atypical case of Klippel-Trénaunay syndrome presenting with crossed-bilateral limb hypertrophy and postaxial polydactyly: a case report. BMC Pediatr. 2019;19:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Deka JB, Deka NK, Shah MV, Bhatnagar N, Nanni AL, Jimenez F. Intraneural hemangioma in Klippel-Trenaunay syndrome: role of musculo-skeletal ultrasound in diagnosis-case report and review of the literature. J Ultrasound. 2020;23:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | John PR. Klippel-Trenaunay Syndrome. Tech Vasc Interv Radiol. 2019;22:100634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Oduber CE, van der Horst CM, Hennekam RC. Klippel-Trenaunay syndrome: diagnostic criteria and hypothesis on etiology. Ann Plast Surg. 2008;60:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 10. | Oluwafemi A, Omokafe L, Maduka Ogechi C, Ganiyu A, Bako I, Oluleke I, Ifeoma I. Klippel-trenaunay syndrome: A rare case presenting in a 5-year-old girl. West Afr J Radiol. 2016;23:136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Kocaman O, Alponat A, Aygün C, Gürbüz Y, Sarisoy HT, Celebi A, Sentürk O, Hülagü S. Lower gastrointestinal bleeding, hematuria and splenic hemangiomas in Klippel-Trenaunay syndrome: a case report and literature review. Turk J Gastroenterol. 2009;20:62-66. [PubMed] |
| 12. | Harna B, Tomar S. Klippel Trenaunay Syndrome. Indian J Pediatr. 2020;87:966-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Maari C, Frieden IJ. Klippel-Trénaunay syndrome: the importance of "geographic stains" in identifying lymphatic disease and risk of complications. J Am Acad Dermatol. 2004;51:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Sreekar H, Dawre S, Petkar KS, Shetty RB, Lamba S, Naik S, Gupta AK. Diverse manifestations and management options in Klippel-Trenaunay syndrome: a single centre 10-year experience. J Plast Surg Hand Surg. 2013;47:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Baba A, Yamazoe S, Okuyama Y, Shimizu K, Kobashi Y, Nozawa Y, Munetomo Y, Mogami T. A rare presentation of Klippel-Trenaunay syndrome with bilateral lower limbs. J Surg Case Rep. 2017;2017:rjx024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Redondo P, Bastarrika G, Aguado L, Martínez-Cuesta A, Sierra A, Cabrera J, Alonso-Burgos A. Foot or hand malformations related to deep venous system anomalies of the lower limb in Klippel-Trénaunay syndrome. J Am Acad Dermatol. 2009;61:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Shaikh OH, Kumbhar US, Jain A, Chakkalakkoombil SV. Klippel-Trenaunay syndrome in a young patient with the involvement of gastrointestinal and genitourinary tracts: an unusual and rare presentation. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 18. | Karakayali F, Basaran C, Soy EA, Karakus S, Yabanoglu H, Moray G, Haberal M. Spontaneous spleen rupture and rectus sheath hematoma in a patient with Klippel-Trenaunay syndrome: report of a case. Surg Today. 2010;40:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | de Luna Díaz Mdel M, de Laguno de Luna A, Osorio D, Arenzana LM, Eslava Y. [Spontaneous spleen rupture in a patient with Klippel-Trenaunay syndrome]. Cir Esp. 2011;89:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Cohen MM Jr. Klippel-Trenaunay syndrome. Am J Med Genet. 2000;93:171-175. [PubMed] [DOI] [Full Text] |
| 21. | Hale EK. Klippel-Trenaunay syndrome. Dermatol Online J. 2002;8:13. [PubMed] |
| 22. | Anlar B, Yalaz K, Erzen C. Klippel-Trenaunay-Weber syndrome: a case with cerebral and cerebellar hemihypertrophy. Neuroradiology. 1988;30:360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Gupte GL, Deshmukh CT, Bharucha BA, Irani SF. Klippel-Trenaunay-Weber syndrome with hydrocephalus: an unusual association. Pediatr Neurosurg. 1995;22:328-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Olcaysu OO, Altun A, Olcaysu E, Marzıoğlu Ozdemır E, Demır B. Unilateral cataract and vitreoretinopathy in a case with klippel-trenaunay syndrome. Case Rep Ophthalmol Med. 2014;2014:312030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Asghar F, Aqeel R, Farooque U, Haq A, Taimur M. Presentation and Management of Klippel-Trenaunay Syndrome: A Review of Available Data. Cureus. 2020;12:e8023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Vahidnezhad H, Youssefian L, Uitto J. Klippel-Trenaunay syndrome belongs to the PIK3CA-related overgrowth spectrum (PROS). Exp Dermatol. 2016;25:17-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Bertino F, Braithwaite KA, Hawkins CM, Gill AE, Briones MA, Swerdlin R, Milla SS. Congenital Limb Overgrowth Syndromes Associated with Vascular Anomalies. Radiographics. 2019;39:491-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Harvey JA, Nguyen H, Anderson KR, Schoch JJ, Bendel EC, Driscoll DJ, Palmer BA, Tollefson MM. Pain, psychiatric comorbidities, and psychosocial stressors associated with Klippel-Trenaunay syndrome. J Am Acad Dermatol. 2018;79:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Redondo P, Bastarrika G, Sierra A, Martínez-Cuesta A, Cabrera J. Efficacy and safety of microfoam sclerotherapy in a patient with Klippel-Trenaunay syndrome and a patent foramen ovale. Arch Dermatol. 2009;145:1147-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Noel AA, Gloviczki P, Cherry KJ Jr, Rooke TW, Stanson AW, Driscoll DJ. Surgical treatment of venous malformations in Klippel-Trénaunay syndrome. J Vasc Surg. 2000;32:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |