Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.909
Peer-review started: October 13, 2022
First decision: November 30, 2022
Revised: December 29, 2022
Accepted: January 12, 2023
Article in press: January 12, 2023
Published online: February 6, 2023
Processing time: 116 Days and 2.9 Hours
Hemophagocytic lymphohistiocytosis (HLH) is a rare life-threatening disorder, often resulting in the immune-mediated injury of multiple organ systems, including primary HLH and secondary HLH (sHLH). Among them, sHLH results from infections, malignant, or autoimmune conditions, which have quite poor outcomes even with aggressive management and are more common in adults.
We report a rare case of a 36-year-old female manifested with sHLH on backgr
The case showed sHLH, thrombotic microvascular, and infection in the whole course of the disease, which was rarely reported by now. The treatment of the patient emphasizes that early recognition and treatment of sHLH in SLE patients was of utmost importance to improve the prognosis and survival rate of patients.
Core Tip: Hemophagocytic lymphohistiocytosis (HLH) is a rare life-threatening disorder, including primary HLH and secondary HLH (sHLH). We report a rare case of a 36-year-old female manifested with sHLH on background with systemic lupus erythematosus (SLE) and related with decreased activity of natural killer cells according to whole exon gene sequencing. Our study expanded the thoughts on the diagnosis and treatment of HLH in SLE patients.
- Citation: Peng LY, Liu JB, Zuo HJ, Shen GF. Unusual presentation of systemic lupus erythematosus as hemophagocytic lymphohistiocytosis in a female patient: A case report. World J Clin Cases 2023; 11(4): 909-917
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/909.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.909
Hemophagocytic lymphohistiocytosis (HLH) is a rare potentially life-threatening disorder, resulting in pathologic immune activation mediated multi-organ dysfunction[1,2]. It can be divided into primary HLH and secondary HLH. Primary HLH is an inherited autosomal recessive disorder that often manifests in the pediatric population[2]. Secondary HLH (sHLH) results from infections, malignancy, or autoimmune condition, and more commonly manifests among adults[3]. The clinical characteristics of HLH vary, but can include prolonged fever, lymphadenopathy, hepatosplenomegaly, and elevated levels of alanine aminotransferase, aspartate aminotransferase, triglyceride, and ferritin[4,5].
Systemic lupus erythematosus (SLE) is an autoimmune condition that is strongly associated with HLH[6]. In SLE patients, the estimated prevalence of co-occurrence of sHLH has been reported to be 0.9%-4.6%[7,8]. Here, we report a rare case of a young female with SLE accompanied by HLH. The patient’s symptoms and laboratory abnormalities improved dramatically after two hospital admissions to our department, and no relapse of HLH symptoms was detected during the 1-year follow-up.
The 36-year-old Han Chinese woman was admitted to our hospital following two-month recurrent high-grade fever (> 39°C).
The patient initially presented to our hospital with Intermittent high-grade fever on January 4, 2021. The fever lasted for more than 20 d peaking at 40.4°C. She previously visited a local hospital and the laboratory results showed a reduced total white blood cells (WBC) count (1.8 × 109/L), and reduced counts of lymphocytes (0.4 × 109/L) and neutrophils (1.24 × 109/L). Dramatically increased C-reactive protein (CRP) levels (54.4 mg/L) were also detected. The patient was treated with broad-spectrum antibiotics (detailed drug names, duration, and dosages were not known). However, after the symptoms did not improve, the patient was finally admitted to our hospital for further treatment.
The patient had been diagnosed with SLE for more than 9 years and was treated with prednisone (10 mg per day). She did not report any history of chronic respiratory disease or surgical procedures. No travel history, infectious exposure was reported. She was allergic to quinolones.
There was nothing of note in the patient’s personal or family history.
Upon admission, the patient was conscious and her temperature was 39.5°C, her heart rate was 119 beats/min, and her blood pressure was 111/86 mmHg. No enlarged lymph nodes were found. Systemic examination found no signs of hepatosplenomegaly, abdominal tenderness, or rebound pain.
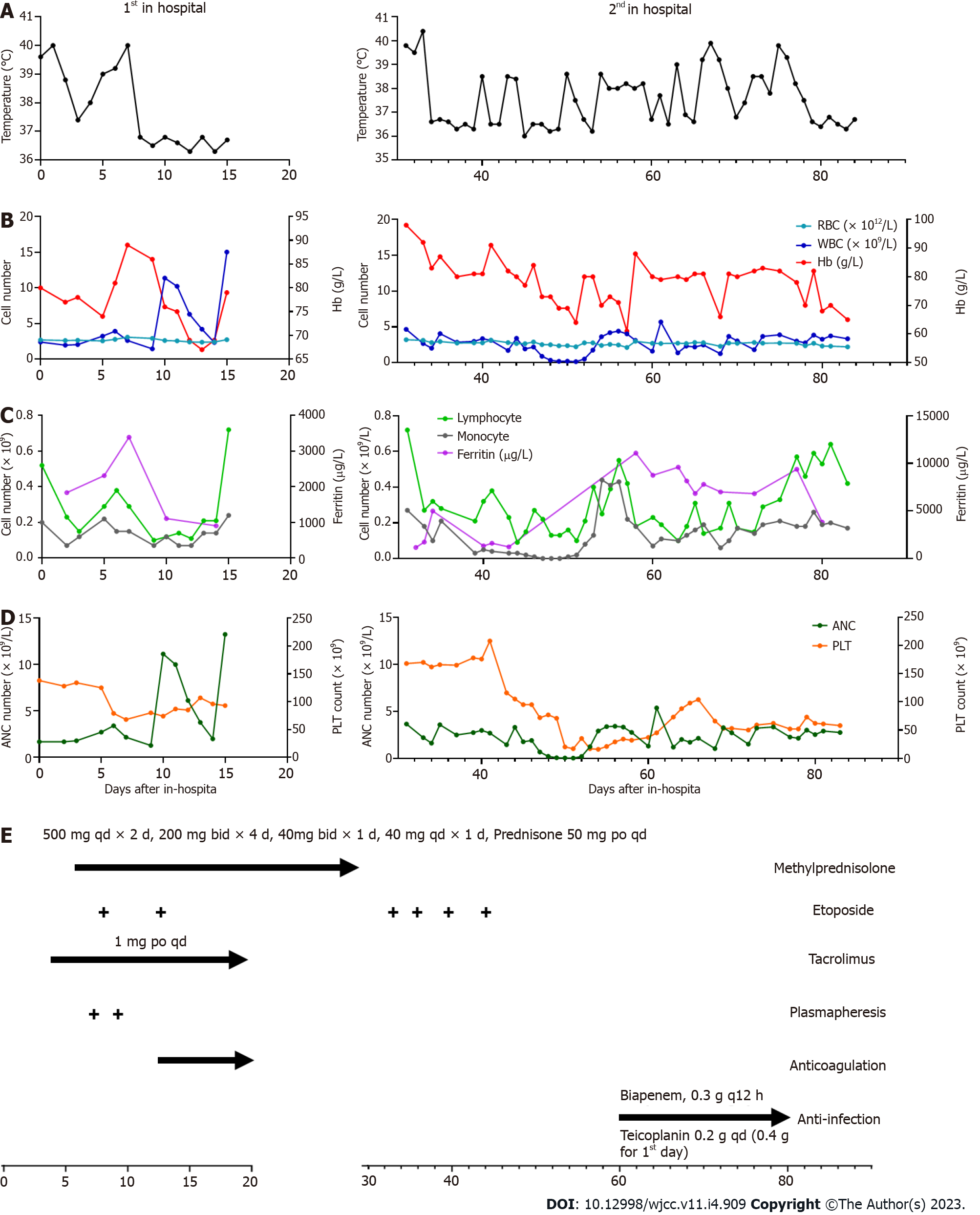
Laboratory results on admission revealed decreased levels of WBCs (2.41× 109/L), neutrophils (1.68 × 109/L), red blood cells (RBCs, 2.71 × 1012/L), hemoglobin (Hb, 80 g/L), and serum calcium (CA, 2.00 mmol/L), and increased levels of ferritin (1835.3 µg/L), CRP (62.04 mg/L), procalcitonin (0.71 ng/mL), erythrocyte sedimentation rate (138 mm/h), interleukin (IL)-10 (5.9 pg/mL), IL-6 (328.56 pg/mL), IL-2R (1060 U/mL), and interferon (IFN)-γ (65.29 pg/mL). The patient tested positive for anti-nuclear antibody (ANA) (titer > 1:1000) and anti-SSA antibody (> 400.00 RU/mL), but negative for other antibodies including anti-dsDNA antibody, anti-Smith antibody, and antiphospholipid antibody. Hypertriglyceridemia was also detected in the patient. The results of other investigations are shown in Figure 1A-D.
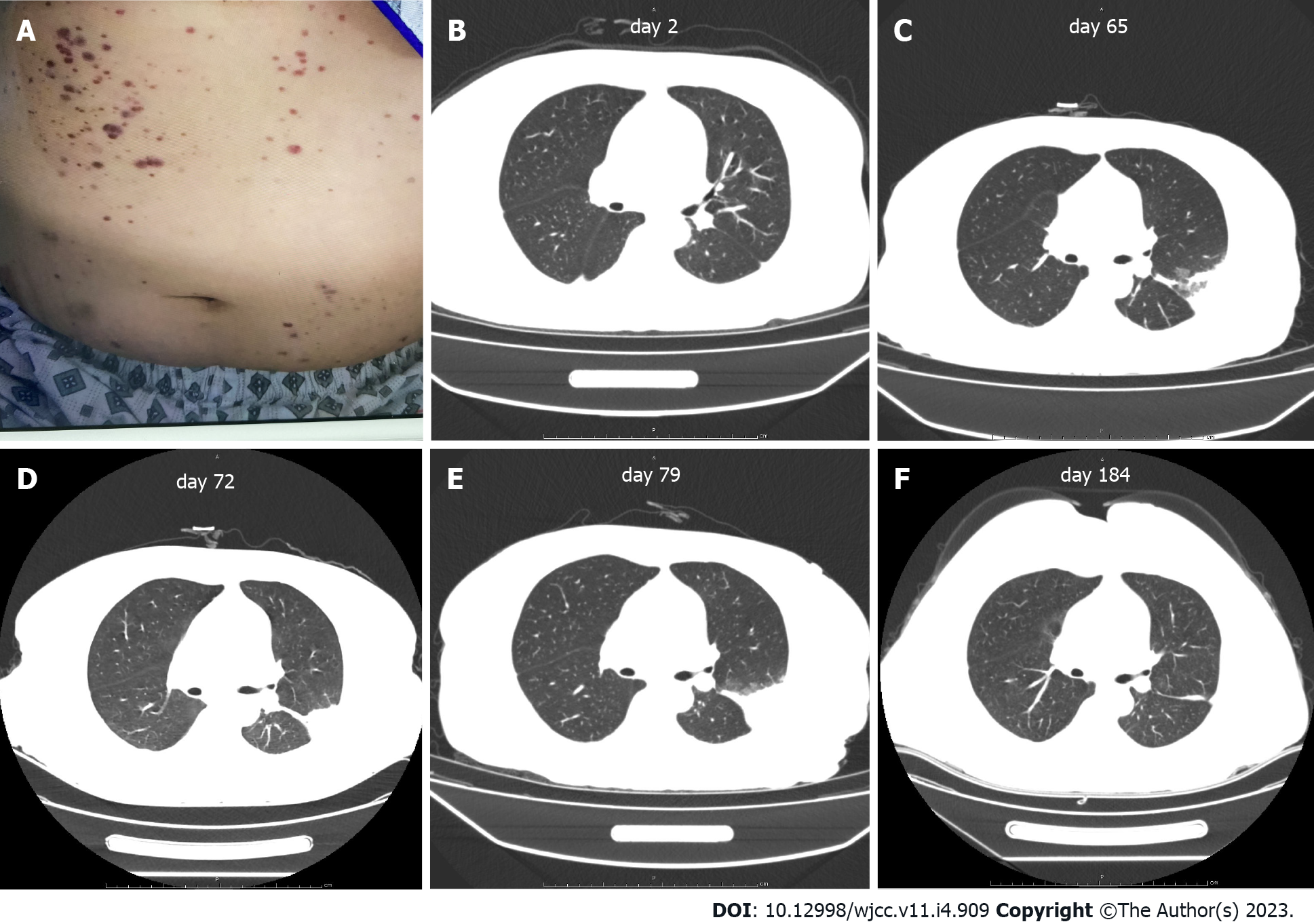
Computed tomography (CT) of the chest revealed patchy shadows in the lower lobe of the left lung (Figure 2B). Ultrasound of the abdomen and pelvis showed no sign of hepatosplenomegaly.
After 3-d of anti-infective treatment (injection of dexamethasone and cefoperazone sodium), the patient’s temperature decreased to 37.4°C. To investigate the cause of the persistent high fever in this patient, bone marrow aspiration was performed three days after admission (day 3) and showed normal with no significant hemophagocytosis (Supplementary Figure 1A). Examination of the peripheral blood revealed an increased proportion of neutrophils, toxic particles within the cytoplasm, variation in the size of mature red blood cells, with some arranged in straight lines, and clearly visible platelets that appeared both scattered and aggregated (Supplementary Figure 1B).
In addition, we performed the whole exon gene sequencing to screen for genetic diseases and revealed missense mutations in the LYST gene [c.910G>A (p.D304N)], ATM gene [c.8071C>T (p.R2691C)], and FERMT1 gene [c.1590A>T (p.K530N)]. Among them, LYST gene is involved in lysosomal transport regulation. The abnormality mutation in LYST gene may cause lysosomal membrane fusion disorder, deposition of giant cytoplasmic particles, inducing immunodeficiency syndrome-related HLH. ATM gene encodes a key kinase of cell cycle checkpoint, which plays an important role in cell cycle regulation and DNA damage response. Abnormality of ATM gene may lead to Ataxia-telangiectasia (A-T), which is characterized by immune deficiency, ataxia, telangiectasia, chromosome instability, tumor susceptibility. The protein encoded by FERMT1 gene is involved in integrin signal transmission and the linkage between actin skeleton and outer matrix. Genetic abnormalities of FERMT1 can lead to FERMT1-related immunodeficiency characterized by skin diseases, photosensitivity, and autoimmune diseases. Next-generation sequencing of microorganism infection in peripheral blood also showed negative results.
The patient was diagnosed with SLE according to clinical guidelines[9], with an SLE Disease Activity Score (SLE- DAS) of 55[10] indicating that she was in the active stage of SLE after a 9-year disease duration. On evaluation, altogether four of the eight diagnostic criteria of HLH were fulfilled[11], although her bone marrow biopsy showed no hemophagocytosis. A diagnosis of HLH secondary to SLE was made (Table 1). The diagnosis of the patient also included thrombotic microangiopathy and severe pulmonary infection. Thrombotic microangiopathy was diagnosed according to a combination of symptoms and signs, including scattered petechiae and ecchymoses over her abdomen and extremities. Severe pulmonary infection was diagnosed based on uncontrolled recurrent fever and chest CT findings. Recovered infection foci in the lungs after continuous antimicrobial treatment also aided the diagnosis of severe pulmonary infection.
| Variables | Patient |
| Baseline characteristics | |
| Age (yr) | 36 |
| Etiology/Trigger | SLE and multiple infections |
| HLH- directed therapies | VP16 and methylprednisolone |
| HLH- 2004 criteria at diagnosis (ref.: Henter-2007) | |
| Fever | Y |
| Splenomegaly | N |
| Cytopenia, affecting 2 of 3 lineages in the peripheral blood | |
| Hemoglobin concentration < 9 g/dL | Y |
| Neutrophil count < 1.0 × 109/L | Y |
| Platelet count < 100 × 109/L | Y |
| Hypertriglyceridemia (fasting ≥ 3.0 mmol/L) and/or hypofibrinogenemia (≤ 150 mg/dL) | Y |
| Hemophagocytosis in bone marrow or spleen or lymph nodes (no evidence of malignancy) | N |
| Low or absent natural killer cell activity | N/A |
| Ferritin ≥ 500 ng/mL | Y |
| Soluble cluster of differentiation 25 (i.e. soluble interleukin 2 receptor) ≥ 2400 U/mL | N |
Initially, the patient received broad-spectrum antimicrobial treatment at a local hospital with no significant improvement in symptom. Then, the patient was admitted to our hospital and after an extensive medical examination diagnosed with systemic autoimmune abnormalities induced by sHLH. She was treated with methylprednisolone (6 d), immunosuppressants (tacrolimus), and antimicrobial therapy. Plasmapheresis and etoposide were applied when the condition was extremely acute and progressive. Along with gamma globulin and leukocyte raising therapy, the patient’s temperature returned to normal. Then methylprednisolone was maintained at 50 mg/d (Figure 1E).
As the disease rapidly progressed, several scattered petechiae and ecchymoses were detected over the patient’s abdomen and extremities (Figure 2A). Besides, patient showed decrease levels of platelet and hemoglobin, and severe abdominal pain. Platelet and plasma transfusions were performed in response to coagulation dysfunction.
Anti-infective therapy
Because of methylprednisolone and immunosuppressant treatment, the patient presented with infection. As a result of granulocyte deficiency and the absence of a definite etiological diagnosis, the infection was difficult to control and, following recurrent episodes of fever, the patient was re-admitted to our hospital. After continuous antimicrobial treatment, infection foci in the lungs were eventually confined (Figure 2B-E).
The patient was discharged after her temperature normalized for 6 d and laboratory abnormalities and the patient’s condition had improved. After 1 year of follow-up, no episodes of fever were reported, and laboratory findings were normal. Chest CT showed that the shadow on the lungs had diminished (Figure 2F).
In this study, we report a case of a young female SLE patient who developed sHLH, thrombotic microvascular disease, and infection of the lungs during the course of the disease. As far as we know, this is the first case of an SLE patient with a disease course accompanied by sHLH, thrombotic microvascular disease, and infection. As a rare case of a young female with SLE accompanied by HLH. The patient presented with thrombotic microangiopathy (TMA) and infection on second admission to our hospital, which was an essential reminder for clinicians during treatment of SLE patients complicated with HLH. Besides, we also did whole exon gene sequencing to screen for genetic diseases.
Primary HLH is generated from genetic mutations disrupting cytotoxic effects such as the normal assembly of perforins and granzymes, proper trafficking and targeting of cells, and the timely cessation of the immune response. sHLH results from a malignant, infectious, or autoimmune stimulus. The pathophysiology of HLH is characterized by abnormal reciprocal activation of cytotoxic T lymphocytes, natural killer cells, and macrophages, and dramatic elevations in cytokine levels. sHLH is also referred to as macrophage activation syndrome (MAS) or more recently MAS-HLH[12]. Nearly 25% of SLE-associated MAS-HLH cases occurred when the first manifestation of the underlying disease was detected with no identifiable trigger[12]. The patient in our study had been diagnosed with SLE for 9 years and had no obvious causes for MAS-HLH, indicating that sHLH may not only accompany the first manifestation of symptoms but may also arise after long-term SLE. Clinicians should therefore be vigilant with patients suffering from intermittent fever regarding infections, malignant lymphoid tumors, and febrile recessive diseases[11]. Tuberculosis, for which there are more than 10 million new cases each year, should be ruled out as almost half of the patients present exclusively with extrapulmonary disease[13]. Our patient received empirical treatment for tuberculosis after several negative attempts to gain laboratory evidence. However, no clinical improvement was observed. Bone marrow biopsy was also performed and showed no involvement of the marrow due to lymphoma.
The previous study systematically reviewed the characteristics of patients with SLE and MAS[14]. Aziz et al[14] demonstrated that MAS development in SLE patients led to highly intensive care unit admissions and in-hospital mortalities with the presence of infection, and thrombocytopenia. Similar to the review, the patient in our case report showed dramatically increased levels of ferritin, which formed an important part of the diagnostic criteria[14]. Despite MAS-HLH being a life-threatening disorder, the complexity of the underlying diseases, triggers, and associated symptoms means that there is currently no standardized treatment protocol for HLH in adults[15]. Patients have shown the beneficial effects of treatment with a combination of corticosteroids with other immunosuppressive medications compared with corticosteroids alone[8,16]. In our case study, we used a combination treatment strategy and received a satisfactory therapeutic effect. However, there is no clear conclusion on which immunosuppressant is preferable for MAS-HLH in SLE patients[8]. Further studies are needed to investigate the detailed treatment strategies for MAS-HLH secondary to SLE.
TMA is a rare and fatal complication in SLE patients[17], occurring in 3%-9% cases of SLE cases[18]. The pathology of TMA in SLE patients is that endothelial injury results in thrombosis in capillaries and arterioles[19]. The trigger factors of TMA in SLE patients included lupus flare, infection, pregnancy, and medication non-compliance[17]. TMA has been divided into thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome. The triggering factor in our case was severe infection. TTP has been associated with more deleterious outcomes in patients with SLE[17]. Importantly, no standardized clinical treatment guideline has been reported for TMA secondary to SLE[17]. The basic treatment strategies currently employed, which include glucocorticoids, immunosuppressive therapy, anticoagulation, anti-platelet agents, and plasmapheresis, result in treatment failure in half of all patients[20]. Our patient was treated on coagulation dysfunction and a positive therapeutic effect was observed. A combination of multiple treatment strategies, including basic treatment and supportive treatment, would maximize the therapeutic effect and improve the prognosis of patients.
Pulmonary manifestations of SLE normally include disorders of the lung parenchyma, pleura, and pulmonary vasculature[21]. Furthermore, immunosuppressive treatment, which is routinely used during SLE management, predisposes to an increased risk of respiratory infections[22]. Respiratory infection often mimics acute pulmonary manifestations secondary to SLE[21]. Pulmonary infection in SLE patients presents with a wide array of symptoms and is difficult to differentiate from other pulmonary disorders related to SLE[23]. Our patient presented with infection foci in the lungs. After timely anti-infection treatment, her body temperature returned to normal, and the lung infection foci were absorbed. Careful screening of complications in SLE patients should be undertaken by clinicians to improve the prognosis of patients.
Our study had some limitations. We did not check nature killer cell activity. These are not routine tests, and it is difficult to rely on such test results to determine HLH diagnosis, as this condition occurs at such low incidence rate. Furthermore, our patient did not have continuous clinical examination data because she was admitted to hospital on two separate occasions.
This case study examines the characteristics of sHLH, infection, and the thrombotic microvascular during the course of sHLH disease in an SLE patient, which has rarely been reported to date. Our findings highlight the importance of the early recognition and treatment of sHLH in SLE cases to improve the prognosis and survival rate of MAS-HLH patients.
This case report also highlights that fever and pancytopenia acted as important clinical features of SLE and sHLH. The features that prompted consideration of sHLH in this patient were fever, pancytopenia, hypertriglyceridemia, and hyperferritinemia coupled with her 9-year history of SLE. Timely recognition, early and effective interventions to treat the triggers and pathological processes, and systematic symptomatic treatment are crucial in curbing the rapid progressive disease course. Although significant advances have been made, much work is still needed in the HLH field to deepen our understanding of this condition and improve patient outcomes.
The authors thank the patient for her cooperation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; El Chazli Y, Egypt; Tanaka H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041-4052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 798] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 2. | La Rosée P, Horne A, Hines M, von Bahr Greenwood T, Machowicz R, Berliner N, Birndt S, Gil-Herrera J, Girschikofsky M, Jordan MB, Kumar A, van Laar JAM, Lachmann G, Nichols KE, Ramanan AV, Wang Y, Wang Z, Janka G, Henter JI. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133:2465-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 651] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 3. | Ramos-Casals M, Brito-Zerón P, López-Guillermo A, Khamashta MA, Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 962] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 4. | Weitzman S. Approach to hemophagocytic syndromes. Hematology Am Soc Hematol Educ Program. 2011;2011:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Chen WT, Liu ZC, Li MS, Zhou Y, Liang SJ, Yang Y. Tuberculosis-associated hemophagocytic lymphohistiocytosis misdiagnosed as systemic lupus erythematosus: A case report. World J Clin Cases. 2022;10:3178-3187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 6. | Patel AR, Desai PV, Banskota SU, Edigin E, Manadan AM. Hemophagocytic Lymphohistiocytosis Hospitalizations in Adults and Its Association With Rheumatologic Diseases: Data From Nationwide Inpatient Sample. J Clin Rheumatol. 2022;28:e171-e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Gupta D, Mohanty S, Thakral D, Bagga A, Wig N, Mitra DK. Unusual Association of Hemophagocytic Lymphohistiocytosis in Systemic Lupus Erythematosus: Cases Reported at Tertiary Care Center. Am J Case Rep. 2016;17:739-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Dall'Ara F, Cavazzana I, Frassi M, Taraborelli M, Fredi M, Franceschini F, Andreoli L, Rossi M, Cattaneo C, Tincani A, Airò P. Macrophage activation syndrome in adult systemic lupus erythematosus: report of seven adult cases from a single Italian rheumatology center. Reumatismo. 2018;70:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, Smolen JS, Wofsy D, Boumpas DT, Kamen DL, Jayne D, Cervera R, Costedoat-Chalumeau N, Diamond B, Gladman DD, Hahn B, Hiepe F, Jacobsen S, Khanna D, Lerstrøm K, Massarotti E, McCune J, Ruiz-Irastorza G, Sanchez-Guerrero J, Schneider M, Urowitz M, Bertsias G, Hoyer BF, Leuchten N, Tani C, Tedeschi SK, Touma Z, Schmajuk G, Anic B, Assan F, Chan TM, Clarke AE, Crow MK, Czirják L, Doria A, Graninger W, Halda-Kiss B, Hasni S, Izmirly PM, Jung M, Kumánovics G, Mariette X, Padjen I, Pego-Reigosa JM, Romero-Diaz J, Rúa-Figueroa Fernández Í, Seror R, Stummvoll GH, Tanaka Y, Tektonidou MG, Vasconcelos C, Vital EM, Wallace DJ, Yavuz S, Meroni PL, Fritzler MJ, Naden R, Dörner T, Johnson SR. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 2019;78:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 890] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 10. | Jesus D, Larosa M, Henriques C, Matos A, Zen M, Tomé P, Alves V, Costa N, Le Guern V, Iaccarino L, Costedoat-Chalumeau N, Doria A, Inês LS. Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) enables accurate and user-friendly definitions of clinical remission and categories of disease activity. Ann Rheum Dis. 2021;80:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3602] [Article Influence: 200.1] [Reference Citation Analysis (1)] |
| 12. | Lorenz G, Schul L, Schraml F, Riedhammer KM, Einwächter H, Verbeek M, Slotta-Huspenina J, Schmaderer C, Küchle C, Heemann U, Moog P. Adult macrophage activation syndrome-haemophagocytic lymphohistiocytosis: 'of plasma exchange and immunosuppressive escalation strategies' - a single centre reflection. Lupus. 2020;29:324-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Furin J, Cox H, Pai M. Tuberculosis. Lancet. 2019;393:1642-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 553] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 14. | Aziz A, Castaneda EE, Ahmad N, Veerapalli H, Rockferry AG, Lankala CR, Hamid P. Exploring Macrophage Activation Syndrome Secondary to Systemic Lupus Erythematosus in Adults: A Systematic Review of the Literature. Cureus. 2021;13:e18822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood. 2015;125:2908-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 16. | Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheumatol. 2014;66:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 17. | Kello N, Khoury LE, Marder G, Furie R, Zapantis E, Horowitz DL. Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: Case series and review of literature. Semin Arthritis Rheum. 2019;49:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Magil AB, McFadden D, Rae A. Lupus glomerulonephritis with thrombotic microangiopathy. Hum Pathol. 1986;17:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Benz K, Amann K. Thrombotic microangiopathy: new insights. Curr Opin Nephrol Hypertens. 2010;19:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Pattanashetti N, Anakutti H, Ramachandran R, Rathi M, Sharma A, Nada R, Gupta KL. Effect of Thrombotic Microangiopathy on Clinical Outcomes in Indian Patients With Lupus Nephritis. Kidney Int Rep. 2017;2:844-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Amarnani R, Yeoh SA, Denneny EK, Wincup C. Lupus and the Lungs: The Assessment and Management of Pulmonary Manifestations of Systemic Lupus Erythematosus. Front Med (Lausanne). 2020;7:610257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 22. | Cuchacovich R, Gedalia A. Pathophysiology and clinical spectrum of infections in systemic lupus erythematosus. Rheum Dis Clin North Am. 2009;35:75-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Hannah JR, D'Cruz DP. Pulmonary Complications of Systemic Lupus Erythematosus. Semin Respir Crit Care Med. 2019;40:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |